Prediction of α-Glucosidase Inhibitory Activity of LC-ESI-TQ-MS/MS-Identified Compounds from Tradescantia pallida Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Extraction
2.4. Identification of Phenolic Compounds
2.5. In Silico Analysis
2.5.1. Protein Preparation
2.5.2. Ligand Preparation
2.5.3. Molecular Docking
2.5.4. Prime MM-GBSA Calculations
2.5.5. MD Simulations
3. Results
3.1. Identification of Polyphenols
3.2. In Silico Analysis
3.2.1. Molecular Docking
3.2.2. Energy Minimization Calculations
3.2.3. MD Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kehm, R.; Rückriemen, J.; Weber, D.; Deubel, S.; Grune, T.; Höhn, A. Endogenous advanced glycation end products in pancreatic islets after short-term carbohydrate intervention in obese, diabetes-prone mice. Nutr. Diabetes 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peytam, F.; Takalloobanafshi, G.; Saadattalab, T.; Norouzbahari, M.; Emamgholipour, Z.; Moghimi, S.; Firoozpour, L.; Bijanzadeh, H.R.; Faramarzi, M.A.; Mojtabavi, S. Design, synthesis, molecular docking, and in vitro α-glucosidase inhibitory activities of novel 3-amino-2, 4-diarylbenzo [4, 5] imidazo [1, 2-a] pyrimidines against yeast and rat α-glucosidase. Sci. Rep. 2021, 11, 11911. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Vizzotto, M. Synergisms in alpha-glucosidase inhibition and antioxidant activity of camellia sinensis l. kuntze and eugenia uniflora l. ethanolic extracts. Pharmacogn. Res. 2017, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442. [Google Scholar] [PubMed]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M. Citrus flavanone narirutin, in vitro and in silico mechanistic antidiabetic potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. A Combination Approach in Inhibiting Type 2 Diabetes-Related Enzymes Using Ecklonia radiata Fucoidan and Acarbose. Pharmaceutics 2021, 13, 1979. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Gardner, Z.; McGuffin, M. American Herbal Products Association’s Botanical Safety Handbook; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of Withania frutescens L. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M. Diabetesmellitus type 2: An international challenge. Ann. Res. Dial. 2016, 1, 1. [Google Scholar]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2021, 21, 1049–1079. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Bester, M.J.; Neitz, A.W.; Gaspar, A.R. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed. Pharmacother. 2018, 107, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Costa, L.; Lean, M.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Maruca, A.; Moraca, F.; Rocca, R.; Molisani, F.; Alcaro, F.; Gidaro, M.C.; Alcaro, S.; Costa, G.; Ortuso, F. Chemoinformatic database building and in silico hit-identification of potential multi-targeting bioactive compounds extracted from mushroom species. Molecules 2017, 22, 1571. [Google Scholar] [CrossRef] [Green Version]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Gavillán-Suárez, J.; Aguilar-Perez, A.; Rivera-Ortiz, N.; Rodríguez-Tirado, K.; Figueroa-Cuilan, W.; Morales-Santiago, L.; Maldonado-Martínez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Chemical profile and in vivo hypoglycemic effects of Syzygiumjambos, Costusspeciosus and Tapeinochilosananassae plant extracts used as diabetes adjuvants in Puerto Rico. BMC Complement. Altern. Med. 2015, 15, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.B.L.; Kwan, Y.M. The biological activities of the spiderworts (Tradescantia). Food Chem. 2020, 317, 126411. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Tsou, G.; Sigel, C.W. Datiscacin, a novel cytotoxic cucurbitacin 20-acetate from Datisca glomerata. J. Org. Chem. 1973, 38, 1420–1421. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Characterization of phenolic profile by LC-ESI-MS/MS and enzyme inhibitory activities of two wild edible garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef] [PubMed]
- De Monte, C.; Carradori, S.; Chimenti, P.; Secci, D.; Mannina, L.; Alcaro, F.; Petzer, A.; Clarina, I.D.; Gidaro, M.C.; Costa, G. New insights into the biological properties of Crocus sativus L.: Chemical modifications, human monoamine oxidases inhibition and molecular modeling studies. Eur. J. Med. Chem. 2014, 82, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef] [Green Version]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure moleculardynamicsalgorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Humphreys, D.D.; Friesner, R.A.; Berne, B.J. A multiple-time-step molecular dynamics algorithm for macromolecules. J. Phys. Chem. 1994, 98, 6885–6892. [Google Scholar] [CrossRef]
- Tian, J.-L.; Si, X.; Wang, Y.-H.; Gong, E.-S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef] [PubMed]
- El Gizawy, H.A.; Abo-Salem, H.M.; Ali, A.A.; Hussein, M.A. Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAA α5, GSK-3β, and p38α MAP-Kinase Genes. ACS Omega 2021, 6, 20492–20511. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound polyphenols from red quinoa prevailed over free polyphenols in reducing postprandial blood glucose rises by inhibiting α-glucosidase activity and starch digestion. Nutrients 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, S.; Xie, J.; Chen, Y.; Dong, R.; Zhang, X.; Liu, S.; Xie, J.; Hu, X.; Yu, Q. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. Lwt 2020, 132, 109943. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Sarma, B.; Singh, U. Ferulic acid may prevent infection of Cicer arietinum by Sclerotium rolfsii. World J. Microbiol. Biotechnol. 2003, 19, 123–127. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Kıvrak, İ. Analytical methods applied to assess chemical composition, nutritional value and in vitro bioactivities of Terfeziaolbiensis and Terfeziaclaveryi from Turkey. Food Anal. Methods 2015, 8, 1279–1293. [Google Scholar] [CrossRef]
- Ramabulana, A.-T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, Y.; Xiong, S.; Sun, P.; Deng, Z. A UPLC–MS/MS method for comparative pharmacokinetics study of morusin and morin in normal and diabetic rats. Biomed. Chromatogr. 2019, 33, e4516. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liao, X.; Chen, Z. Determination of bioactive components in the fruits of Cercis chinensis Bunge by HPLC-MS/MS and quality evaluation by principal components and hierarchical cluster analyses. J. Pharm. Anal. 2021, 11, 465–471. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Wu, L.; Lin, X.; Hu, X.; Wang, L. Phenolic profiles and screening of potential α-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESI-qTOF-MS/MS and molecular docking analysis. Ind. Crops Prod. 2020, 154, 112673. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Chaturvedi, N.; Singh, A.; Mishra, A. Characterization, inhibitory activity and mechanism of polyphenols from faba bean (gallic-acid and catechin) on α-glucosidase: Insights from molecular docking and simulation study. Prep. Biochem. Biotechnol. 2020, 50, 123–132. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.; Hou, T. End-point binding free energy calculation with MM/PBSA and MM/GBSA: Strategies and applications in drug design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Azam, F.; Abodabos, H.S.; Taban, I.M.; Rfieda, A.R.; Mahmood, D.; Anwar, M.J.; Khan, S.; Sizochenko, N.; Poli, G.; Tuccinardi, T. Rutin as promising drug for the treatment of Parkinson’s disease: An assessment of MAO-B inhibitory potential by docking, molecular dynamics and DFT studies. Mol. Simul. 2019, 45, 1563–1571. [Google Scholar] [CrossRef]
- Azam, F.; Alabdullah, N.H.; Ehmedat, H.M.; Abulifa, A.R.; Taban, I.; Upadhyayula, S. NSAIDs as potential treatment option for preventing amyloid β toxicity in Alzheimer’s disease: An investigation by docking, molecular dynamics, and DFT studies. J. Biomol. Struct. Dyn. 2018, 36, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine natural compounds as potents inhibitors against the main protease of SARS-CoV-2—A molecular dynamic study. J. Biomol. Struct. Dyn. 2021, 39, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Swargiary, A.; Roy, M.K.; Mahmud, S. Phenolic compounds as α-glucosidase inhibitors: A docking and molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2022, 1–10, Online ahead of print. [Google Scholar] [CrossRef]
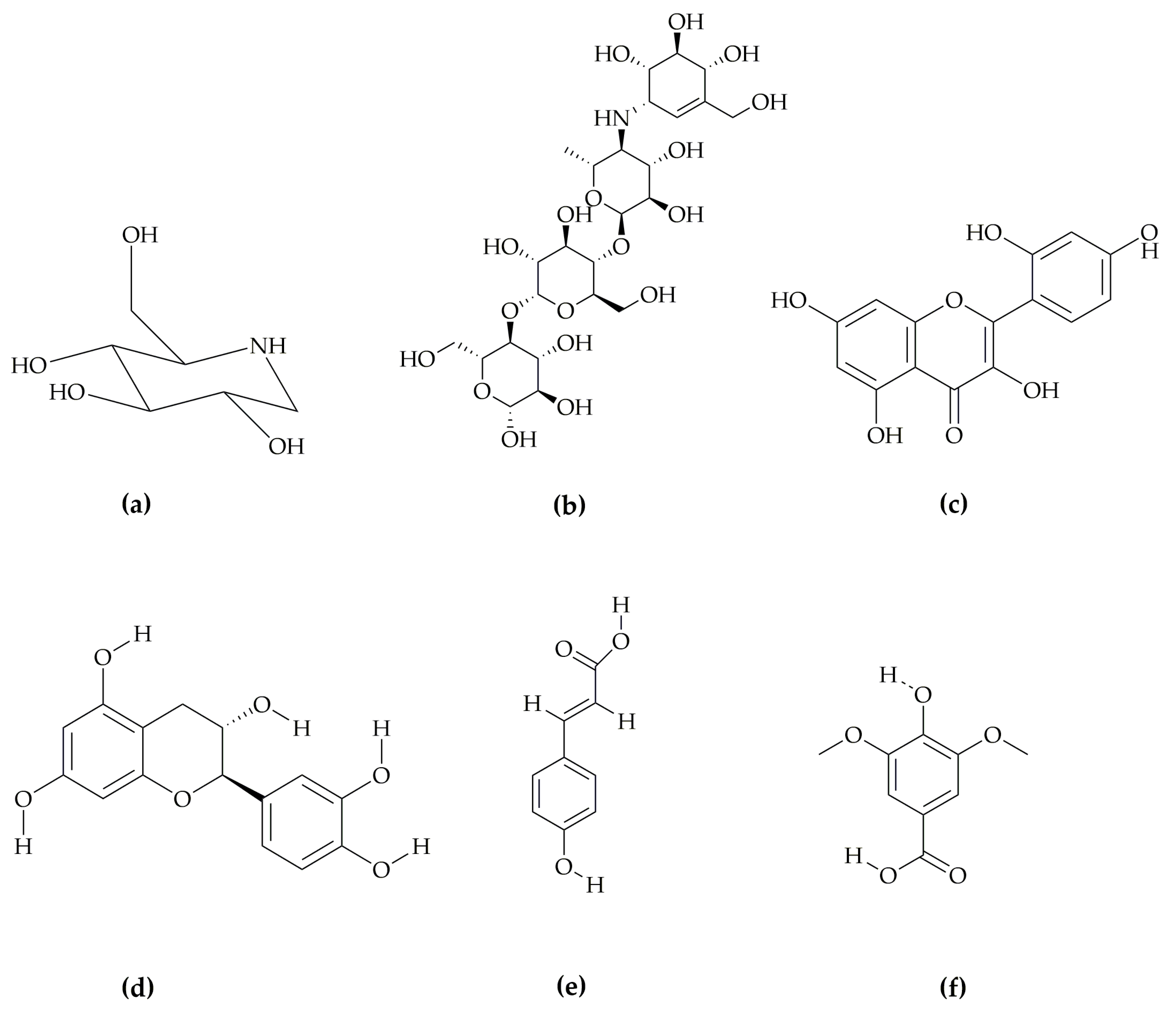
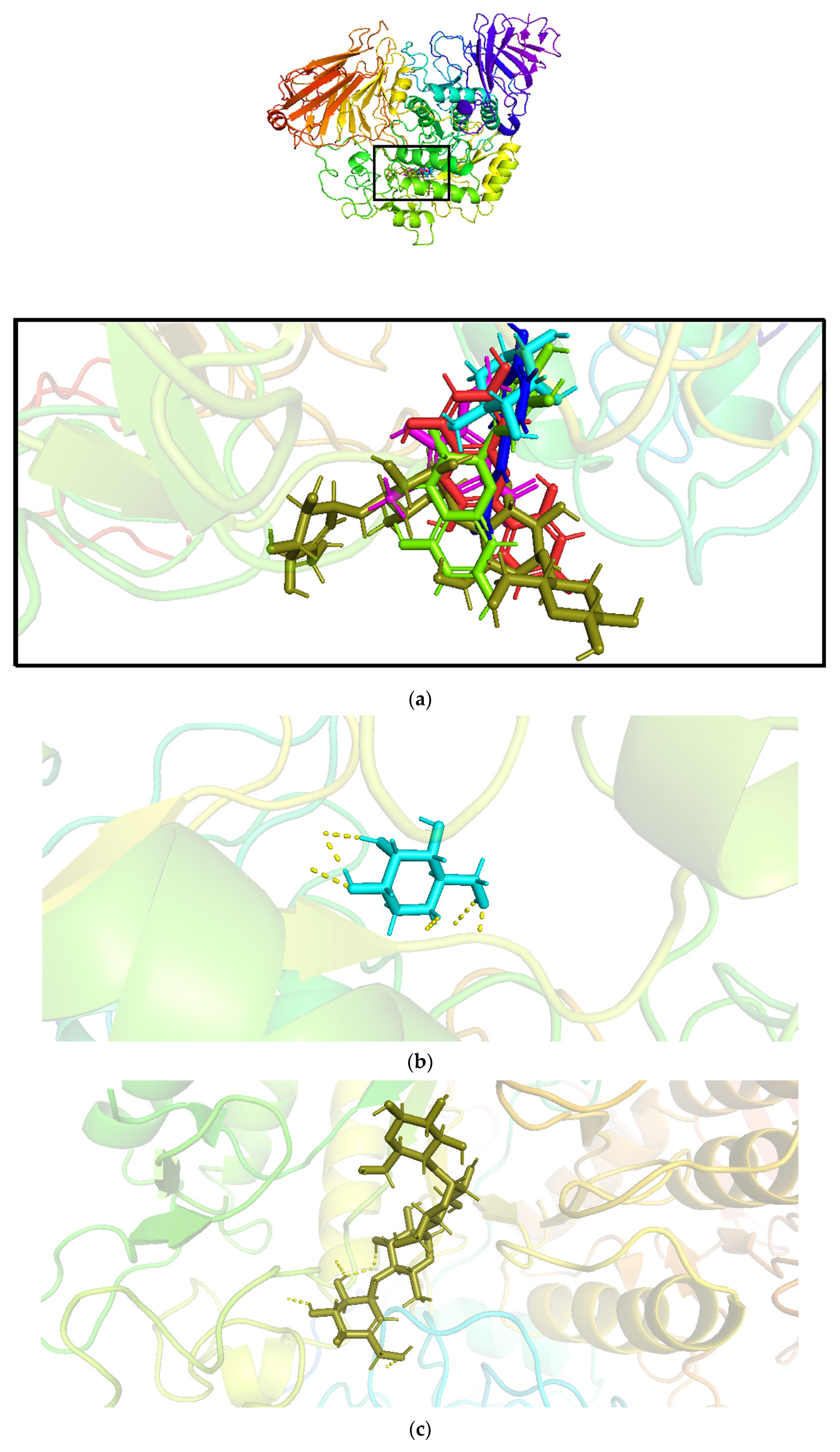


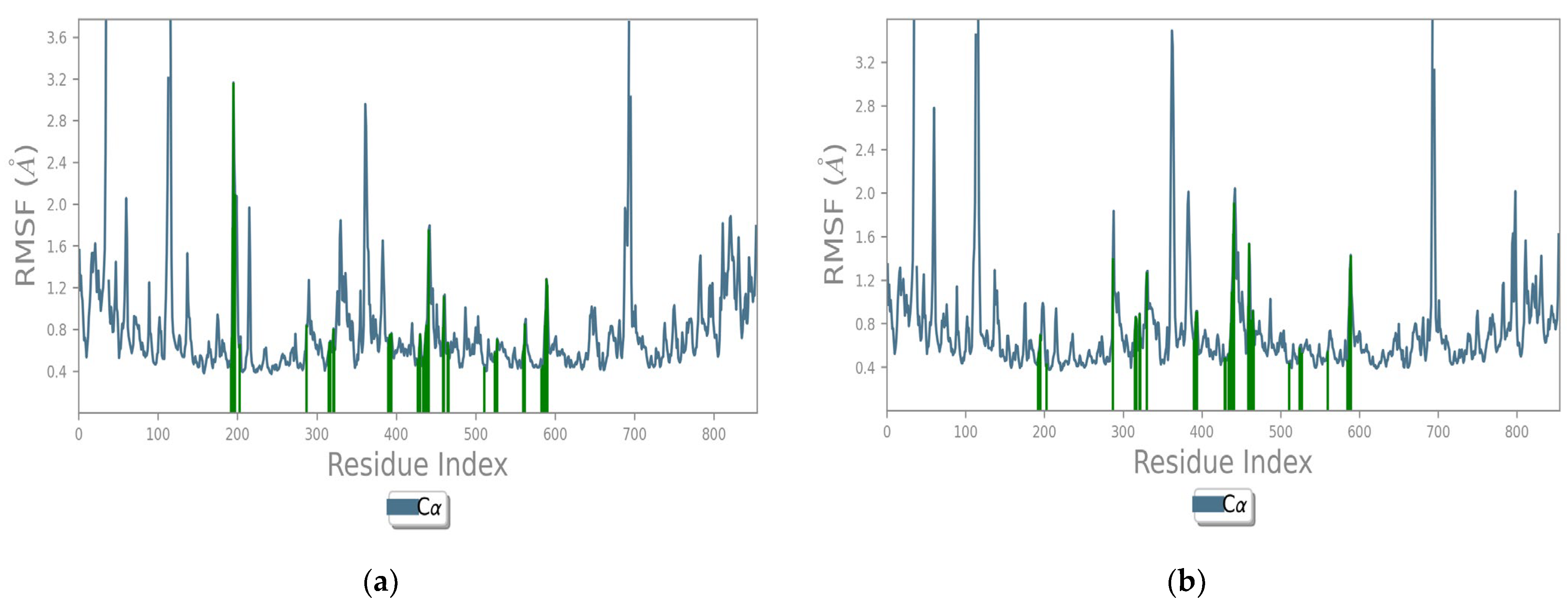
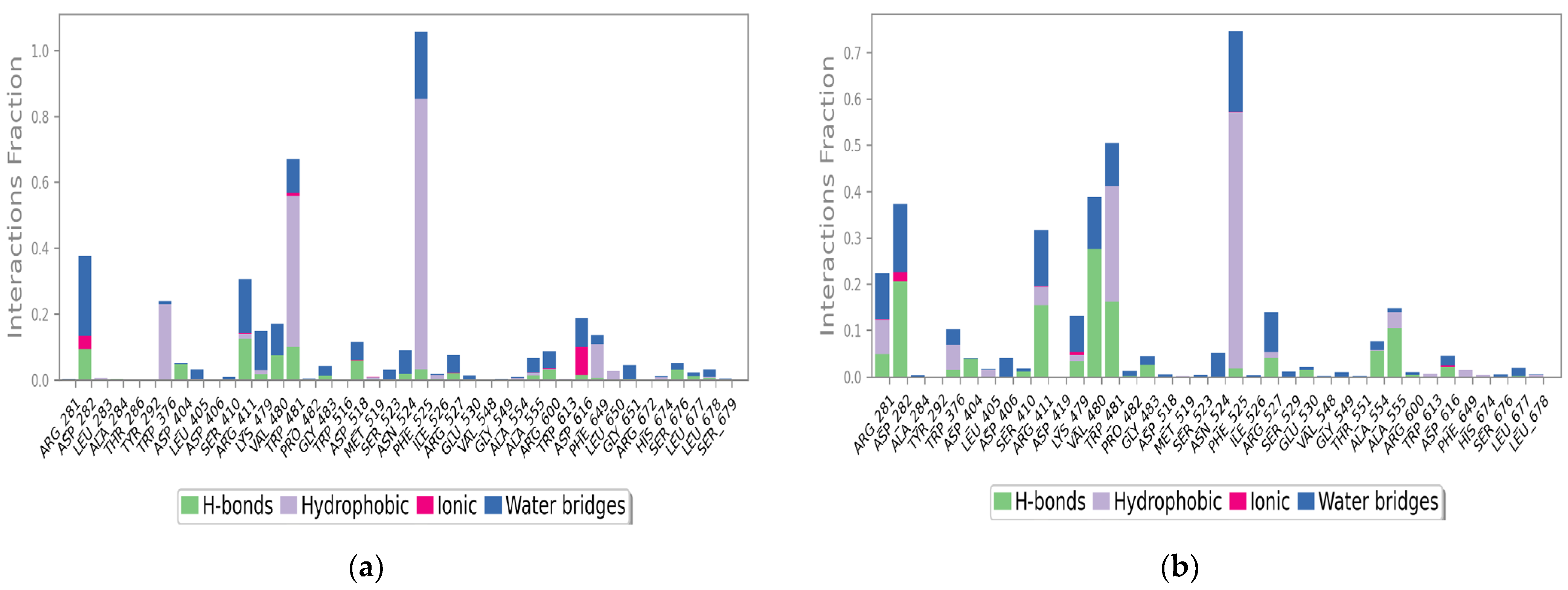
| Time(min) | 10 | 20 | 30 | 40 | 50 | 56 | 60 | 66 |
|---|---|---|---|---|---|---|---|---|
| A (%) | 94 | 89 | 80 | 70 | 59 | 40 | 20 | 6 |
| B (%) | 6 | 11 | 20 | 30 | 41 | 60 | 80 | 94 |
| Ligand–Protein Complex | Water Molecules | Na+ | Cl− |
|---|---|---|---|
| Morin–5NN5 | 22,059 | 84 | 61 |
| Catechin–5NN5 | 22,084 | 85 | 62 |
| Compound Name | Molecular Formula | Retention Time | Prec Ion | Pro Ion | Frag | CE | Polarity |
|---|---|---|---|---|---|---|---|
| (min) | (m/z) | (V) | |||||
| p-coumaric acid | 5.2 | 163.01 | 119.00 | 105 | 17 | Neg | |
| Trans-ferulic acid | 7.8 | 193.00 | 134.01 | 55 | 17 | Neg | |
| 4-hydroxybenzoic acid | 8 | 144.00 | 99.03 | 40 | 19 | Neg | |
| Vanillic acid | 16.1 | 167.01 | 108.00 | 45 | 20 | Neg | |
| Naringenin | 18 | 271.01 | 151.00 | 75 | 22 | Neg | |
| Gallic acid | 20 | 169.04 | 125.01 | 55 | 18 | Neg | |
| Quercetin | 20.9 | 301.00 | 151.00 | 105 | 24 | Neg | |
| Morin | 27.7 | 301.06 | 150.75 | 105 | 24 | Neg | |
| Syringic acid | 42 | 197.01 | 182.08 | 90 | 15 | Neg | |
| Catechin | 42.9 | 289.06 | 203.12 | 90 | 20 | Neg | |
| Epicatechin | 43.3 | 289.11 | 203.00 | 85 | 21 | Neg | |
| Catechol | 44.2 | 109.99 | 108.00 | 30 | Neg | ||
| Chlorogenic acid | 47.3 | 353.01 | 191.00 | 50 | 20 | Neg |
| Ligand | Binding Affinity | Glide Score | Glide Energy | Glide Evdw | XP-Hbond | XP Penalties | Glide–Ligand Efficiency | Hydrogen Bonding | Hydrogen Bond Distance |
|---|---|---|---|---|---|---|---|---|---|
| Kcal/mol | Å | ||||||||
| 5NN5 | |||||||||
| 1-DNJ | −6.325 | −6.329 | −26.474 | −5.250 | −1.920 | 0.003 | −0.575 | ASP404 O—ligand H | 1.9, 2.2 |
| ASP518 O—ligand H | 2.2 | ||||||||
| ARG600 H—ligand O | 1.7 | ||||||||
| ASP616 O—ligand H | 2.2 | ||||||||
| HIP674 H—ligand O | 2.5 | ||||||||
| Standard | −6.187 | −6.694 | −21.722 | −3.337 | −4.245 | 0.507 | −0.141 | ARG281 H—ligand O | 2.1 |
| ASP282 O—ligand H | 1.4, 2.2 | ||||||||
| SER523 O—ligand H | 2.7 | ||||||||
| ASN524 O—ligand H | 2.2 | ||||||||
| FTP1 | −5.704 | −5.959 | −32.474 | −22.588 | −1.914 | 0.254 | −0.259 | ASP282 O—ligand H | 2.5 |
| ARG600 H—ligand O | 2.1, 2.4 | ||||||||
| ASP518 O—ligand H | 1.6 | ||||||||
| FTP2 | −5.096 | −5.096 | −35.033 | −19.426 | −1.775 | 0 | −0.243 | ASP616 O—ligand H | 2.0 |
| ARG600 H—ligand O | 1.9 | ||||||||
| ASP404 O—ligand H | 1.6, 1.8 | ||||||||
| FTP3 | −3.082 | −3.082 | −19.143 | −15.676 | −0.346 | 0 | −0.257 | ASP518 O—ligand H | 1.9 |
| FTP4 | −2.944 | −2.944 | −19.011 | −14.862 | −1.341 | 0 | −0.210 | ARG600 H—ligand O | 2.0, 2.3, 2.5 |
| ASP282 O—ligand H | 2.0 | ||||||||
| Ligand | Ligand Efficiency | Ligand Efficiency sa | Ligand Efficiency ln | ΔG Bind (NS) | ΔG Bind (NS) Coulomb | ΔG Bind (NS) Hbond | ΔG Bind (NS) Lipo | ΔG Bind (NS) Packing | ΔG Bind (NS) SolvGB | ΔG Bind (NS) vdW |
|---|---|---|---|---|---|---|---|---|---|---|
| Kcal/mol | ||||||||||
| 5NN5 | ||||||||||
| Standard | −0.141 | −0.496 | −1.293 | −31.300 | −25.240 | −2.800 | −14.760 | 0 | 48.500 | −50.910 |
| FTP1 | −0.210 | −0.507 | −0.809 | 3.600 | 64.730 | −2.160 | −8.160 | −0.350 | −27.550 | −22.900 |
| FTP2 | −0.243 | −0.669 | −1.260 | −21.190 | −21.590 | −3.110 | −15.840 | −2.330 | 43.740 | −31.070 |
| FTP3 | −0.257 | −0.588 | −0.884 | −0.120 | 60.96 | −0.750 | −13.640 | −1.820 | −24.780 | −20.090 |
| FTP4 | −0.243 | −0.669 | −1.260 | −21.190 | −21.590 | −3.110 | −15.840 | −2.330 | 43.740 | −31.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiaz, F.; Islam, M.; Saeed, H.; Ahmed, A.; Hashmi, F.K.; Khan, K.M.; Dar, U.I.; Ullah, K.; Rana, S.M.; Saleem, B.; et al. Prediction of α-Glucosidase Inhibitory Activity of LC-ESI-TQ-MS/MS-Identified Compounds from Tradescantia pallida Leaves. Pharmaceutics 2022, 14, 2578. https://doi.org/10.3390/pharmaceutics14122578
Imtiaz F, Islam M, Saeed H, Ahmed A, Hashmi FK, Khan KM, Dar UI, Ullah K, Rana SM, Saleem B, et al. Prediction of α-Glucosidase Inhibitory Activity of LC-ESI-TQ-MS/MS-Identified Compounds from Tradescantia pallida Leaves. Pharmaceutics. 2022; 14(12):2578. https://doi.org/10.3390/pharmaceutics14122578
Chicago/Turabian StyleImtiaz, Fariha, Muhammad Islam, Hamid Saeed, Abrar Ahmed, Furqan Khurshid Hashmi, Kashif Maqbool Khan, Umair Ikram Dar, Kalim Ullah, Sibghat Mansoor Rana, Bushra Saleem, and et al. 2022. "Prediction of α-Glucosidase Inhibitory Activity of LC-ESI-TQ-MS/MS-Identified Compounds from Tradescantia pallida Leaves" Pharmaceutics 14, no. 12: 2578. https://doi.org/10.3390/pharmaceutics14122578







