In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Evaluation of the Oils
2.1.1. Density (ρ)
2.1.2. Dynamic Viscosity (η)
2.1.3. Melatonin Carrier Capacity Evaluated as Plipid/water Coefficient
2.1.4. Melatonin Degradation and Half-Life
2.1.5. Initial Peroxide Value (IP) and Oil Oxidation under Stress Conditions
2.2. In Vivo Evaluation of the Oils
2.3. Statistical Data Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorini, O.; Lorio, S.; Ciliberti, R.M.L.; Licata, M.; Armocida, G. Olive oil in pharmacological and cosmetic traditions. J. Cosmet. Dermatol. 2019, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Bielfeldt, S.; Blaak, J.; Staib, P.; Simon, I.; Wohlfart, R.; Manger, C.; Wilhelm, K.P. Observer-blind randomized controlled study of a cosmetic blend of safflower, olive and other plant oils in the improvement of scar and striae appearance. Int. J. Cosmet. Sci. 2018, 40, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budiyanto, A.; Ahmed, N.U.; Wu, A.; Bito, T.; Nikaido, O.; Osawa, T.; Ueda, M.; Ichihashi, M. Protective effect of topically applied olive oil against photocarcinogenesis following UVB exposure of mice. Carcinogenesis 2000, 21, 2085–2090. [Google Scholar] [CrossRef] [Green Version]
- Kiechl-Kohlendorfer, U.; Berger, C.; Inzinger, R. The effect of daily treatment with an olive oil/lanolin emollient on skin integrity in preterm infants: A randomized controlled trial. Pediatr. Dermatol. 2008, 25, 174–178. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Massei, G.; Hartley, S.E. Disarmed by domestication? Induced responses to browsing in wild and cultivated olive. Oecologia 2000, 122, 225–231. [Google Scholar] [CrossRef]
- Leon, L.; Rosa, R.; Velasco, L.; Belaj, A. Using wild olives in breeding programs: Implications on oil quality composition. Front. Plant. Sci. 2018, 9, 232. [Google Scholar] [CrossRef]
- Espínola, F.; Vidal, A.M.; Espínola, J.M.; Moya, M. Processing Effect and Characterization of Olive Oils from Spanish Wild Olive Trees (Olea europaea var. sylvestris). Molecules 2021, 26, 1304. [Google Scholar] [CrossRef] [PubMed]
- Espejo Maqueda, J. Estudio Analítico Comparado Entre el Aceite de Acebuchina y el Aceite de Oliva Virgen. Ph.D. Thesis, University of Seville, Seville, Spain, 2005. [Google Scholar]
- Hannachi, H.; Elfalleh, W.; Marzouk, S. Oil, protein, antioxidants and free radical scavenging activity of stone from wild olive trees (Olea europaea L.). Pak. J. Pharm. Sci. 2013, 26, 503–510. [Google Scholar]
- Marchena, A.M.; Franco, L.; Romero, A.M.; Barriga, C.; Rodríguez, A.B. Lycopene and Melatonin: Antioxidant Compounds in Cosmetic Formulations. Ski. Pharmacol. Physiol. 2020, 33, 237–243. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef] [Green Version]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, D.J.; Mraz-Robinson, D.; Granger, C. Efficacy and safety of a 3-in-1 antiaging night facial serum containing melatonin, bakuchiol, and ascorbyl tetraisopalmitate through clinical and histological analysis. J. Cosmet. Dermatol. 2020, 19, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. Use of Cutometer to assess epidermal hydration. Ski. Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. Evaluation of the inhibitory activity of topical indomethacin, betamethasone valerate and emollients on UVL-induced inflammation by means of non-invasive measurements of the skin elasticity. Photodermatol. Photoimmunol. Photomed. 2001, 17, 184–188. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity--in vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Ferrillo, M.; Vastarella, M.; Cantelli, M.; Mazzella, C.; Fabbrocini, G. Instrumental, clinical and subjective evaluation of the efficacy of a cosmetic treatment for home use. J. Cosmet. Laser Ther. 2019, 21, 190–195. [Google Scholar] [CrossRef]
- Lubart, R.; Yariv, I.; Fixler, D.; Lipovsky, A. Topical Hyaluronic Acid Facial Cream with New Micronized Molecule Technology Effectively Penetrates and Improves Facial Skin Quality: Results from In-vitro, Ex-vivo, and In-vivo (Open-label) Studies. J. Clin. Aesthet. Dermatol. 2019, 12, 39–44. [Google Scholar]
- Yilmaz, E.; Borchert, H.H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema--an in vivo study. Int. J. Pharm. 2006, 307, 232–238. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.C.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q. A Randomized, Active Comparator-controlled Clinical Trial of a Topical Botanical Cream for Skin Hydration, Elasticity, Firmness, and Cellulite. J. Clin. Aesthetic Dermatol. 2018, 11, 51–57. [Google Scholar]
- Muizzuddin, N.; Benjamin, R. Beauty from within: Oral administration of a sulfur-containing supplement methylsulfonylmethane improves signs of skin ageing. Int. J. Vitam. Nutr. Res. 2020, 21, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Matji, A.; Donato, N.; Gagol, A.; Morales, E.; Carvajal, L.; Serrano, D.R.; Worku, Z.A.; Healy, A.M.; Torrado, J.J. Predicting the critical quality attributes of ibuprofen tablets via modelling of process parameters for roller compaction and tabletting. Int. J. Pharm. 2019, 565, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Alonso, T.R.; Gagol, A.; Scherer, M.; Matji, A.; Torrado-Santiago, S.; Serrano, D.; Garcia-Arieta, A.; Torrado, J.J. A multivariate investigation into the relationship between pharmaceutical characteristics and patient preferences of bioequivalent ibuprofen tablets. Patient Prefer. Adherence 2018, 12, 1927–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Pharmacopoeia. European Directorate for the Quality of Medicines & Health Care, 10th ed.; European Pharmacopoeia: Strasbourg, France, 2021; pp. 27–169. [Google Scholar]
- USP 38. NF 33, The United States Pharmacopoeial Convention; Spanish Edition; USP: Rockville, MD, USA, 2015; pp. 6571–6574. [Google Scholar]
- Microcaya Dermoanalyzer Probes. Available online: https://www.microcaya.com/productos/analizadores-de-piel/para-investigacion/33-cutometer-dual-mpa-580 (accessed on 25 July 2022).
- Jiménez-López, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Grajzer, M.; Prescha, A.; Korzonek, K.; Wojakowska, A.; Dziadas, M.; Kulma, A.; Grajeta, H. Characteristics of rose hip (Rosa canina L.) cold-pressed oil and its oxidative stability studied by the differential scanning calorimetry method. Food Chem. 2015, 188, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; López-Muñoz, F.; de los Ríos, C.; Egea, J.; Gil-Martín, E.; Pita, R.; Torrado, J.J.; Serrano, D.R.; Juberías, A. Toxicology of blister agents: Is melatonin a potential therapeutic option? Diseases 2021, 9, 27. [Google Scholar] [CrossRef]
- Kovacs, A.; Péter-Héderi, D.; Perei, K.; Budai-Szűcs, M.; Léber, A.; Gácsi, A.; Csányi, E.; Berkó, S. Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care. Pharmaceutics 2020, 12, 729. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.R.; Kwon, D.I.; Park, M.S.; Lim, D.H. Depth profiling of epidermal hydration inducing improvement of skin roughness and elasticity: In vivo study by con-focal Raman spectroscopy. J. Cosmet. Dermatol. 2022, 21, 4810–4817. [Google Scholar] [CrossRef]
- Kim, M.A.; Kim, E.J.; Lee, H.K. Use of SkinFibrometer® to measure skin elasticity and its correlation with Cutometer® and DUB® Skinscanner. Ski. Res. Technol. 2018, 24, 466–471. [Google Scholar] [CrossRef] [PubMed]
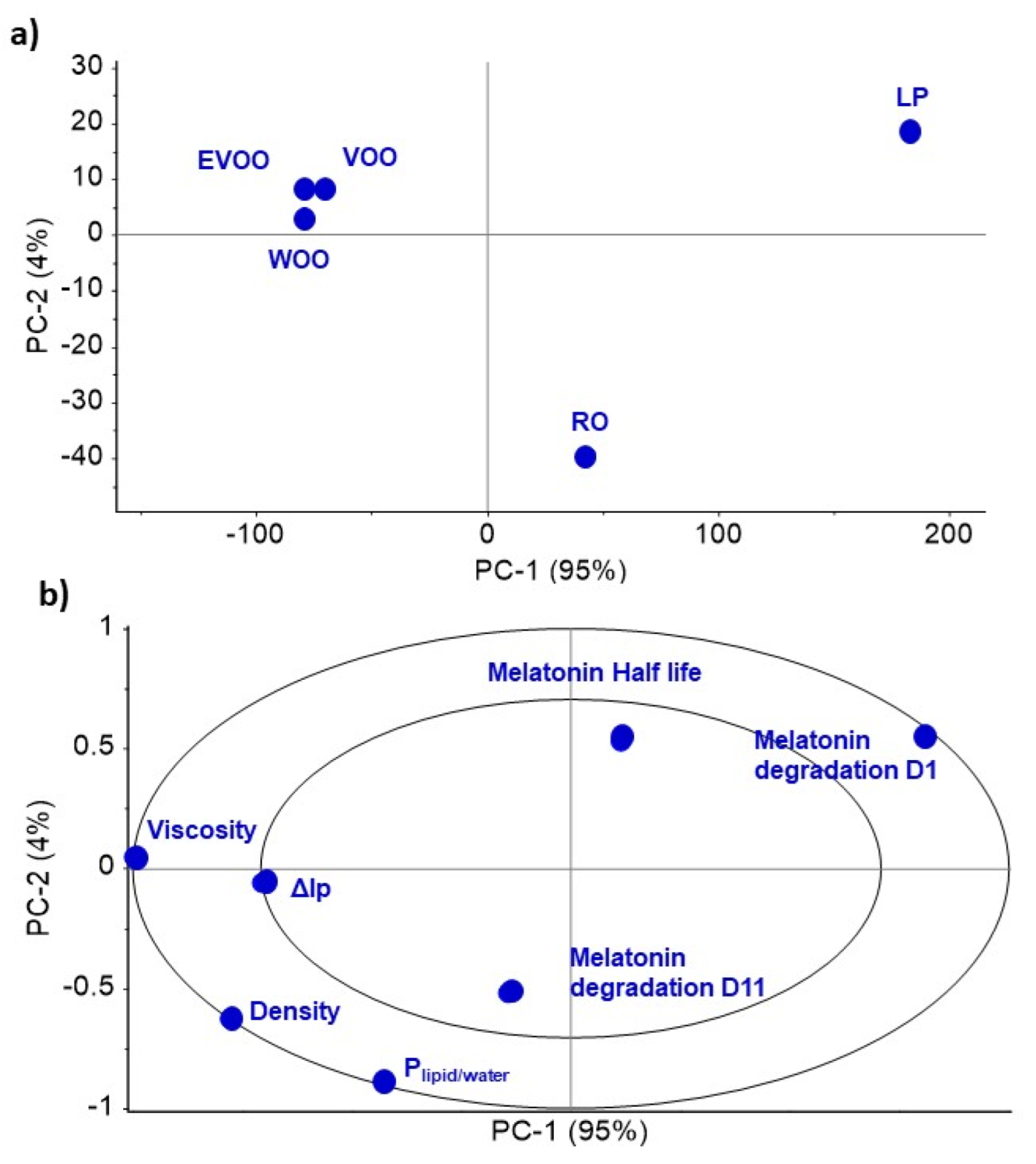


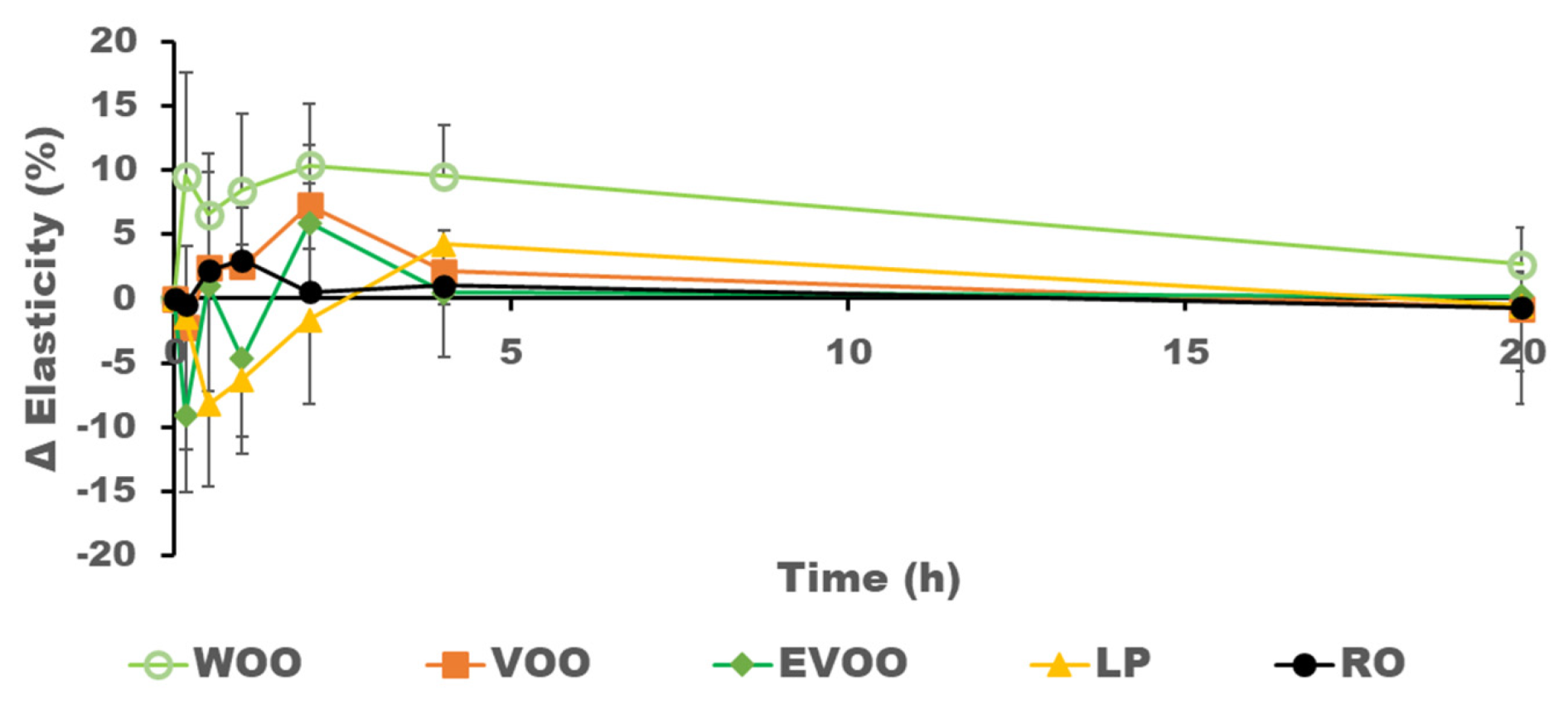
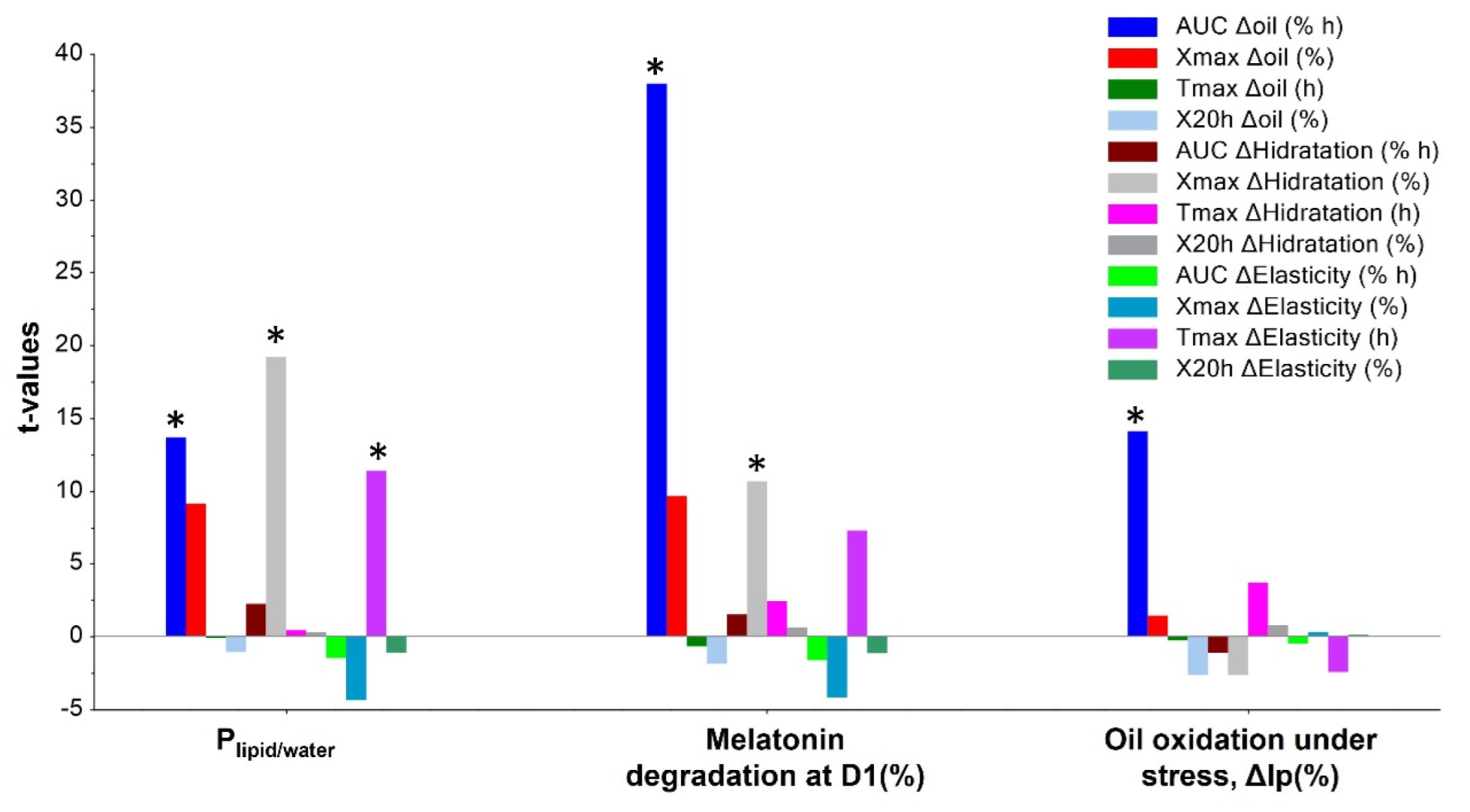
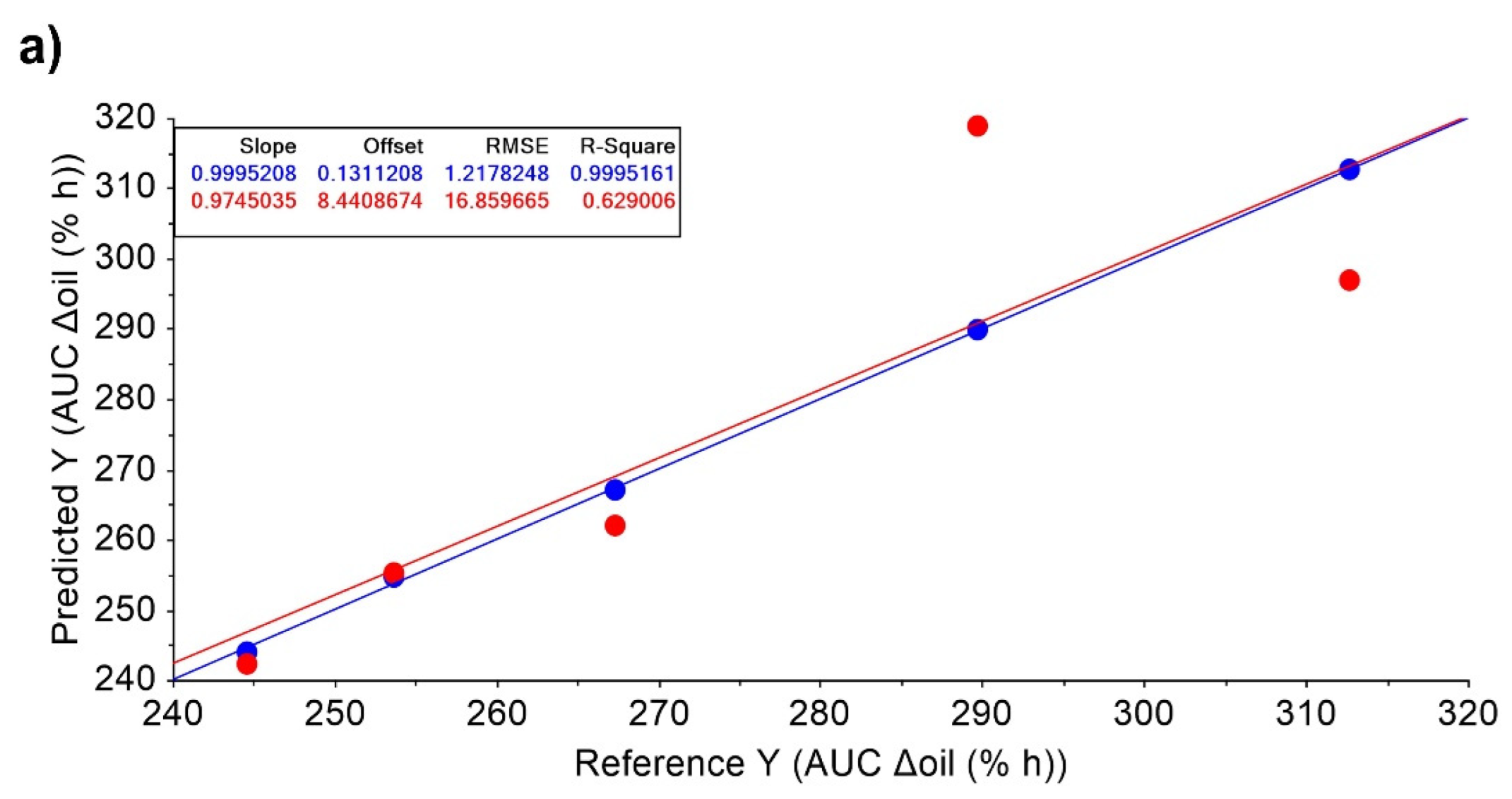

| Subject | Period 1 | Period 2 | Period 3 | Period 4 | Period 5 |
|---|---|---|---|---|---|
| 1 | LP | RO | WOO | EVOO | VOO |
| 2 | LP | RO | WOO | EVOO | VOO |
| 3 | VOO | LP | RO | WOO | EVOO |
| 4 | VOO | LP | RO | WOO | EVOO |
| 5 | WOO | EVOO | LP | RO | VOO |
| 6 | WOO | EVOO | LP | RO | VOO |
| 7 | RO | WOO | VOO | LP | EVOO |
| 8 | RO | WOO | VOO | LP | EVOO |
| Parameter | RO | LP | EVOO | WOO | VOO |
|---|---|---|---|---|---|
| Density (mg/mL) | 930.3 ± 1.1 | 844.5 ± 2.3 | 912.8 ± 3.4 | 917.3 ± 2.3 | 913.4 ± 2.8 |
| Dynamic Viscosity (mPa s) | 279.1 ± 31.2 | 157.6 ± 3.8 | 408.9 ± 10 | 410.3 ± 31.4 | 404.9 ± 14.5 |
| Melatonin Plipid/water coefficient | 0.31 ± 0.02 | 0.01 ± 0.002 | 0.17 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.002 |
| Melatonin degradation day 1 (%) | 3.5 ± 1.9 | 22.8 ± 0.2 | 8.6 ± 2.6 | 3.2 ± 0.9 | 4.9 ± 1.9 |
| Melatonin degradation day 11 (%) | 65.5 ± 3 | 59.4 ± 2.6 | 65.6 ± 5.6 | 63.5 ± 1.7 | 55.8 ± 0.3 |
| Melatonin degradation half-life (days) | 7.2 | 9.1 | 7.1 | 8.1 | 10.2 |
| Initial Peroxide Value Ip (mEq O/kg) | 40 ± 2.1 | 0.9 ± 0.3 | 23 ± 3.6 | 20.2 ± 0.6 | 8.1 ± 0.7 |
| Oil oxidation under stress ΔIp (%) | 16 ± 4.2 | 0.0 ± 1.5 | 42.5 ± 2.8 | 17.5 ± 4.5 | 14.1 ± 2.3 |
| Parameter | RO | LP | EVOO | WOO | VOO |
|---|---|---|---|---|---|
| AUC Δoil (% h) | 267.3 ± 90.4 | 312.7 ± 140.9 | 289.7 ± 51.6 | 244.6 ± 144.2 | 253.7 ± 128.3 |
| Xmax Δoil (%) | 94 ± 30.4 | 90.7 ± 20.9 | 89.4 ± 40 | 79.9 ± 23.1 | 83 ± 27.4 |
| Tmax Δoil (h) | 0.33 ± 0.23 | 0.22 ± 0.12 | 0.3 ± 0.18 | 0.31 ± 0.18 | 0.47 ± 0.34 |
| X20h Δoil (%) | 0 ± 0.1 | 0.4 ± 0.2 | −2.2 ± 1.8 | 1.1 ± 0.7 | 2.2 ± 1.3 |
| AUC ΔHydration (% h) | 168 ± 28.9 | 116.2 ± 59.9 | 75.3 ± 59.4 | 41.7 ± 26.4 | 128.3 ± 75.4 |
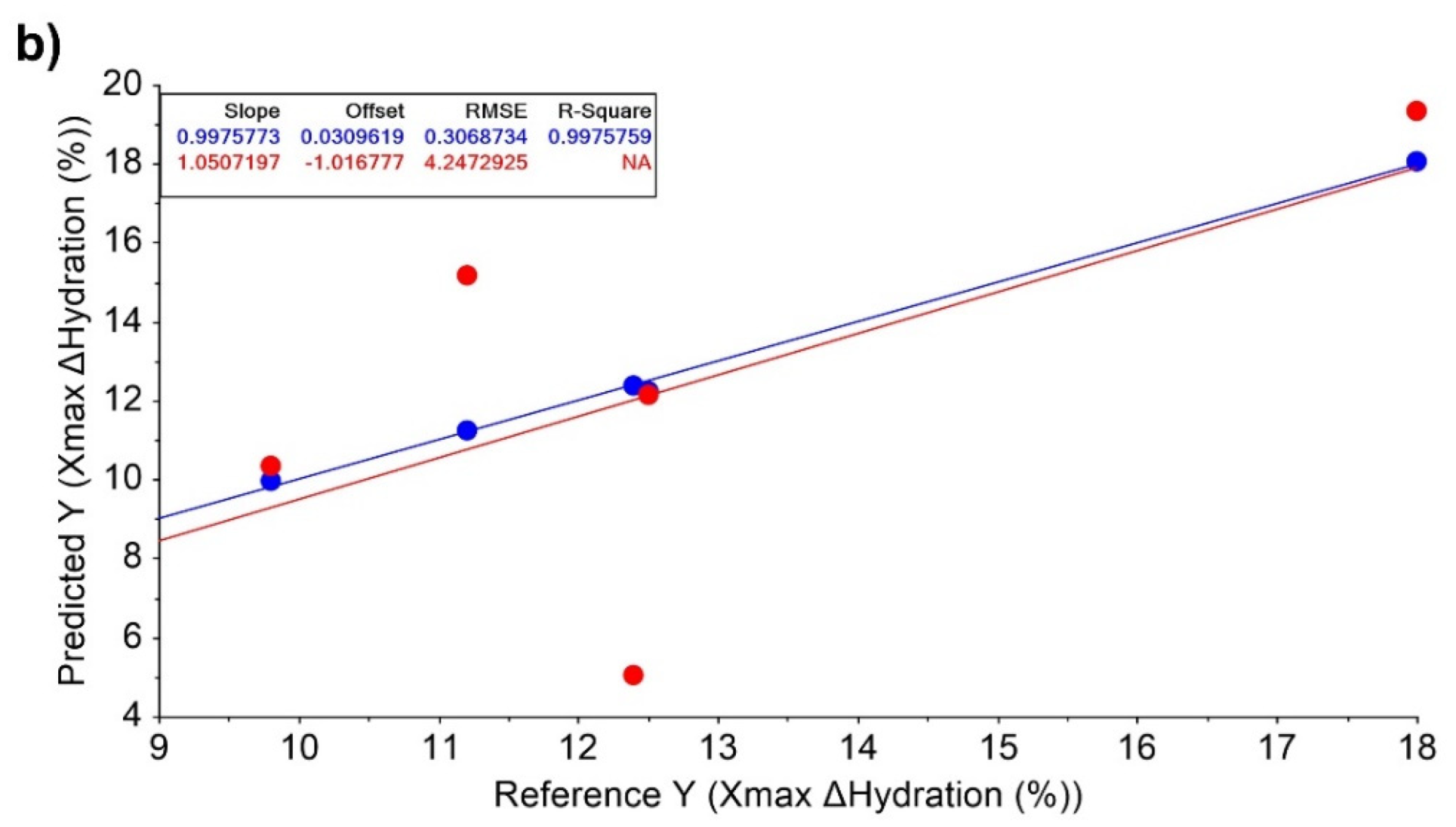
| Xmax ΔHydration (%) | 18 ± 8.5 | 11.2 ± 6.1 | 12.4 ± 5 | 9.8 ± 7.3 | 12.5 ± 9.4 |
| Tmax ΔHydration (h) | 2.5 ± 2.11 | 5.33 ± 4.31 | 9.31 ± 7.08 | 1.35 ± 0.91 | 3.46 ± 2.71 |
| X20h ΔHydration (%) | 2 ± 0.2 | 3.6 ± 2.1 | 7.9 ± 4.1 | −2 ± 0.9 | 6.4 ± 4.2 |
| AUC ΔElasticity (% h) | 7.2 ± 5.8 | 22.9 ± 11.4 | 9.2 ± 7.2 | 133.8 ± 90.1 | 26.3 ± 19.1 |
| Xmax ΔElasticity (%) | 7.8 ± 6.3 | 10.4 ± 8.5 | 12.6 ± 7 | 19.1 ± 13.4 | 13.2 ± 9.9 |
| Tmax ΔElasticity (h) | 11 ± 9.72 | 4.48 ± 4.03 | 4.14 ± 3.1 | 1.78 ± 1.41 | 3.8 ± 2.66 |
| X20h ΔElasticity (%) | −0.7 ± 0.6 | −0.53 ± 0.4 | 0.19 ± 0.15 | 2.7 ± 2.1 | −0.7 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Torrado, M.; Kara, A.; Torrado, S.; Romero, A.; Juberías, A.; Torrado, J.J.; Serrano, D.R. In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration. Pharmaceutics 2022, 14, 2615. https://doi.org/10.3390/pharmaceutics14122615
Rodríguez-Torrado M, Kara A, Torrado S, Romero A, Juberías A, Torrado JJ, Serrano DR. In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration. Pharmaceutics. 2022; 14(12):2615. https://doi.org/10.3390/pharmaceutics14122615
Chicago/Turabian StyleRodríguez-Torrado, Marta, Aytug Kara, Susana Torrado, Alejandro Romero, Antonio Juberías, Juan J. Torrado, and Dolores R. Serrano. 2022. "In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration" Pharmaceutics 14, no. 12: 2615. https://doi.org/10.3390/pharmaceutics14122615
APA StyleRodríguez-Torrado, M., Kara, A., Torrado, S., Romero, A., Juberías, A., Torrado, J. J., & Serrano, D. R. (2022). In Vitro and In Vivo Characteristics of Olive Oil as Excipient for Topical Administration. Pharmaceutics, 14(12), 2615. https://doi.org/10.3390/pharmaceutics14122615







