Effect of Dispersion Medium on Pharmacokinetic Profile of Rotigotine Crystalline Suspension following Subcutaneous Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RTG Crystalline Suspensions Using Bead-Milling Technology
2.3. Characterization of RTG Crystalline Suspensions
2.3.1. Morphological Observations
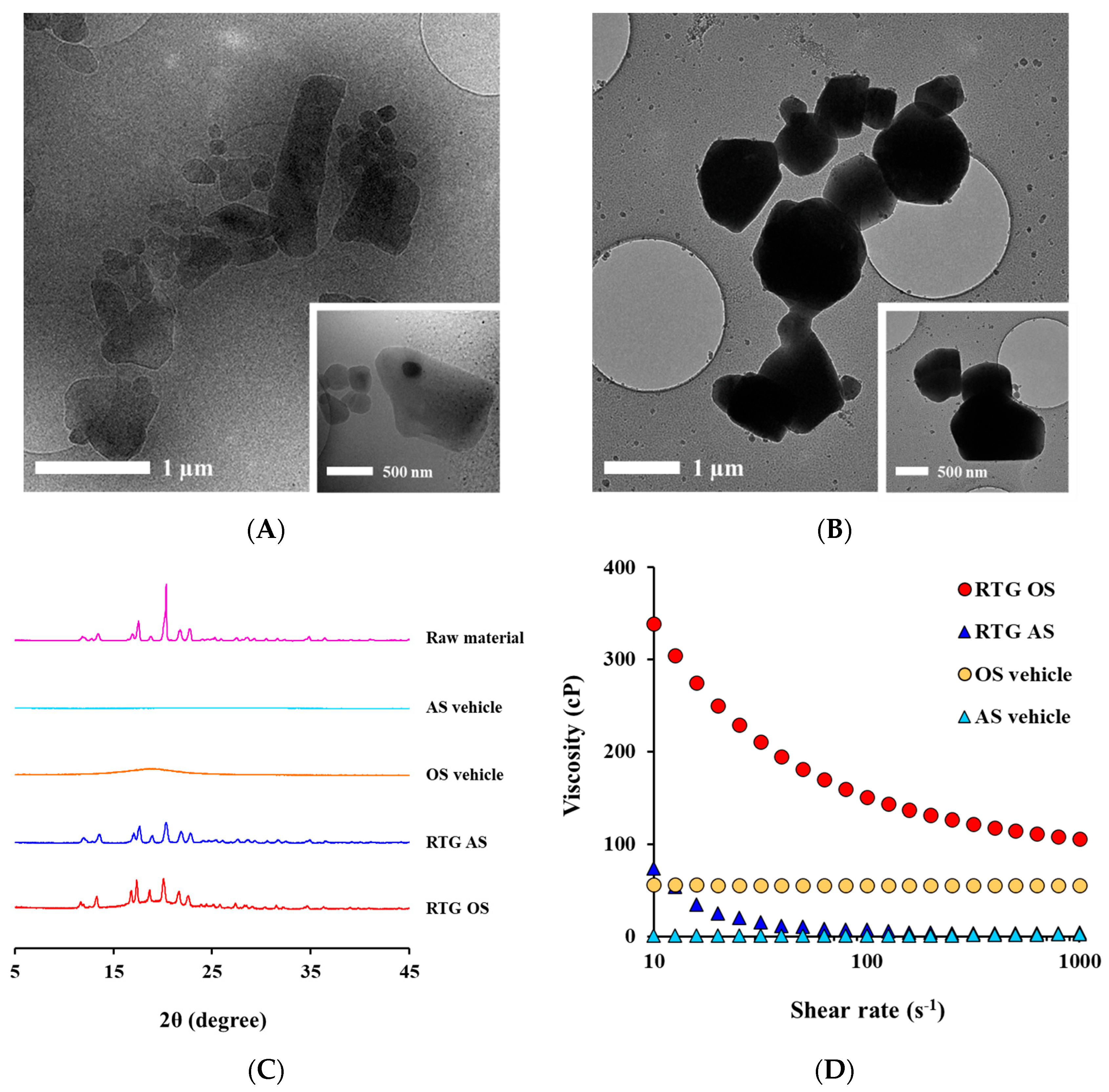
2.3.2. X-ray diffractometry (XRD)
2.3.3. Drug Content Analysis
2.3.4. Determination of Crystal Size of RTG Crystalline Suspensions
2.3.5. Viscosity of RTG Crystalline Suspensions
2.4. In Vitro Dissolution Profile of RTG Crystalline Suspensions
2.5. In Vivo Macroscopic and Histological Observations of Injected Sites following SC Injection
2.6. In Vivo Pharmacokinetic Profile of RTG Crystalline Suspensions in Rats
2.7. Statistical Analysis
3. Results and Discussion
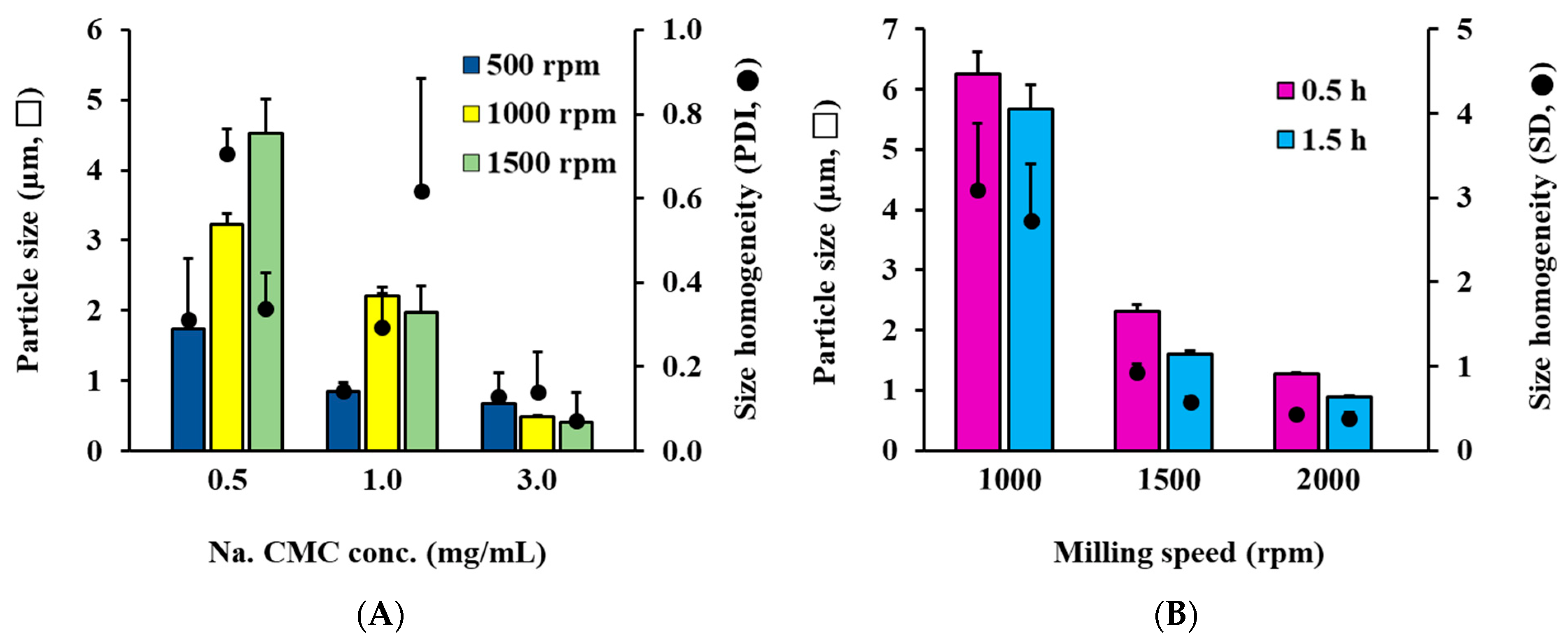
3.1. Selection of Dispersant and Control of Particle Size of RTG AS System
3.2. Selection of Oily Vehicle and Control of Particle Size of RTG-Loaded OS System
3.3. Morphological and Physical Characteristics of RTG-Loaded AS and OS
3.4. In Vitro Dissolution Profile of RTG-Loaded AS and OS
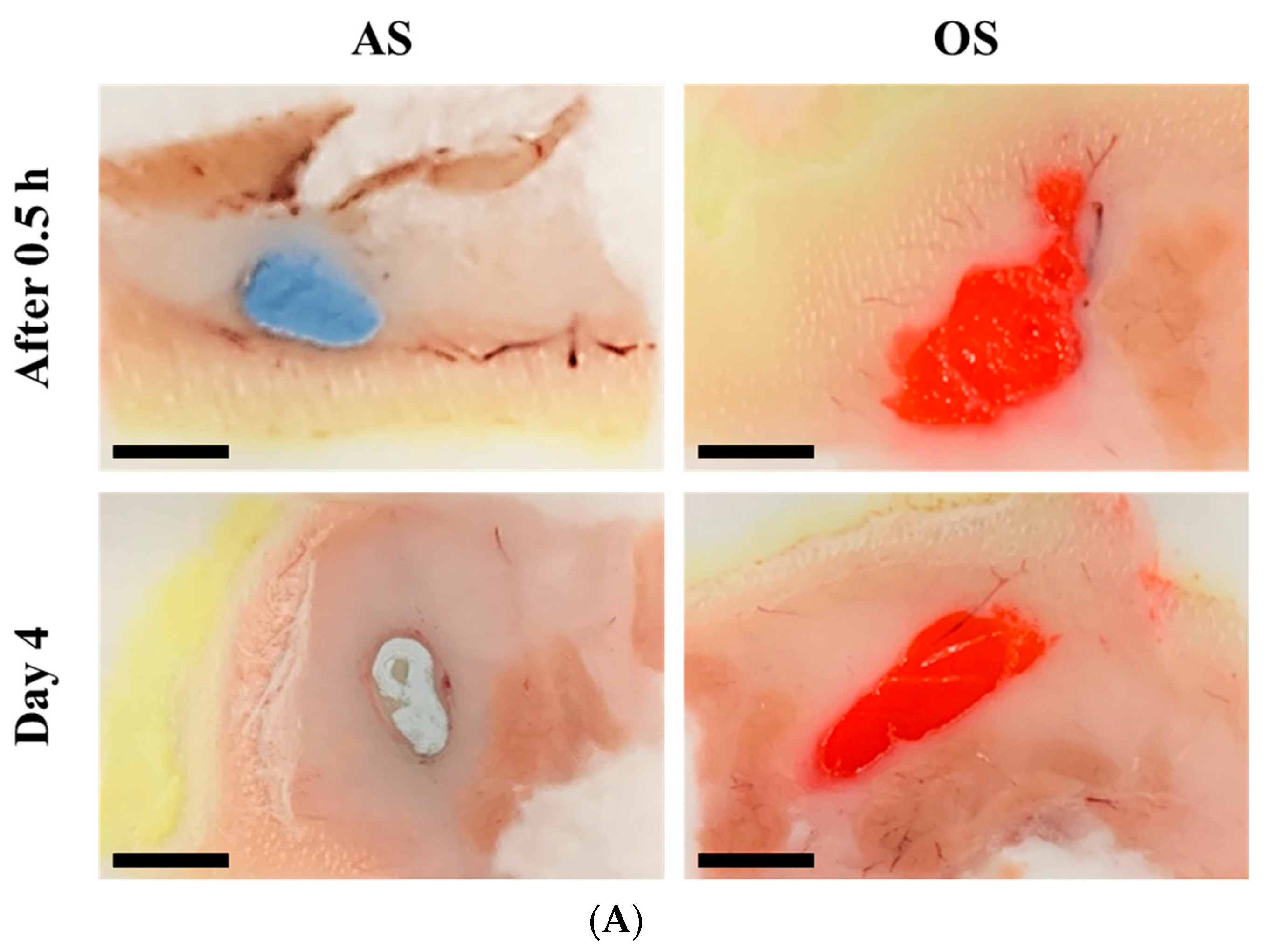
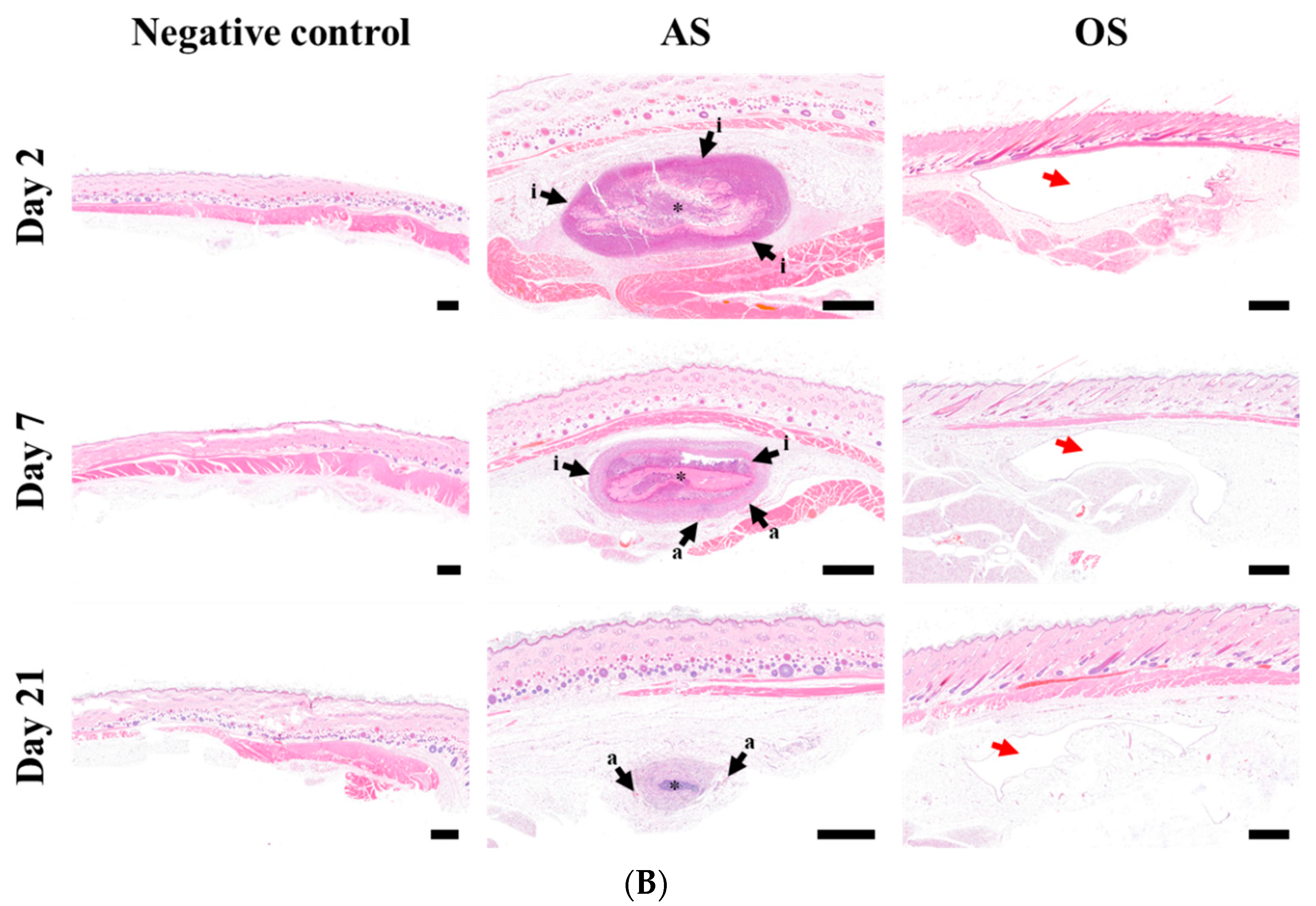
3.5. Macroscopic and Histopathological Observation of Injection Site following SC Injection
3.6. In Vivo Pharmacokinetic Profile of RTG following SC Injection of AS or OS in Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frampton, J.E. Rotigotine transdermal patch: A review in Parkinson’s disease. CNS Dugs 2019, 33, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Raeder, V.; Boura, I.; Leta, V.; Jenner, P.; Reichmann, H.; Trenkwalder, C.; Klingelhoefer, L.; Chaudhuri, K. Rotigotine transdermal patch for motor and non-motor Parkinson’s disease: A review of 12 years’ clinical experience. CNS Drugs 2021, 35, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Swart, P.; De Zeeuw, R. Extensive gastrointestinal metabolic conversion limits the oral bioavailability of the dopamine D2 agonist N-0923 in freely moving rats. Pharmazie 1992, 47, 613–615. [Google Scholar] [PubMed]
- Angelo Antonini, M.; Bernardi, L.; Daniela Calandrella, M.; Francesca Mancini, M.; Plebani, M. Rotigotine transdermal patch in the management of Parkinson’s disease (PD) and its night-time use for PD-related sleep disorders. Funct. Neurol. 2010, 25, 21. [Google Scholar]
- Md, S.; Karim, S.; Saker, S.R.; Gie, O.A.; Hooi, L.C.; Yee, P.H.; Kang, A.W.; Zhe, C.K.; Ian, N.; Aldawsari, H.M. Current status and challenges in rotigotine delivery. Curr. Pharm. Des. 2020, 26, 2222–2232. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Zhang, L.; Li, B.; Wang, D.; Zhang, L.; Wang, T.; Fu, F. Rotigotine-loaded microspheres exerts the antinociceptive effect via central dopaminergic system. Eur. J. Pharmacol. 2021, 910, 174443. [Google Scholar] [CrossRef]
- Durham, S.H.; Chahine, E.B. Cabotegravir-rilpivirine: The first complete long-acting injectable regimen for the treatment of HIV-1 infection. Ann. Pharmacother. 2021, 55, 1397–1409. [Google Scholar] [CrossRef]
- Ho, M.J.; Jeong, M.Y.; Jeong, H.T.; Kim, M.S.; Park, H.J.; Kim, D.Y.; Lee, H.C.; Song, W.H.; Kim, C.H.; Lee, C.H. Effect of particle size on in vivo performances of long-acting injectable drug suspension. J. Control. Release 2022, 341, 533–547. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, A.; Wang, X.; Belhadj, Z.; Wang, J.; Zhang, Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm. Sin. B 2021, 11, 2396–2415. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kraft, J.C.; McConnachie, L.A.; Koehn, J.; Kinman, L.; Sun, J.; Collier, A.C.; Collins, C.; Shen, D.D.; Ho, R.J. Mechanism-based pharmacokinetic (MBPK) models describe the complex plasma kinetics of three antiretrovirals delivered by a long-acting anti-HIV drug combination nanoparticle formulation. J. Control. Release 2018, 275, 229–241. [Google Scholar] [CrossRef] [PubMed]
- van′ t Klooster, G.; Hoeben, E.; Borghys, H.; Looszova, A.; Bouche, M.P.; van Velsen, F.; Baert, L. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob. Agents Chemother. 2010, 54, 2042–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, S.W.; Larsen, C. Critical factors influencing the in vivo performance of long-acting lipophilic solutions—impact on in vitro release method design. AAPS J. 2009, 11, 762–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalicharan, R.; Oussoren, C.; Schot, P.; de Rijk, E.; Vromans, H. The contribution of the in-vivo fate of an oil depot to drug absorption. Int. J. Pharm. 2017, 528, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chikaura, H.; Nakashima, Y.; Fujiwara, Y.; Komohara, Y.; Takeya, M.; Nakanishi, Y. Effect of particle size on biological response by human monocyte-derived macrophages. Biosurf. Biotribol. 2016, 2, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.W.; Frost, A.B.; Østergaard, J.; Marcher, H.; Larsen, C. On the mechanism of drug release from oil suspensions in vitro using local anesthetics as model drug compounds. Eur. J. Pharm. Sci. 2008, 34, 37–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kalicharan, R.; Baron, P.; Oussoren, C.; Bartels, L.; Vromans, H. Spatial distribution of oil depots monitored in human muscle using MRI. Int. J. Pharm. 2016, 505, 52–60. [Google Scholar] [CrossRef]
- Hagedorn, M.; Bögershausen, A.; Rischer, M.; Schubert, R.; Massing, U. Dual centrifugation–a new technique for nanomilling of poorly soluble drugs and formulation screening by an DoE-approach. Int. J. Pharm. 2017, 530, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Bunjes, H. Influence of process and formulation parameters on the preparation of solid lipid nanoparticles by dual centrifugation. Int. J. Pharm. X 2021, 3, 100085. [Google Scholar] [CrossRef]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation-process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.; Rao, B.T.; Kumar, R.K. A stability indica-ting of rotigotine in bulk drugs by HPLC assay method. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 848–857. [Google Scholar]
- Ryu, S.; Jin, M.; Lee, H.K.; Wang, M.H.; Baek, J.S.; Cho, C.W. Effects of lipid nanoparticles on physicochemical properties, cellular uptake, and lymphatic uptake of 6-methoxflavone. J. Pharm. Investig. 2022, 52, 233–241. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Liew, Q.X.; Ng, K.Y.; Chowdhury, E.H. Active targeting via ligand-anchored pH-responsive strontium nanoparticles for efficient nucleic acid delivery into breast cancer cells. J. Pharm. Investig. 2022, 52, 243–257. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Fambri, L.; Pegoretti, A. Multifunctional epoxy/carbon fiber laminates for thermal energy storage and release. Compos. Sci. Technol. 2018, 158, 101–111. [Google Scholar] [CrossRef]
- De Coninck, E.; Marchesini, F.H.; Vanhoorne, V.; De Beer, T.; Vervaet, C. Viscosity of API/fatty acid suspensions: Pitfalls during analysis. Int. J. Pharm. 2020, 584, 119447. [Google Scholar] [CrossRef]
- Lei, F.; Zhang, H.; Luo, R.; Fei, Q.; Bai, L.; He, N. Sustained ocular delivery of desmopressin acetate via thermoreversible in situ gel formulation: Preparation and in vitro/in vivo evaluation. J. Pharm. Investig. 2022, 52, 639–648. [Google Scholar] [CrossRef]
- Kaul, S.; Jain, N.; Nagaich, U. Ultra deformable vesicles for boosting transdermal delivery of 2-arylpropionic acid class drug for management of musculoskeletal pain. J. Pharm. Investig. 2022, 52, 217–231. [Google Scholar] [CrossRef]
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.P. In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Tam, B.M.; Yang, L.L.; Bogėa, T.H.; Ross, B.; Martens, G.; Moritz, O.L. Preparation of Xenopus laevis retinal cryosections for electron microscopy. Exp. Eye Res. 2015, 136, 86–90. [Google Scholar] [CrossRef]
- Wilson, N.; Esfandiary, E.; Bedi, K. Cryosections of pre-irradiated adult rat spinal cord tissue support axonal regeneration in vitro. Int. J. Dev. Neurosci. 2000, 18, 735–741. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Zhang, L.; Fu, X.; Chen, J.; Hong, D. A modified tape transfer approach for rapidly preparing high-quality cryosections of undecalcified adult rodent bones. J. Orthop. Translat. 2021, 26, 92–100. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, H.C.; Jang, Y.J.; Kim, J.H.; Lee, H.R.; Kang, M.J.; Choi, Y.S. A simple and efficient method to determine montelukast in rat plasma using liquid-liquid extraction and tandem mass spectrometry. Mass Spectrom. Lett. 2020, 11, 71–76. [Google Scholar]
- Liedtke, S.; Wissing, S.; Müler, R.; Mäer, K. Influence of high pressure homogenisation equipment on nanodispersions characteristics. Int. J. Pharm. 2000, 196, 183–185. [Google Scholar] [CrossRef]
- Medarević, D.; Djuriš, J.; Ibrić, S.; Mitrić, M.; Kachrimanis, K. Optimization of formulation and process parameters for the production of carvedilol nanosuspension by wet media milling. Int. J. Pharm. 2018, 540, 150–161. [Google Scholar] [CrossRef]
- Niwa, T.; Miura, S.; Danjo, K. Universal wet-milling technique to prepare oral nanosuspension focused on discovery and preclinical animal studies–development of particle design method. Int. J. Pharm. 2011, 405, 218–227. [Google Scholar] [CrossRef]
- Hagedorn, M.; Liebich, L.; Böershausen, A.; Massing, U.; Hoffmann, S.; Mende, S.; Rischer, M. Rapid development of API nano-formulations from screening to production combining dual centrifugation and wet agitator bead milling. Int. J. Pharm. 2019, 565, 187–198. [Google Scholar] [CrossRef]
- Drugbank. Available online: https://go.drugbank.com/drugs/DB05271 (accessed on 21 April 2021).
- Kostanski, J.W.; Matsuda, T.; Manoj, N.; Naringrekar, V.H. Controlled Release Sterile Injectable Aripiprazole Formulation and Method. U.S. Patent 7807680B2, 5 October 2010. [Google Scholar]
- Malhotra, G.; Singh, S.; Ansari, K.A. Paliperidone Palmitate Particles and Compositions Thereof. WO Patent 2016199170, 15 December 2016. [Google Scholar]
- Paquette, S.M.; Dawit, H.; Hickey, M.B.; Merisko-Liversidge, E.; Almarsson, O.; Deaver, D.R. Long-acting atypical antipsychotics: Characterization of the local tissue response. Pharm. Res. 2014, 31, 2065–2077. [Google Scholar] [CrossRef] [Green Version]
- Mundhra, D.B.; Pan, R. Pharmaceutical Compositions. U.S. Patent 20130171214A1, 4 July 2013. [Google Scholar]
- Phillips, D.J.; Pygall, S.R.; Cooper, V.B.; Mann, J.C. Overcoming sink limitations in dissolution testing: A review of traditional methods and the potential utility of biphasic systems. J. Pharm. Pharm. 2012, 64, 1549–1559. [Google Scholar] [CrossRef]
- McConville, C.; Smith, J.M.; McCoy, C.F.; Srinivasan, P.; Mitchell, J.; Holder, A.; Otten, R.A.; Butera, S.; Doncel, G.F.; Friend, D.R. Lack of in vitro–in vivo correlation for a UC781-releasing vaginal ring in macaques. Drug Deliv. Transl. Res. 2015, 5, 27–37. [Google Scholar] [CrossRef]
- Darville, N.; Van Heerden, M.; Erkens, T.; De Jonghe, S.; Vynckier, A.; De Meulder, M.; Vermeulen, A.; Sterkens, P.; Annaert, P.; Van den Mooter, G. Modeling the time course of the tissue responses to intramuscular long-acting paliperidone palmitate nano-/microcrystals and polystyrene microspheres in the rat. Toxicol. Pathol. 2016, 44, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.W.; Rinvar, E.; Svendsen, O.; Lykkesfeldt, J.; Friis, G.J.; Larsen, C. Determination of the disappearance rate of iodine-125 labelled oils from the injection site after intramuscular and subcutaneous administration to pigs. Int. J. Pharm. 2001, 230, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.; Ajulo, D.; Tamburrini, V.; Le Gall, G.; Kimpe, K.; Holm, R.; Belton, P.; Qi, S. Lipid based intramuscular long-acting injectables: Current state of the art. Eur. J. Pharm. Sci. 2022, 178, 106253. [Google Scholar] [CrossRef] [PubMed]
- Darville, N.; Van Heerden, M.; Mariën, D.; De Meulder, M.; Rossenu, S.; Vermeulen, A.; Vynckier, A.; De Jonghe, S.; Sterkens, P.; Annaert, P. The effect of macrophage and angiogenesis inhibition on the drug release and absorption from an intramuscular sustained-release paliperidone palmitate suspension. J. Control. Release 2016, 230, 95–108. [Google Scholar] [CrossRef]
- European Medicine Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/neupro (accessed on 3 November 2021).
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Glennon, B.; He, Y.; Donnellan, P. Dissolution kinetics of a bcs class ii active pharmaceutical ingredient: Diffusion-based model validation and prediction. ACS Omega 2021, 6, 8056–8067. [Google Scholar] [CrossRef]
- Serafini, A.; Lorenzut, S.; Gigli, G.L.; Merlino, G.; Valente, M. The use of rotigotine in the treatment of restless legs syndrome. Ther. Adv. Neurol. Disord. 2010, 3, 241–248. [Google Scholar] [CrossRef]


| Stabilizer a | Appearance b | Particle size (nm) c,d | Homogeneity (PDI) d,e |
|---|---|---|---|
| Na. CMC | Homogeneous | 374.8 ± 1.68 | 0.206 ± 0.015 |
| Tween 80 | Homogeneous | 414.7 ± 3.39 | 0.319 ± 0.017 |
| Poloxamer 188 | Homogeneous | 1099 ± 303.6 | 0.334 ± 0.279 |
| Kolliphor HS 15 | Homogeneous | 3021 ± 222 | 0.407 ± 0.035 |
| Kolliphor RH 40 | Homogeneous | 447.5 ± 5.69 | 0.236 ± 0.082 |
| Kolliphor EL | Homogeneous | 424.4 ± 4.49 | 0.295 ± 0.027 |
| PEG 4000 | Aggregated | 7148 ± 715.5 | 0.564 ± 0.121 |
| PVP K17 | Aggregated | 3198 ± 130.8 | 0.665 ± 0.197 |
| Vehicle a | Appearance b | Particle size (nm) c,d | Fraction Dissolved (% w/w) d |
|---|---|---|---|
| Castor oil | Homogeneous | 945.3 ± 19.2 | 27.2 ± 2.8 |
| Cottonseed oil | Homogeneous | 831.0 ± 21.2 | 21.2 ± 0.2 |
| Soybean oil | Homogeneous | 896.5 ± 44.5 | 22.3 ± 0.2 |
| Corn oil | Homogeneous | 835.4 ± 38.3 | 22.9 ± 0.0 |
| Sesame oil | Homogeneous | 878.2 ± 28.1 | 19.6 ± 0.0 |
| Peanut oil | Homogeneous | 835.7 ± 16.1 | 20.8 ± 0.1 |
| Miglyol 810N | Homogeneous | 724.2 ± 9.7 | 45.9 ± 1.0 |
| Tricaprylin | Homogeneous | 3234.5 ± 576.4 | 48.6 ± 0.1 |
| Parameters | AS | OS |
|---|---|---|
| RTG conc. (mg/mL) a | 100.9 ± 0.09 | 100.7 ± 0.53 |
| Suspended (mg/mL) a | 100.8 ± 0.09 | 81.1 ± 0.53 |
| Dissolved (mg/mL) a | 0.07 ± 0.00 | 19.6 ± 0.04 |
| Particle size (nm) a | 853.7 ± 19.9 b | 878.3 ± 28.1 c |
| Zeta potential (mV) d,a | −39.97 ± 1.46 | not determined |
| Viscosity (cP) e | 73.8 | 338.4 |
| Parameters | RTG AS | RTG OS |
|---|---|---|
| AUC(0–21 days) (ng·day/mL) | 95.1 ± 6.4 | 104.3 ± 28.0 |
| Cmax (ng/mL) | 61.5 ± 12.1 | 103.4 ± 22.7 * |
| Tmax (day) | 0.28 ± 0.13 | 0.43 ± 0.15 |
| T1/2α a (day) | 0.76 ± 0.28 | 0.54 ± 0.18 |
| T1/2β b (day) | 2.95 ± 0.66 | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Ho, M.J.; Joung, M.Y.; Choi, Y.S.; Kang, M.J. Effect of Dispersion Medium on Pharmacokinetic Profile of Rotigotine Crystalline Suspension following Subcutaneous Injection. Pharmaceutics 2022, 14, 2630. https://doi.org/10.3390/pharmaceutics14122630
Kim MS, Ho MJ, Joung MY, Choi YS, Kang MJ. Effect of Dispersion Medium on Pharmacokinetic Profile of Rotigotine Crystalline Suspension following Subcutaneous Injection. Pharmaceutics. 2022; 14(12):2630. https://doi.org/10.3390/pharmaceutics14122630
Chicago/Turabian StyleKim, Min Seop, Myoung Jin Ho, Min Yeong Joung, Yong Seok Choi, and Myung Joo Kang. 2022. "Effect of Dispersion Medium on Pharmacokinetic Profile of Rotigotine Crystalline Suspension following Subcutaneous Injection" Pharmaceutics 14, no. 12: 2630. https://doi.org/10.3390/pharmaceutics14122630






