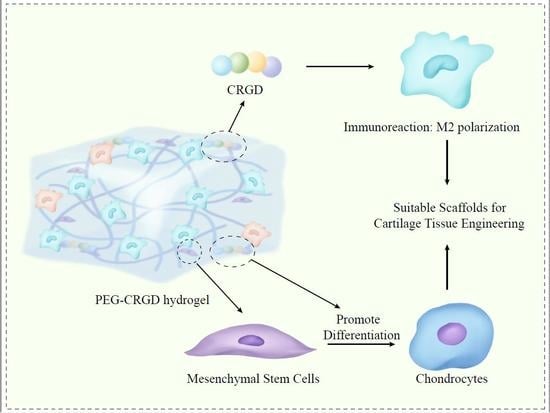
Immunomodulatory PEG-CRGD Hydrogels Promote Chondrogenic Differentiation of PBMSCs
Abstract
:1. Introduction
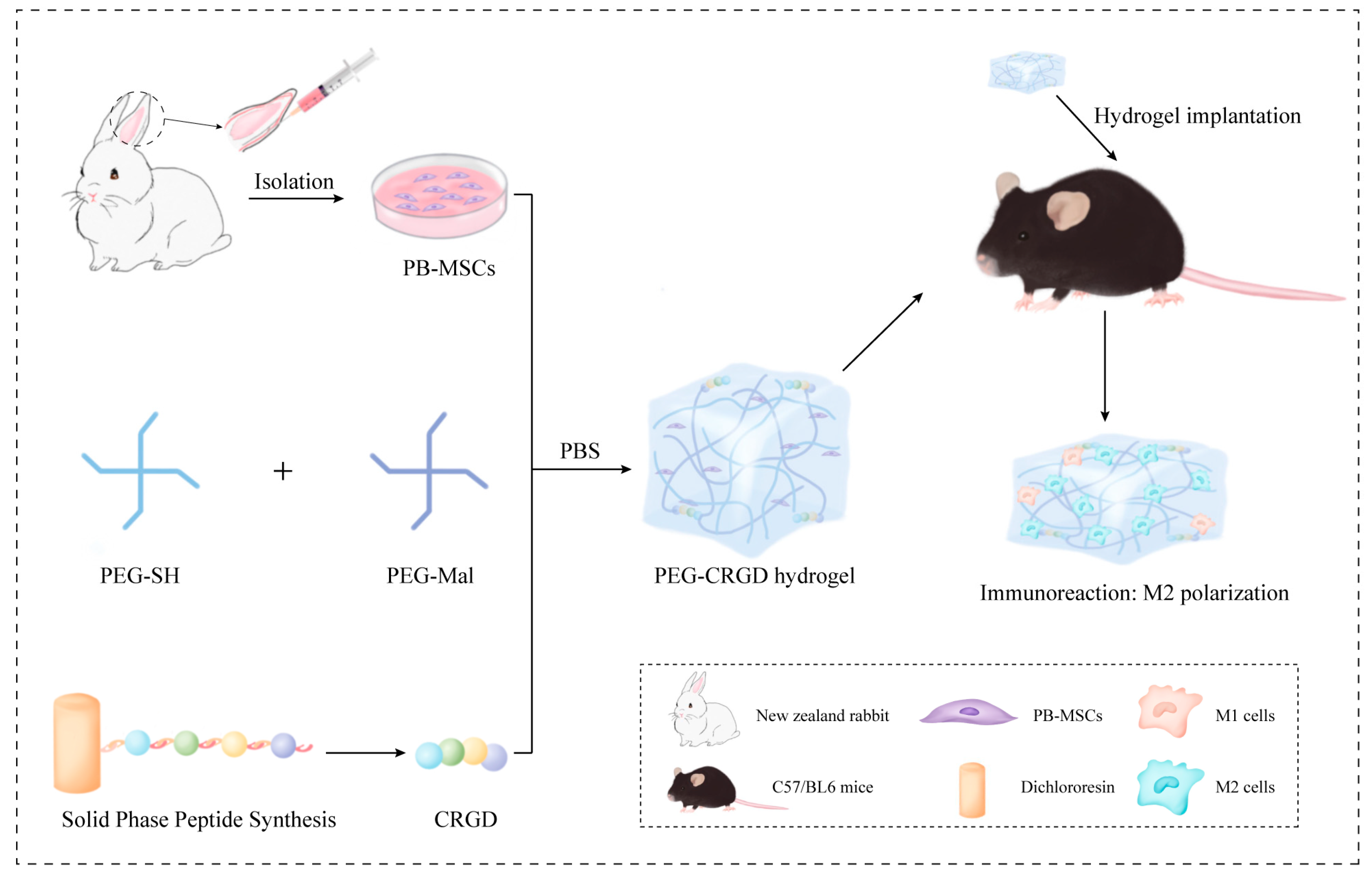
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Hydrogels
2.3. Scanning Electron Microscopy
2.4. Rheology Measurement of PEG-CRGD Hydrogels
2.5. Compressive Testing of PEG-CRGD Hydrogels
2.6. Swelling Properties of PEG-CRGD Hydrogels
2.7. Cell Survival and Proliferation in PEG-CRGD Hydrogels
2.8. Cell Adhesion on Hydrogels
2.9. Chondrogenic in PEG-CRGD Hydrogels
2.10. Reverse Transcription Quantitative Polymerase Chain Reaction (qRT-PCR)
2.11. Macroscopic and Histological Analysis
2.12. Statistical Analysis
3. Results and Discussions
3.1. Morphological Observation and Characterization of Hydrogels
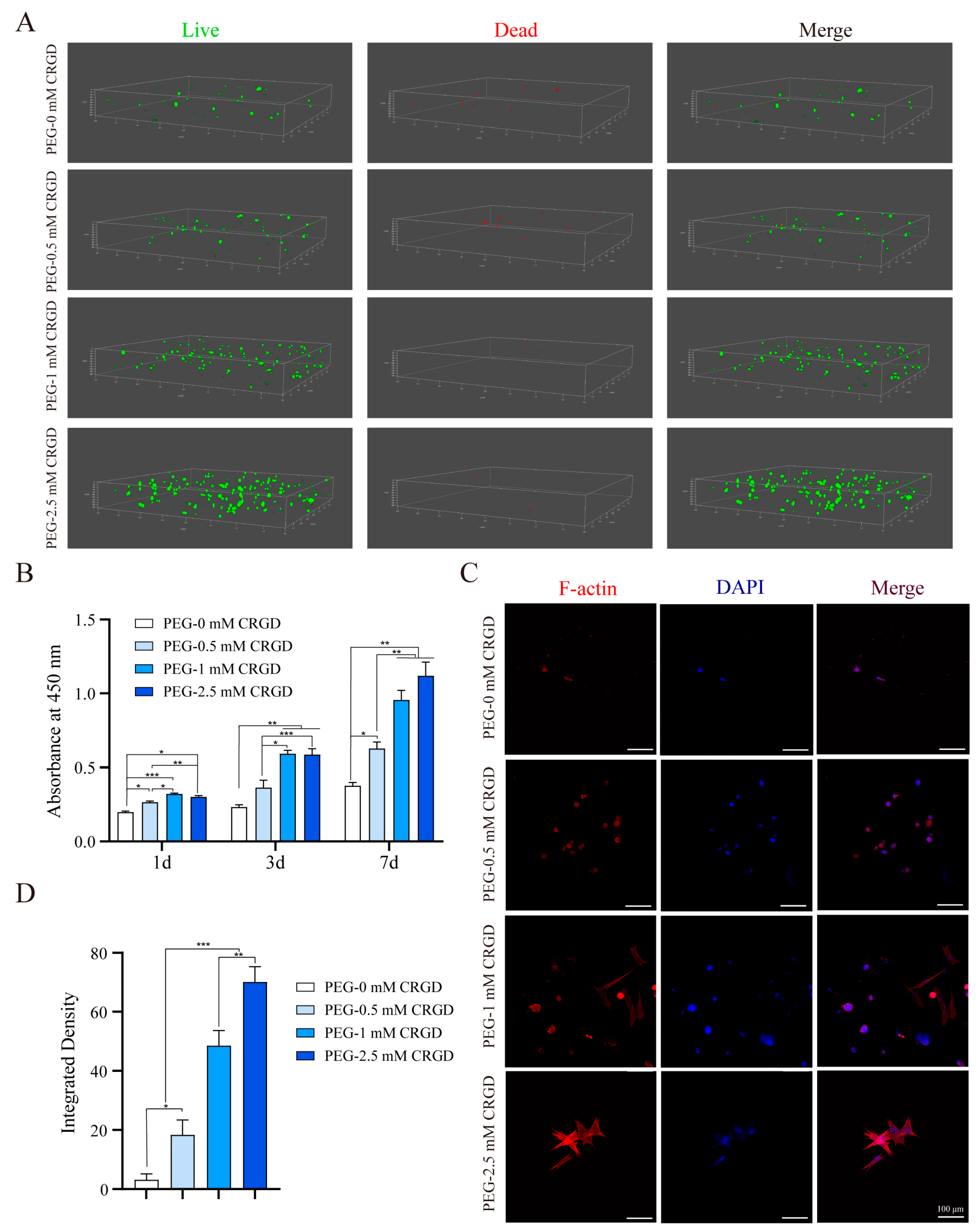
3.2. Biocompatibility of MSCs on Hydrogel Scaffolds
3.3. The Effect of Gradient Concentration of RGD on Cell Adhesion
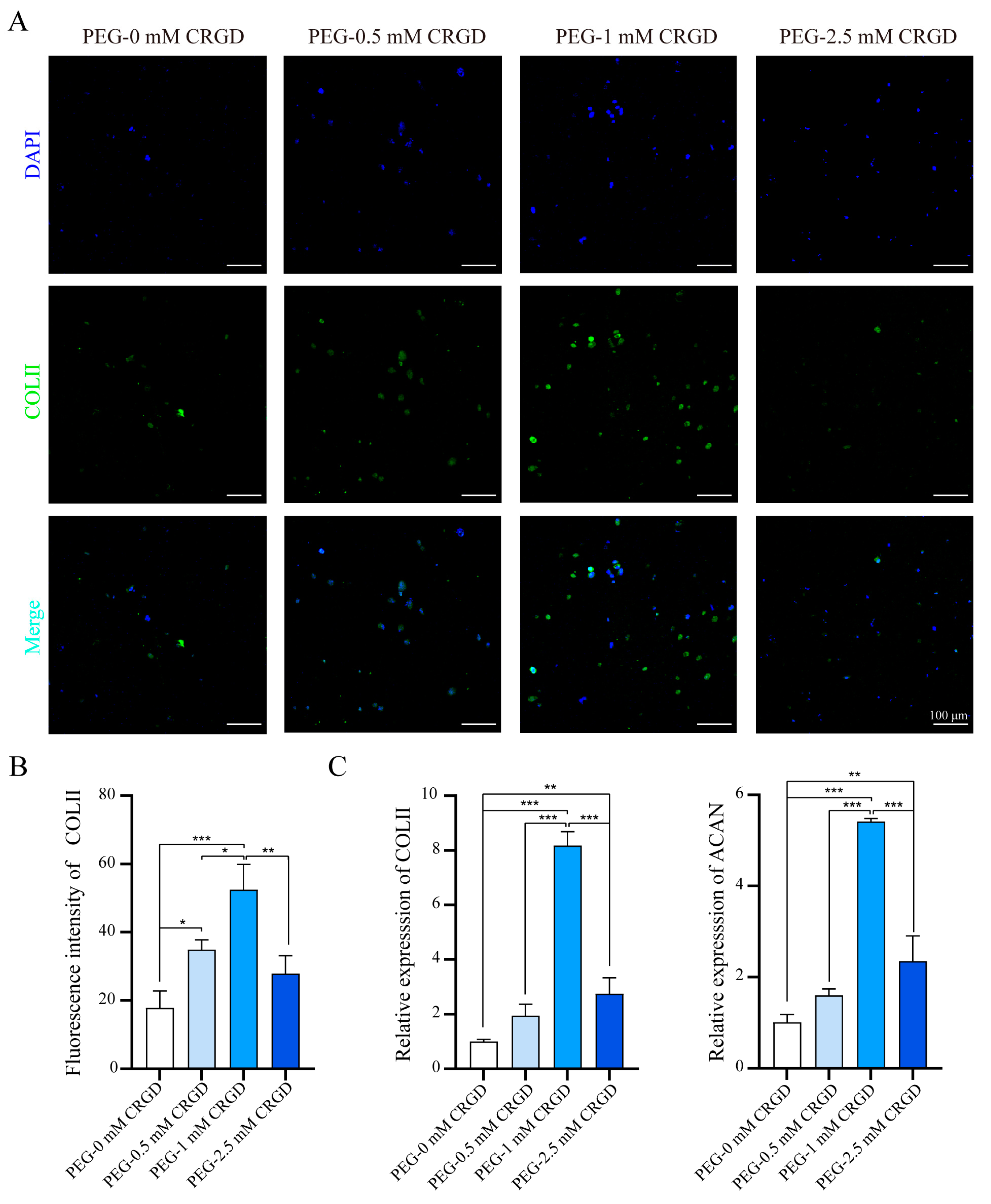
3.4. The Effect of Gradient Concentration of CRGD on Cell Chondrogenesis
3.5. The Effect of CRGD Gradient Concentration on Immunophenotype
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Z.C.; Yuan, F.Z.; Deng, R.H.; Yan, X.; Mao, F.B.; Chen, Y.R.; Lu, H.; Yu, J.K. An immunomodulatory polypeptide hydrogel for osteochondral defect repair. Bioact. Mater. 2022, 19, 678–689. [Google Scholar] [CrossRef]
- Fu, J.N.; Wang, X.; Yang, M.; Chen, Y.R.; Zhang, J.Y.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Scaffold-Based Tissue Engineering Strategies for Osteochondral Repair. Front. Bioeng. Biotechnol. 2021, 9, 812383. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.T.; Nagalla, R.R.; Wang, S.W.; Liu, W.F. Extracellular Matrix-Based Strategies for Immunomodulatory Biomaterials Engineering. Adv. Healthc. Mater. 2019, 8, e1801578. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Tang, Q.; Lim, T.; Shen, L.Y.; Zheng, G.; Wei, X.J.; Zhang, C.Q.; Zhu, Z.Z. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials 2021, 268, 120605. [Google Scholar] [CrossRef]
- Yuan, F.Z.; Wang, H.F.; Guan, J.; Fu, J.N.; Yang, M.; Zhang, J.Y.; Chen, Y.R.; Wang, X.; Yu, J.K. Fabrication of Injectable Chitosan-Chondroitin Sulfate Hydrogel Embedding Kartogenin-Loaded Microspheres as an Ultrasound-Triggered Drug Delivery System for Cartilage Tissue Engineering. Pharmaceutics 2021, 13, 1487. [Google Scholar] [CrossRef]
- Min, Q.; Liu, J.; Zhang, Y.; Yang, B.; Wan, Y.; Wu, J. Dual Network Hydrogels Incorporated with Bone Morphogenic Protein-7-Loaded Hyaluronic Acid Complex Nanoparticles for Inducing Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Pharmaceutics 2020, 12, 613. [Google Scholar] [CrossRef]
- Schuurmans, C.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2021, 268, 120602. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef]
- Wu, H.; Shang, Y.; Sun, W.; Ouyang, X.; Zhou, W.; Lu, J.; Yang, S.; Wei, W.; Yao, X.; Wang, X.; et al. Seamless and early gap healing of osteochondral defects by autologous mosaicplasty combined with bioactive supramolecular nanofiber-enabled gelatin methacryloyl (BSN-GelMA) hydrogel. Bioact. Mater. 2023, 19, 88–102. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxid. Med. Cell Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [Green Version]
- Chahal, A.S.; Schweikle, M.; Lian, A.M.; Reseland, J.E.; Haugen, H.J.; Tiainen, H. Osteogenic potential of poly(ethylene glycol)-amorphous calcium phosphate composites on human mesenchymal stem cells. J. Tissue Eng. 2020, 11, 2041731420926840. [Google Scholar] [CrossRef]
- Maynard, S.A.; Gelmi, A.; Skaalure, S.C.; Pence, I.J.; Lee-Reeves, C.; Sero, J.E.; Whittaker, T.E.; Stevens, M.M. Nanoscale Molecular Quantification of Stem Cell-Hydrogel Interactions. ACS Nano 2020, 14, 17321–17332. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.C.; Liu, Y.; Chen, Y.R.; Deng, R.H.; Zhang, Z.N.; Yu, J.K.; Yuan, F.Z. Function and Mechanism of RGD in Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Zhang, Z.; Elisseeff, J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006, 12, 2695–2706. [Google Scholar] [CrossRef]
- Salinas, C.N.; Cole, B.B.; Kasko, A.M.; Anseth, K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007, 13, 1025–1034. [Google Scholar] [CrossRef]
- Salinas, C.N.; Anseth, K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 2008, 29, 2370–2377. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. Part A 2015, 21, 757–766. [Google Scholar] [CrossRef]
- Vonwil, D.; Schuler, M.; Barbero, A.; Ströbel, S.; Wendt, D.; Textor, M.; Aebi, U.; Martin, I. An RGD-restricted substrate interface is sufficient for the adhesion, growth and cartilage forming capacity of human chondrocytes. Eur. Cell Mater. 2010, 20, 316–328. [Google Scholar] [CrossRef]
- Smith, C.L.; Childers, E.P.; Bernard, S.L.; Weiner, S.D.; Becker, M.L. Maximizing phenotype constraint and extracellular matrix production in primary human chondrocytes using arginine-glycine-aspartate concentration gradient hydrogels. Acta Biomater. 2013, 9, 7420–7428. [Google Scholar] [CrossRef] [Green Version]
- Akalin, O.B.; Bayraktar, H. Alteration of cell motility dynamics through collagen fiber density in photopolymerized polyethylene glycol hydrogels. Int. J. Biol. Macromol. 2020, 157, 414–423. [Google Scholar] [CrossRef]
- Carberry, B.J.; Hergert, J.E.; Yavitt, F.M.; Hernandez, J.J.; Speckl, K.F.; Bowman, C.N.; McLeod, R.R.; Anseth, K.S. 3D printing of sacrificial thioester elastomers using digital light processing for templating 3D organoid structures in soft biomatrices. Biofabrication 2021, 13, 044104. [Google Scholar] [CrossRef]
- Heggli, M.; Tirelli, N.; Zisch, A.; Hubbell, J.A. Michael-type addition as a tool for surface functionalization. Bioconjug. Chem. 2003, 14, 967–973. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.W.; Chen, Y.H.; Wu, D.Y.; Wang, J.B.; Lv, M.M.; Wang, X.S.; Sun, J.; Zhang, Z.Y. Development of an Accurate and Proactive Immunomodulatory Strategy to Improve Bone Substitute Material-Mediated Osteogenesis and Angiogenesis. Theranostics 2018, 8, 5482–5500. [Google Scholar] [CrossRef]
- Fu, W.L.; Zhou, C.Y.; Yu, J.K. A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am. J. Sports Med. 2014, 42, 592–601. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tian, Q.; Wang, L.; Hedrick, J.L.; Hui, J.H.; Yang, Y.Y.; Ee, P.L. Injectable Biodegradable Poly(ethylene glycol)/RGD Peptide Hybrid Hydrogels for in vitro Chondrogenesis of Human Mesenchymal Stem Cells. Macromol. Rapid Commun. 2010, 31, 1148–1154. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired Nanocomposite Hydrogels with Highly Ordered Structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Deng, R.-H.; Yuan, F.-Z.; Zhang, J.-Y.; Zhang, Z.-N.; Chen, Y.-R.; Yu, J.-K. Immunomodulatory PEG-CRGD Hydrogels Promote Chondrogenic Differentiation of PBMSCs. Pharmaceutics 2022, 14, 2622. https://doi.org/10.3390/pharmaceutics14122622
Yang M, Deng R-H, Yuan F-Z, Zhang J-Y, Zhang Z-N, Chen Y-R, Yu J-K. Immunomodulatory PEG-CRGD Hydrogels Promote Chondrogenic Differentiation of PBMSCs. Pharmaceutics. 2022; 14(12):2622. https://doi.org/10.3390/pharmaceutics14122622
Chicago/Turabian StyleYang, Meng, Rong-Hui Deng, Fu-Zhen Yuan, Ji-Ying Zhang, Zi-Ning Zhang, You-Rong Chen, and Jia-Kuo Yu. 2022. "Immunomodulatory PEG-CRGD Hydrogels Promote Chondrogenic Differentiation of PBMSCs" Pharmaceutics 14, no. 12: 2622. https://doi.org/10.3390/pharmaceutics14122622
APA StyleYang, M., Deng, R.-H., Yuan, F.-Z., Zhang, J.-Y., Zhang, Z.-N., Chen, Y.-R., & Yu, J.-K. (2022). Immunomodulatory PEG-CRGD Hydrogels Promote Chondrogenic Differentiation of PBMSCs. Pharmaceutics, 14(12), 2622. https://doi.org/10.3390/pharmaceutics14122622