The Application of Black Phosphorus Nanomaterials in Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Method
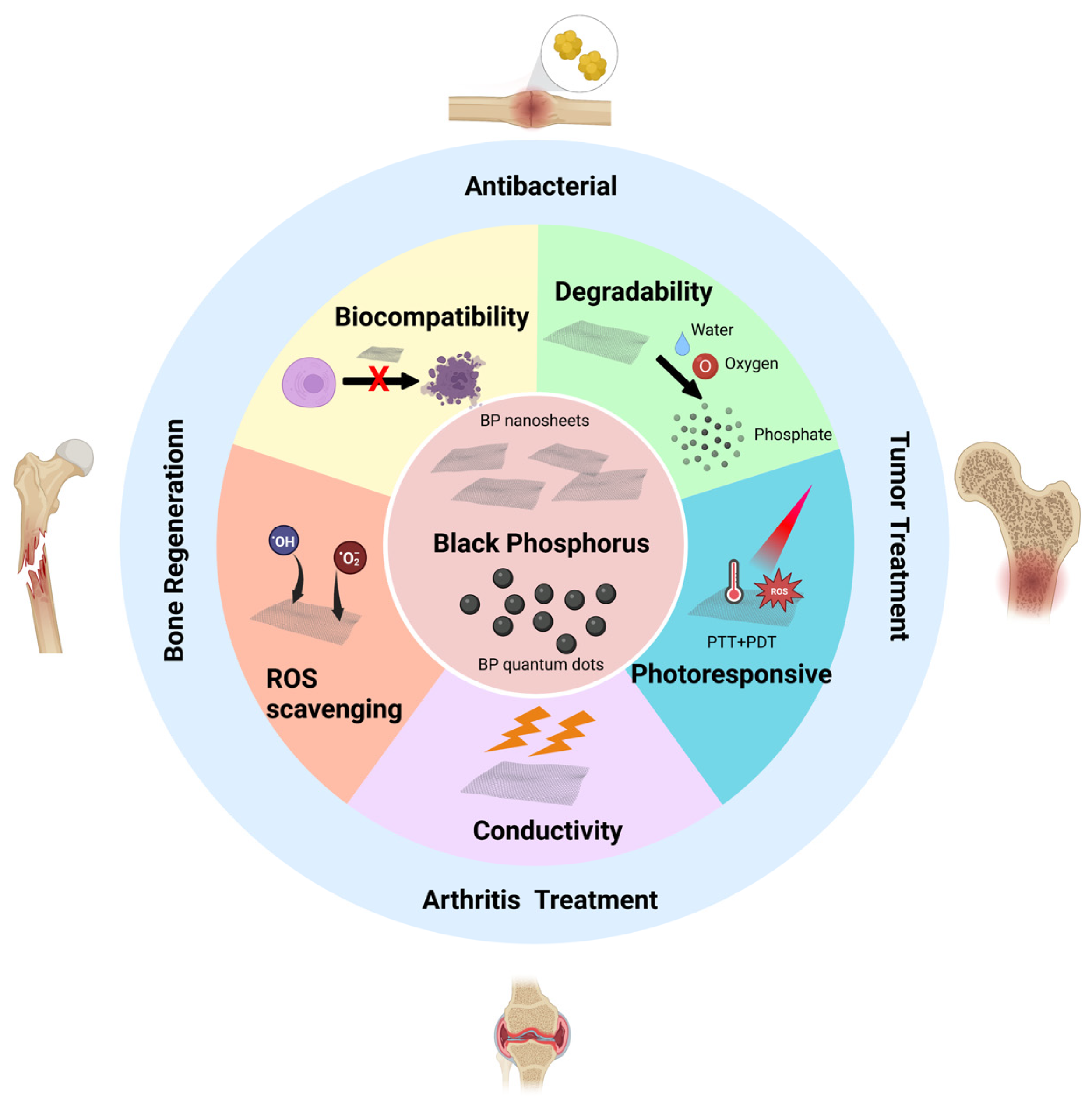
3. Structure and Properties of Black Phosphorus
3.1. Structure of Black Phosphorus
3.2. Biocompatibility of Black Phosphorus
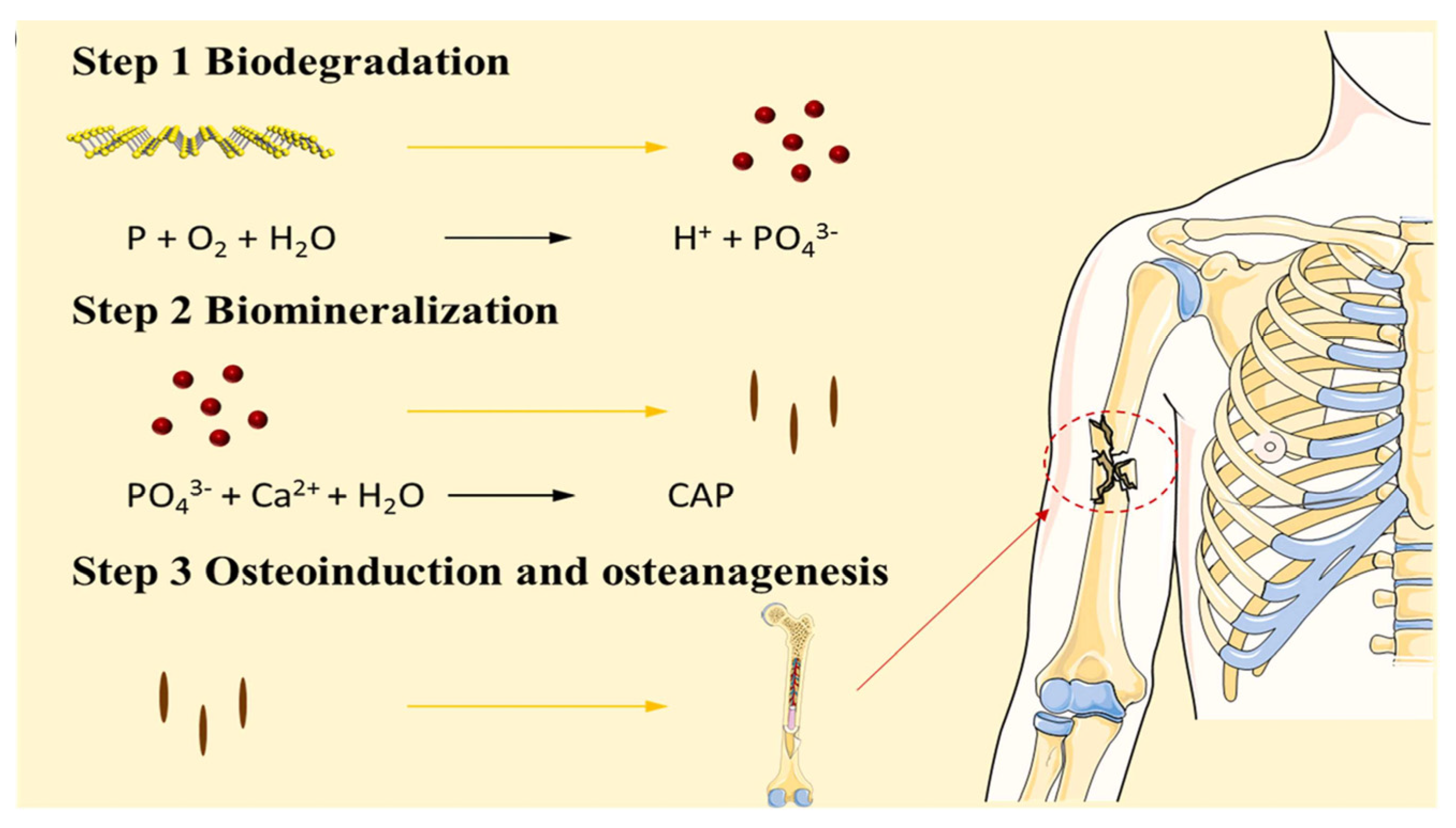
3.3. Degradability of Black Phosphorus
3.4. Photoresponsivity of Black Phosphorus
3.5. The Oxidative Stress Regulation Ability of Black Phosphorus
3.6. Conductivity of Black Phosphorus
4. The Advantages of Black Phosphorus in Bone Tissue Engineering
5. Research Progress of Black Phosphorus in Bone Tissue Engineering
5.1. Bone Regeneration Materials Based on the Biomineralization Function of Black Phosphorus
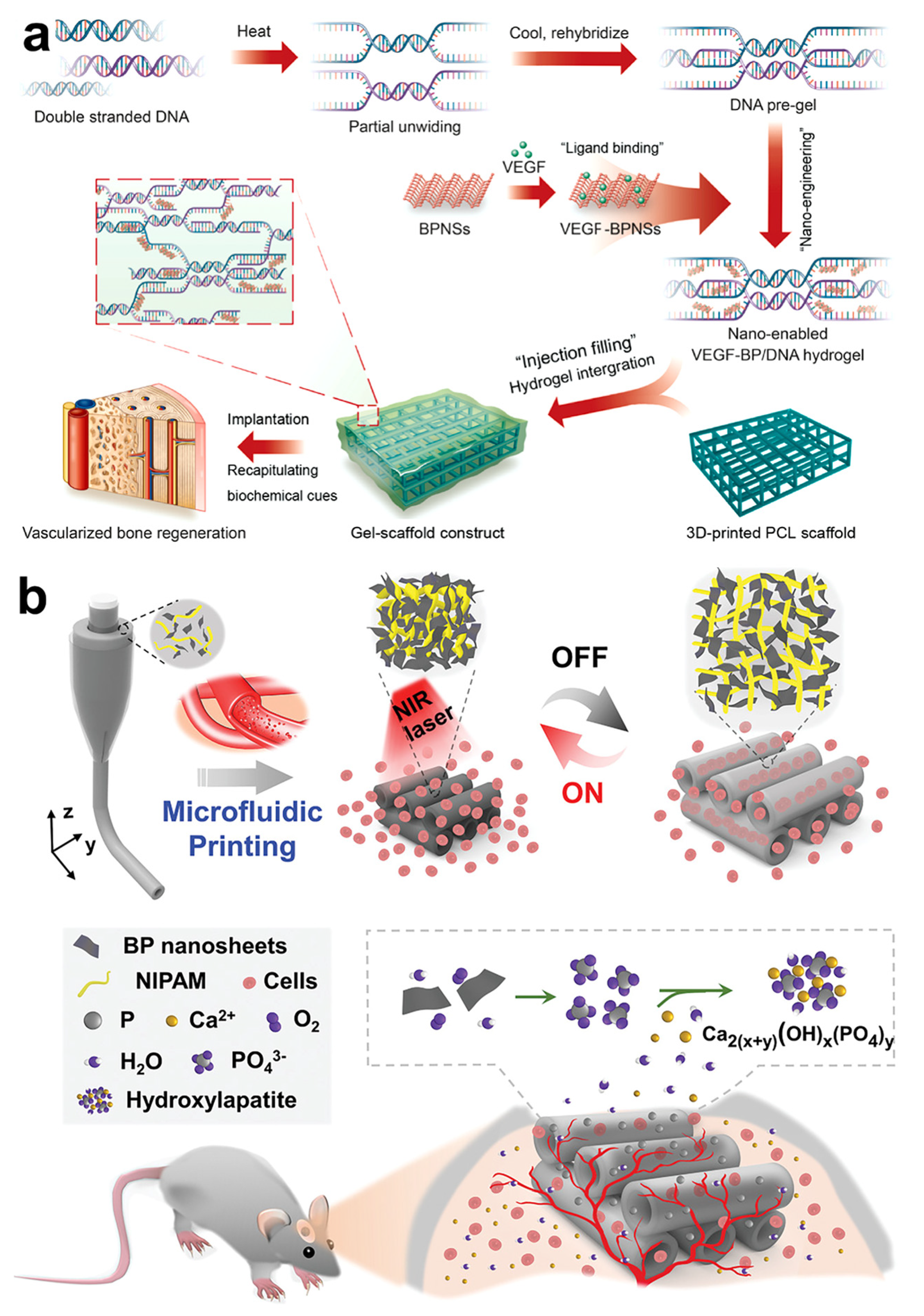
5.2. Vascularized Bone Regeneration Material Based on Black Phosphorus
5.3. Bone Regeneration Material Based on Photothermal Responsiveness of Black Phosphorus
5.4. Antibacterial Bone Regeneration Material Based on Black Phosphorus
5.5. Antitumor Bone Regeneration Materials Based on Black Phosphorus
5.6. Applications of Black Phosphorus-Based Biomaterials in Other Orthopedic Diseases
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calvo, M.S.; Lamberg-Allardt, C.J. Phosphorus. Adv. Nutr. 2015, 6, 860–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.R.; Anderson, C. Dietary Phosphorus Intake and the Kidney. Annu. Rev. Nutr. 2017, 37, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Houston, D.A.; Farquharson, C.; MacRae, V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.K.; Yu, Y.J.; Ye, G.J.; Ge, Q.Q.; Ou, X.D.; Wu, H.; Feng, D.L.; Chen, X.H.; Zhang, Y.B. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Kou, L.Z.; Chen, C.F.; Smith, S.C. Phosphorene: Fabrication, Properties, and Applications. J. Phys. Chem. Lett. 2015, 6, 2794–2805. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Du, Y.C.; Deng, Y.X.; Ye, P.D. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.J.; Shi, Z.; Shi, X.Y.; Zhang, K.; Zhang, H. Recent progress in black phosphorus and black-phosphorus-analogue materials: Properties, synthesis and applications. Nanoscale 2019, 11, 14491–14527. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.Q.; Wu, J.; Gu, Z.P. Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Appl. Mater. Interfaces 2019, 11, 2908–2916. [Google Scholar] [CrossRef]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Yuan, W.E.; Cheng, Y.; Yang, Y.Q.; Qu, X.H.; Fan, C.Y. Concentrically Integrative Bioassembly of a Three-Dimensional Black Phosphorus Nanoscaffold for Restoring Neurogenesis, Angiogenesis, and Immune Homeostasis. Nano Lett. 2019, 19, 8990–9001. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, X.Y.; Zhao, Y.T.; Bai, L.; He, Z.; Tong, Q.; Xie, X.Q.; Zhu, H.R.; Cai, D.Z.; Zhou, Y.; et al. Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication 2020, 12, 035004. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X.N. Black Phosphorus and its Biomedical Applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Liao, Q.; Wu, L.; Luo, Y.X.; Zhang, W.; Guan, M.; Pan, H.B.; Tong, L.P.; Chu, P.K.; Wang, H.Y. ZnL2-BPs Integrated Bone Scaffold under Sequential Photothermal Mediation: A Win-Win Strategy Delivering Antibacterial Therapy and Fostering Osteogenesis Thereafter. ACS Nano 2021, 15, 17854–17869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Tang, J.J.; Li, C.; Lu, Y.; Cheng, L.L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.Z.; Dai, C.B.; Li, Y.; Yin, Y.M.; Gong, L.; Machuki, J.O.; Yang, Y.; Qiu, S.; Guo, K.J.; Gao, F.L. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials 2020, 239, 119851. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Shao, J.D.; Abd El Raouf, M.; Xie, H.H.; Huang, H.; Wang, H.Y.; Chu, P.K.; Yu, X.F.; Yang, Y.; AbdEl-Aal, A.M.; et al. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.P.; Liao, Q.; Zhao, Y.T.; Huang, H.; Gao, A.; Zhang, W.; Gao, X.Y.; Wei, W.; Guan, M.; Chu, P.K.; et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials 2019, 193, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.B.; Xie, Q.Y.; Wei, W.; Zhao, J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat. Commun. 2018, 9, 2480. [Google Scholar] [CrossRef]
- Mo, J.B.; Xu, Y.; Wang, X.X.; Wei, W.; Zhao, J. Exploiting the protein corona: Coating of black phosphorus nanosheets enables macrophage polarization via calcium influx. Nanoscale 2020, 12, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Gui, R.J.; Jin, H.; Wang, Z.H.; Li, J.H. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.A.; Li, R.Y.; Li, S.H.; Li, Y.H.; Wang, X.Y.; Qin, Y.G. Advanced Black Phosphorus Nanomaterials for Bone Regeneration. Int. J. Nanomed. 2020, 15, 2045–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.N.; Chen, S.; Wang, Z.Z.; Yang, Z.Y.; Liu, F.; Xu, Y.H.; Wang, J.H.; Yi, Y.; Zhang, H.; Liao, L.; et al. Metal-Ion-Modified Black Phosphorus with Enhanced Stability and Transistor Performance. Adv. Mater. 2017, 29, 1703811. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.P.; Liu, Z.J.; Han, Y.J.; Wang, L.Q.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.M.; Liu, Z.D.; Tan, C.L.; Luo, Z.M.; Li, H.; Lin, J.D.; Sun, L.Q.; Chen, W.; Xu, Z.C.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Miao, Y.H.; Wang, X.J.; Sun, J.; Yan, Z. Recent advances in the biomedical applications of black phosphorus quantum dots. Nanoscale Adv. 2021, 3, 1532–1550. [Google Scholar] [CrossRef]
- Tayari, V.; Hemsworth, N.; Fakih, I.; Favron, A.; Gaufres, E.; Gervais, G.; Martel, R.; Szkopek, T. Two-dimensional magnetotransport in a black phosphorus naked quantum well. Nat. Commun. 2015, 6, 7702. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Zhu, X.B.; Yu, X.H.; Zeng, X.W.; Xiao, Q.L.; Zhang, X.D.; Ji, X.Y.; Wang, X.S.; Shi, J.J.; Zhang, H.; et al. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2017, 29, 1603276. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.M.; Kasper, F.K.; Mikos, A.G. Phosphorous-containing polymers for regenerative medicine. Biomed. Mater. 2014, 9, 025014. [Google Scholar] [CrossRef]
- Latiff, N.M.; Teo, W.Z.; Sofer, Z.; Fisher, A.C.; Pumera, M. The Cytotoxicity of Layered Black Phosphorus. Chem. Eur. J. 2015, 21, 13991–13995. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhang, Z.M.; Zhang, S.Y.; Li, D.Y.; Ma, W.; Ma, C.X.; Wu, F.C.; Zhao, Q.; Yan, Q.F.; Xing, B.S. Size Effect on the Cytotoxicity of Layered Black Phosphorus and Underlying Mechanisms. Small 2017, 13, 1701210. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, G.; Gong, K.K.; Wang, J.; Ge, X.X.; Liu, X.Q.; Guo, S.J.; Wang, F. MnO2-Laden Black Phosphorus for MRI-Guided Synergistic PDT, PTT, and Chemotherapy. Matter 2019, 1, 496–512. [Google Scholar] [CrossRef] [Green Version]
- Doganov, R.A.; O’Farrell, E.C.T.; Koenig, S.P.; Yeo, Y.T.; Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Watanabe, K.; Taniguchi, T.; et al. Transport properties of pristine few-layer black phosphorus by van der Waals passivation in an inert atmosphere. Nat. Commun. 2015, 6, 6647. [Google Scholar] [CrossRef] [Green Version]
- Favron, A.; Gaufres, E.; Fossard, F.; Phaneuf-L’Heureux, A.L.; Tang, N.Y.W.; Levesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Neto, A.H.C. Oxygen Defects in Phosphorene. Phys. Rev. Lett. 2015, 114, 046801. [Google Scholar] [CrossRef] [Green Version]
- Li, W.H.; Li, S.T.; Zhang, J.W.; Zhong, H.M.; Liang, J.; Huang, S.J.; Liao, G.Z.; Zhang, B.; Liu, C.L. Fabrication and evaluation of bone morphogenetic protein-2 microspheres coated black phosphorus nanosheets@polylactic-glycolic acid copolymers scaffold: A multifunctional antibacterial photothermal scaffold for bone regeneration. Int. J. Biol. Macromol. 2022, 210, 350–364. [Google Scholar] [CrossRef]
- Koenig, S.P.; Doganov, R.A.; Seixas, L.; Carvalho, A.; Tan, J.Y.; Watanabe, K.; Taniguchi, T.; Yakovlev, N.; Neto, A.H.C.; Ozyilmaz, B. Electron Doping of Ultrathin Black Phosphorus with Cu Adatoms. Nano Lett. 2016, 16, 2145–2151. [Google Scholar] [CrossRef]
- Ryder, C.R.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.J.; Schatz, G.C.; Hersam, M.C. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.S.; Yang, B.C.; Wang, Y.; Zhang, J.Y.; Zeng, Z.M.; Liu, Z.Y.; Wang, W.H. Enhanced stability of black phosphorus field-effect transistors with SiO2 passivation. Nanotechnology 2015, 26, 435702. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.W.; Luo, M.M.; Liu, G.; Wang, X.S.; Tao, W.; Lin, Y.X.; Ji, X.Y.; Nie, L.; Mei, L. Polydopamine-Modified Black Phosphorous Nanocapsule with Enhanced Stability and Photothermal Performance for Tumor Multimodal Treatments. Adv. Sci. 2018, 5, 1800510. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Hu, K.; Li, Z.; Wang, C.; Yu, M.; Li, Z.G.; Li, Z. Black Phosphorus Nanosheets Passivation Using a Tripeptide. Small 2018, 14, 1801701. [Google Scholar] [CrossRef]
- Liang, X.; Ye, X.Y.; Wang, C.; Xing, C.Y.; Miao, Q.W.; Xie, Z.J.; Chen, X.L.; Zhang, X.D.; Zhang, H.; Mei, L. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Control. Release 2019, 296, 150–161. [Google Scholar] [CrossRef]
- Deng, X.R.; Liu, H.X.; Xu, Y.; Chan, L.; Xie, J.; Xiong, Z.S.; Tang, Z.; Yang, F.; Chen, T.F. Designing highly stable ferrous selenide-black phosphorus nanosheets heteronanostructure via P-Se bond for MRI-guided photothermal therapy. J. Nanobiotechnol. 2021, 19, 1–20. [Google Scholar] [CrossRef]
- Sa, B.S.; Li, Y.L.; Qi, J.S.; Ahuja, R.; Sun, Z.M. Strain Engineering for Phosphorene: The Potential Application as a Photocatalyst. J. Phys. Chem. C 2014, 118, 26560–26568. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, H.; Xiao, X.; Liang, D.; Liang, X.Y.; Mi, L.; Wang, J.F.; Liu, J. Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors. Acta Biomater. 2020, 107, 260–271. [Google Scholar] [CrossRef]
- Huang, M.; Gu, Z.Y.; Zhang, J.G.; Zhang, D.; Zhang, H.; Yang, Z.G.; Qu, J.L. MXene and black phosphorus based 2D nanomaterials in bioimaging and biosensing: Progress and perspectives. J. Mater. Chem. B 2021, 9, 5195–5220. [Google Scholar] [CrossRef]
- Zhang, W.N.; Shen, Z.X.; Wu, Y.; Zhang, W.Z.; Zhang, T.G.; Yu, B.Y.; Zheng, X.C.; Tian, J.W. Renal-clearable and biodegradable black phosphorus quantum dots for photoacoustic imaging of kidney dysfunction. Anal. Chim. Acta 2022, 1204, 339737. [Google Scholar] [CrossRef]
- Sun, Z.B.; Xie, H.H.; Tang, S.Y.; Yu, X.F.; Guo, Z.N.; Shao, J.D.; Zhang, H.; Huang, H.; Wang, H.Y.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.Z.; Shao, W.; Chen, S.C.; Xie, J.F.; Zhang, X.D.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Shao, J.D.; Li, Z.B.; Ren, Q.Z.; Yu, X.F.; Liu, S.J. Black Phosphorus-Based Multimodal Nanoagent: Showing Targeted Combinatory Therapeutics against Cancer Metastasis. Nano Lett. 2019, 19, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, L.; Chen, H.J.; Chen, Y.Z.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.N.; Chen, G.P. Composite scaffolds of black phosphorus nanosheets and gelatin with controlled pore structures for photothermal cancer therapy and adipose tissue engineering. Biomaterials 2021, 275, 120923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.Q.; Xu, Y.L.; Ji, W.; Zhou, S.Y.; Li, L.F.; Qiu, L.H.; Qian, Z.Z.; Wang, X.H.; Zhang, H.L. Biomimetic black phosphorus quantum dots-based photothermal therapy combined with anti-PD-L1 treatment inhibits recurrence and metastasis in triple-negative breast cancer. J. Nanobiotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Xu, S.B.; Hu, Y.A.; Zhao, X.J.; Chang, L.N.; Chen, Z.H.; Mei, X.F. Preparation of NIR-responsive, ROS-generating and antibacterial black phosphorus quantum dots for promoting the MRSA-infected wound healing in diabetic rats. Acta Biomater. 2022, 137, 199–217. [Google Scholar] [CrossRef]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mat. Sci. Eng. C 2021, 130, 112433. [Google Scholar] [CrossRef]
- Li, D.Z.; Chen, K.W.; Tang, H.; Hu, S.S.; Xin, L.J.; Jing, X.; He, Q.Q.; Wang, S.; Song, J.L.; Mei, L.; et al. A Logic-Based Diagnostic and Therapeutic Hydrogel with Multistimuli Responsiveness to Orchestrate Diabetic Bone Regeneration. Adv. Mater. 2022, 34, 2108430. [Google Scholar] [CrossRef]
- Reybier, K.; Ayala, S.; Alies, B.; Rodrigues, J.V.; Rodriguez, S.B.; La Penna, G.; Collin, F.; Gomes, C.M.; Hureau, C.; Faller, P. Free Superoxide is an Intermediate in the Production of H2O2 by Copper(I)-A Peptide and O-2. Angew. Chem. Int. Ed. 2016, 55, 1085–1089. [Google Scholar] [CrossRef]
- Chen, W.S.; Ouyang, J.; Yi, X.Y.; Xu, Y.; Niu, C.C.; Zhang, W.Y.; Wang, L.Q.; Sheng, J.P.; Deng, L.; Liu, Y.N.; et al. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Adv. Mater. 2018, 30, 1703458. [Google Scholar] [CrossRef]
- Hou, J.J.; Wang, H.; Ge, Z.L.; Zuo, T.T.; Chen, Q.; Liu, X.G.; Mou, S.; Fan, C.H.; Xie, Y.; Wang, L.H. Treating Acute Kidney Injury with Antioxidative Black Phosphorus Nanosheets. Nano Lett. 2020, 20, 1447–1454. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhao, Q.Q.; Qin, J.C.; Guo, Y.Y.; Zhang, C.; Li, Y.H. Urokinase loaded black phosphorus nanosheets for sequential thrombolysis and reactive oxygen species scavenging in ischemic stroke treatment. Biomater. Sci. 2022, 10, 4656–4666. [Google Scholar] [CrossRef]
- Mu, X.Y.; Wang, J.Y.; Bai, X.T.; Xu, F.J.; Liu, H.X.; Yang, J.; Jing, Y.Q.; Liu, L.F.; Xue, X.H.; Dai, H.T.; et al. Black Phosphorus Quantum Dot Induced Oxidative Stress and Toxicity in Living Cells and Mice. ACS Appl. Mater. Interfaces 2017, 9, 20399–20409. [Google Scholar] [CrossRef]
- Zhang, X.D.; Li, L.F.; Ouyang, J.; Zhang, L.Q.; Xue, J.J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Huang, X.; Xing, J.; Wang, Z.G.; Han, J.; Wang, R.X.; Li, C.H.; Xiao, C.R.; Lu, F.; Zhai, J.X.; Zhou, Z.N.; et al. 0D/1D Heterojunction Implant with Electro-Mechanobiological Coupling Cues Promotes Osteogenesis. Adv. Funct. Mater. 2021, 31, 2106249. [Google Scholar] [CrossRef]
- Nekounam, H.; Allahyari, Z.; Gholizadeh, S.; Mirzaei, E.; Shokrgozar, M.A.; Faridi-Majidi, R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2020, 117, 111226. [Google Scholar] [CrossRef]
- Amaral, P.E.M.; Nieman, G.P.; Schwenk, G.R.; Jing, H.; Zhang, R.; Cerkez, E.B.; Strongin, D.; Ji, H.F. High Electron Mobility of Amorphous Red Phosphorus Thin Films. Angew. Chem. Int. Ed. 2019, 58, 6766–6771. [Google Scholar] [CrossRef]
- Hughes, E.A.B.; Robinson, T.E.; Bassett, D.B.; Cox, S.C.; Grover, L.M. Critical and diverse roles of phosphates in human bone formation. J. Mater. Chem. B 2019, 7, 7460–7470. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.D.; Xie, H.H.; Huang, H.; Li, Z.B.; Sun, Z.B.; Xu, Y.H.; Xiao, Q.L.; Yu, X.F.; Zhao, Y.T.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Shao, J.D.; Ruan, C.S.; Xie, H.H.; Chu, P.K.; Yu, X.F. Photochemical Activity of Black Phosphorus for Near-Infrared Light Controlled In Situ Biomineralization. Adv. Sci. 2020, 7, 2000439. [Google Scholar] [CrossRef]
- Tang, C.Z.; Wei, Y.; Gu, L.; Zhang, Q.H.; Li, M.; Yuan, G.H.; He, Y.; Huang, L.; Liu, Y.; Zhang, Y.F. Biomineral Precursor Formation Is Initiated by Transporting Calcium and Phosphorus Clusters from the Endoplasmic Reticulum to Mitochondria. Adv. Sci. 2020, 7, 1902536. [Google Scholar] [CrossRef]
- Xu, H.C.; Liu, X.F.; Park, S.; Terzic, A.; Lu, L.C. Size-dependent osteogenesis of black phosphorus in nanocomposite hydrogel scaffolds. J. Biomed. Mater. Res. A 2022, 110, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wang, J.; Qiu, S.L.; Chen, W.J.; Cheng, L.; Du, W.X.; Wang, J.H.; Han, L.F.; Song, L.; Hu, Y. Biodegradable L-lysine-modified amino black phosphorus/poly (1-lactide-co epsilon-caprolactone) nanofibers with enhancements in hydrophilicity, shape recovery and osteodifferentiation properties. Colloids Surf. B Biointerfaces 2022, 209, 112209. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Liu, X.F.; George, M.N.; Miller, A.L.; Park, S.; Xu, H.; Terzic, A.; Lu, L.C. Black phosphorus incorporation modulates nanocomposite hydrogel properties and subsequent MC3T3 cell attachment, proliferation, and differentiation. J. Biomed. Mater. Res. A 2021, 109, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Qian, G.W.; Yao, J.; Cai, W.L.; Peng, S.P.; Shuai, C.J. Polydopamine-decorated black phosphorous to enhance stability in polymer scaffold. Nanotechnology 2021, 32, 455701. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Miller, A.L.; Park, S.; George, M.N.; Waletzki, B.E.; Xu, H.C.; Terzic, A.; Lu, L.C. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef]
- Xu, D.L.; Gan, K.F.; Wang, Y.; Wu, Z.T.; Wang, Y.L.; Zhang, S.; Peng, Y.J.; Fang, X.G.; Wei, H.; Zhang, Y.S.; et al. A Composite Deferoxamine/Black Phosphorus Nanosheet/Gelatin Hydrogel Scaffold for Ischemic Tibial Bone Repair. Int. J. Nanomed. 2022, 17, 1015–1030. [Google Scholar] [CrossRef]
- Miao, Y.L.; Chen, Y.H.; Luo, J.S.; Liu, X.; Yang, Q.; Shi, X.T.; Wang, Y.J. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2022, 21, 97–109. [Google Scholar] [CrossRef]
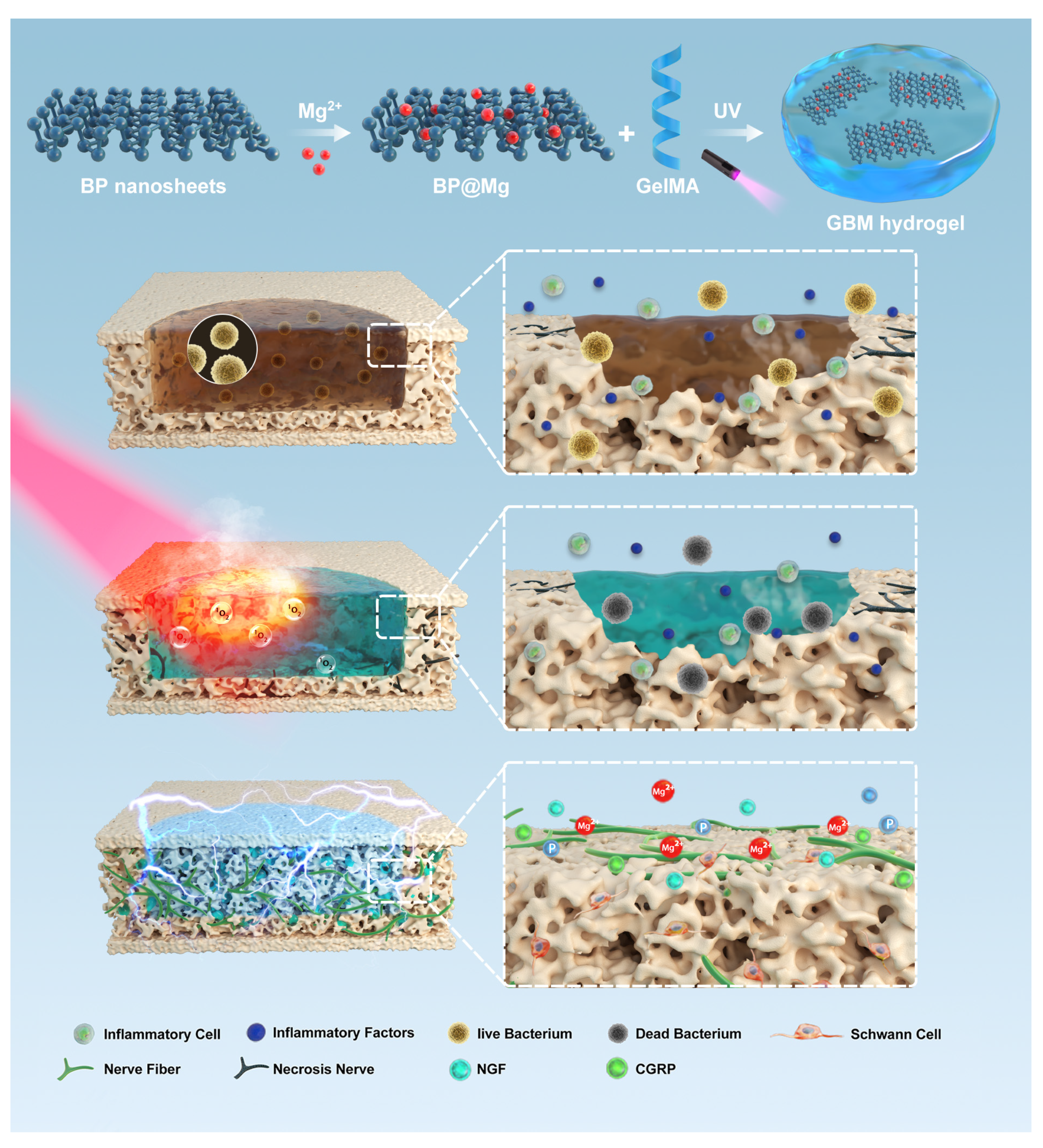
- Xu, Y.; Xu, C.; He, L.; Zhou, J.J.; Chen, T.W.; Ouyang, L.; Guo, X.D.; Qu, Y.Z.; Luo, Z.Q.; Duan, D.Y. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef]
- Chen, S.Y.; Guo, R.Y.; Liang, Q.S.; Xiao, X.F. Multifunctional modified polylactic acid nanofibrous scaffold incorporating sodium alginate microspheres decorated with strontium and black phosphorus for bone tissue engineering. J. Biomat. Sci.-Polym. E 2021, 32, 1598–1617. [Google Scholar] [CrossRef]
- Li, Z.R.; Zhang, X.C.; Ouyang, J.; Chu, D.D.; Han, F.Q.; Shi, L.Q.; Liu, R.X.; Guo, Z.H.; Gu, G.X.; Tao, W.; et al. Ca2+-supplying black phosphorus-based scaffolds fabricated with microfluidic technology for osteogenesis. Bioact. Mater. 2021, 6, 4053–4064. [Google Scholar] [CrossRef]
- Miao, Y.L.; Shi, X.T.; Li, Q.T.; Hao, L.J.; Liu, L.; Liu, X.; Chen, Y.H.; Wang, Y.J. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019, 7, 4046–4059. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, X.; Li, Y.; Zhao, Y.; Xue, M.; Guo, Q.; Zheng, G.; Chen, X.; Lin, H.; Guo, X. Black-Phosphorus-Nanosheet-Reinforced Coating of Implants for Sequential Biofilm Ablation and Bone Fracture Healing Acceleration. ACS Appl. Mater. Interfaces 2022, 14, 47036–47051. [Google Scholar] [CrossRef]
- Jing, X.; Xu, C.; Su, W.; Ding, Q.; Ye, B.; Su, Y.; Yu, K.; Zeng, L.; Yang, X.; Qu, Y.; et al. Photosensitive and Conductive Hydrogel Induced Innerved Bone Regeneration for Infected Bone Defect Repair. Adv. Healthc. Mater. 2022, e2201349. [Google Scholar] [CrossRef]
- Yang, B.W.; Yin, J.H.; Chen, Y.; Pan, S.S.; Yao, H.L.; Gao, Y.S.; Shi, J.L. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds: A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30, 1705611. [Google Scholar] [CrossRef]
- Ma, Y.L.; Jiang, L.; Hu, J.; Zhu, E.J.; Zhang, N. Developing a Versatile Multiscale Therapeutic Platform for Osteosarcoma Synergistic Photothermo-Chemotherapy with Effective Osteogenicity and Antibacterial Capability. ACS Appl. Mater. Interfaces 2022, 14, 44065–44083. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, Z.W.; Zhao, J.W.; Wang, F.; Lu, M.; Deng, L.F.; Cui, W.G. Black phosphorus-based 2D materials for bone therapy. Bioact. Mater. 2020, 5, 1026–1043. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, J.H.; Jang, H.J.; Bin Lee, Y.; Kim, B.; Kang, M.S.; Shin, Y.C.; Shin, D.M.; Hong, S.W.; Han, D.W. Spontaneously promoted osteogenic differentiation of MC3T3-E1 preosteoblasts on ultrathin layers of black phosphorus. Mater. Sci. Eng. C 2021, 128, 112309. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.Y.; Xie, J.L.; Agren, H.; Zhang, H. Black Phosphorus/Polymers: Status and Challenges. Adv. Mater. 2021, 33, 2100113. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Wu, G.S.; Wang, Y.D.; Wang, T.; Ma, Y.; Fei, B. Ultra-strong polyethyleneimine-graphene oxide nanocomposite film via synergistic interactions and its use for humidity sensing. Compos. Part A Appl. Sci. Manuf. 2018, 115, 341–347. [Google Scholar] [CrossRef]
- Wang, H.; Lin, C.C.; Zhang, X.R.; Lin, K.L.; Wang, X.D.; Shen, S.G. Mussel-Inspired Polydopamine Coating: A General Strategy To Enhance Osteogenic Differentiation and Osseointegration for Diverse Implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.F.; Yao, S.; Liu, M.; Yang, Y.S.; Ji, Y.H.; Cui, W.; Qu, Y.Z.; Guo, X.D. Composite Scaffolds of Mineralized Natural Extracellular Matrix on True Bone Ceramic Induce Bone Regeneration Through Smad1/5/8 and ERK1/2 Pathways. Tissue Eng. Part A 2018, 24, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A.R. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Wang, Z.M.; Zhao, J.; Tang, W.Z.; Hu, L.Q.; Chen, X.; Su, Y.P.; Zou, C.; Wang, J.H.; Lu, W.W.; Zhen, W.X.; et al. Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small 2019, 15, 1901560. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, X.F.; Gaihre, B.; Li, Y.; Lu, L.C. Mesenchymal stem cell spheroids incorporated with collagen and black phosphorus promote osteogenesis of biodegradable hydrogels. Mat. Sci. Eng. C 2021, 121, 111812. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.J.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.P.; Bunker, C.E. Graphene Oxide: A Nonspecific Enhancer of Cellular Growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Hehir, S.; McAfee, M.; Breen, A. Biomedical Applications of Electrospun Graphene Oxide. ACS Biomater. Sci. Eng. 2021, 7, 1278–1301. [Google Scholar] [CrossRef]
- Liu, X.F.; George, M.N.; Li, L.L.; Gamble, D.; Miller, A.L.; Gaihre, B.P.; Waletzki, B.E.; Lu, L.C. Injectable Electrical Conductive and Phosphate Releasing Gel with Two-Dimensional Black Phosphorus and Carbon Nanotubes for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 4653–4665. [Google Scholar] [CrossRef]
- Tan, L.; Hu, Y.; Li, M.H.; Zhang, Y.C.; Xue, C.C.; Chen, M.H.; Luo, Z.; Cai, K.Y. Remotely-activatable extracellular matrix-mimetic hydrogel promotes physiological bone mineralization for enhanced cranial defect healing. Chem. Eng. J. 2022, 431, 133382. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.J.; Zhang, Z.Y.; Jiang, X.Q. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801433. [Google Scholar] [CrossRef]
- Barabaschi, G.D.G.; Manoharan, V.; Li, Q.; Bertassoni, L.E. Engineering Pre-vascularized Scaffolds for Bone Regeneration. Adv. Exp. Med. Biol. 2015, 881, 79–94. [Google Scholar]
- Han, X.Y.; Sun, M.J.; Chen, B.; Saiding, Q.; Zhang, J.Y.; Song, H.L.; Deng, L.F.; Wang, P.; Gong, W.M.; Cui, W.G. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact. Mater. 2021, 6, 1639–1652. [Google Scholar] [CrossRef]
- Donneys, A.; Weiss, D.M.; Deshpande, S.S.; Ahsan, S.; Tchanque-Fossuo, C.N.; Sarhaddi, D.; Levi, B.; Goldstein, S.A.; Buchman, S.R. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone 2013, 52, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef]
- Wang, X.C.; Yu, Y.R.; Yang, C.Y.; Shao, C.M.; Shi, K.Q.; Shang, L.R.; Ye, F.F.; Zhao, Y.J. Microfluidic 3D Printing Responsive Scaffolds with Biomimetic Enrichment Channels for Bone Regeneration. Adv. Funct. Mater. 2021, 31, 2105190. [Google Scholar] [CrossRef]
- Shi, W.T.; Wang, Z.; Bian, L.; Wu, Y.Q.; HuiYa, M.; Zhou, Y.J.; Zhang, Z.J.; Wang, Q.; Zhao, P.; Lu, X.J. Periodic Heat Stress Licenses EMSC Differentiation into Osteoblasts via YAP Signaling Pathway Activation. Stem. Cells Int. 2022, 2022, 3715471. [Google Scholar] [CrossRef]
- Boyan, B.D.; Asmussen, N.C.; Lin, Z.; Schwartz, Z. The Role of Matrix-Bound Extracellular Vesicles in the Regulation of Endochondral Bone Formation. Cells 2022, 11, 1619. [Google Scholar] [CrossRef]
- Simao, A.M.S.; Yadav, M.C.; Narisawa, S.; Bolean, M.; Pizauro, J.M.; Hoylaerts, M.F.; Ciancaglini, P.; Millan, J.L. Proteoliposomes Harboring Alkaline Phosphatase and Nucleotide Pyrophosphatase as Matrix Vesicle Biomimetics. J. Biol. Chem. 2010, 285, 7598–7609. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Hu, X.X.; Zhang, L.L.; Zhu, C.L.; Wang, J.; Li, Y.X.; Wang, Y.L.; Wang, C.; Zhang, Y.F.; Yuan, Q. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat. Commun. 2019, 10, 2829. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Patel, K.D.; Leong, K.W.; Kim, H.W. Progress in Nanotheranostics Based on Mesoporous Silica Nanomaterial Platforms. ACS Appl. Mater. Interfaces 2017, 9, 10309–10337. [Google Scholar] [CrossRef]
- Zhang, D.W.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yue, H.B.; Liu, J.; Zhao, Q.L.; He, Z.; Li, K.; Lu, B.H.; Huang, W.H.; Wei, Y.; Tang, Y.J.; et al. Advanced reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of irregular shapes. Biofabrication 2020, 12, 045025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Peng, X.; Wang, D.Q.; Zhang, H.B.; Xin, Q.W.; Wu, M.Z.; Xu, X.Y.; Sun, F.; Xing, Z.Y.; Wang, L.N.; et al. Chloroplast-inspired Scaffold for Infected Bone Defect Therapy: Towards Stable Photothermal Properties and Self-Defensive Functionality. Adv. Sci. 2022, 9, 2204535. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.A.; Wang, H.; Lou, Y.; Fang, X.; Li, S.H.; Wang, X.Y.; Gao, X.; Qin, Y.G. Chemotactic ion-releasing hydrogel for synergistic antibacterial and bone regeneration. Mater. Today Chem. 2022, 24, 100894. [Google Scholar] [CrossRef]
- Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: Material properties and animal models. Biomater. Transl. 2021, 2, 197–213. [Google Scholar]
- Li, Z.; Meyers, C.A.; Chang, L.; Lee, S.; Li, Z.; Tomlinson, R.; Hoke, A.; Clemens, T.L.; James, A.W. Fracture repair requires TrkA signaling by skeletal sensory nerves. J. Clin. Investig. 2019, 129, 5137–5150. [Google Scholar] [CrossRef] [Green Version]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Gimza, B.D.; Cassat, J.E. Mechanisms of Antibiotic Failure During Osteomyelitis. Front. Immunol. 2021, 12, 638085. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Shanaghi, A.; Mehrjou, B.; Ahmadian, Z.; Souri, A.R.; Chu, P.K. Enhanced corrosion resistance, antibacterial properties, and biocompatibility by hierarchical hydroxyapatite/ciprofloxacin-calcium phosphate coating on nitrided NiTi alloy. Mat. Sci. Eng. C 2021, 118, 111524. [Google Scholar] [CrossRef]
- Sun, X.H.; Yu, C.C.; Zhang, L.; Cao, J.C.; Kaleli, E.H.; Xie, G.X. Tribological and Antibacterial Properties of Polyetheretherketone Composites with Black Phosphorus Nanosheets. Polymers 2022, 14, 1242. [Google Scholar] [CrossRef]
- Kumar, V.; Brent, J.R.; Shorie, M.; Kaur, H.; Chadha, G.; Thomas, A.G.; Lewis, E.A.; Rooney, A.P.; Nguyen, L.; Zhong, X.L.; et al. Nanostructured Aptamer-Functionalized Black Phosphorus Sensing Platform for Label-Free Detection of Myoglobin, a Cardiovascular Disease Biomarker. ACS Appl. Mater. Interfaces 2016, 8, 22860–22868. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.P.; Zhang, H.; Kim, J.S. Omnipotent phosphorene: A next-generation, two-dimensional nanoplatform for multidisciplinary biomedical applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.Q.; Wang, J.; Wang, Y.L.; Chen, A.Y.; Wang, C.; Mo, W.T.; Li, Y.X.; Yuan, Q.; Zhang, Y.F. Photon-Responsive Antibacterial Nanoplatform for Synergistic Photothermal-/Pharmaco-Therapy of Skin Infection. ACS Appl. Mater. Interfaces 2019, 11, 300–310. [Google Scholar] [CrossRef]
- Fan, W.P.; Huang, P.; Chen, X.Y. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.M.; Cui, Z.D.; Yang, X.J.; Yeung, K.W.K.; Pan, H.B.; Zheng, Y.F.; Wang, X.B.; Wu, S.L. In Situ Disinfection through Photoinspired Radical Oxygen Species Storage and Thermal-Triggered Release from Black Phosphorous with Strengthened Chemical Stability. Small 2018, 14, 1703197. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Kim, J.J.; Kim, T.H.; Kim, J.H.; Shin, U.S.; Lee, E.J.; Knowles, J.C.; Kim, H.W. Multifunctional hybrid nanocarrier: Magnetic CNTs ensheathed with mesoporous silica for drug delivery and imaging system. ACS Appl. Mater. Interfaces 2014, 6, 2201–2208. [Google Scholar] [CrossRef]
- Liu, X.; Chen, K.; Li, X.Y.; Xu, Q.C.; Weng, J.; Xu, J. Electron Matters: Recent Advances in Passivation and Applications of Black Phosphorus. Adv. Mater. 2021, 33, 2005924. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Zeng, Q.S.; Long, Y.; Liu, Z. Black Phosphorus Nanosheets: Synthesis, Characterization and Applications. Small 2016, 12, 3480–3502. [Google Scholar] [CrossRef]
- Qu, G.B.; Liu, W.; Zhao, Y.T.; Gao, J.; Xia, T.; Shi, J.B.; Hu, L.G.; Zhou, W.H.; Gao, J.J.; Wang, H.Y.; et al. Improved Biocompatibility of Black Phosphorus Nanosheets by Chemical Modification. Angew. Chem. Int. Ed. 2017, 56, 14488–14493. [Google Scholar] [CrossRef] [PubMed]


| Target | Material Design | Main Results | Reference |
|---|---|---|---|
| Biomineralization | BPNs-GelMA | Promoted the expression of osteogenesis-related genes and accelerated bone repair. | [10] |
| BPNs-OPF | Exhibited a controllable degradation rate and phosphate release capacity. Enhanced the adhesion, proliferation, and osteogenic differentiation of pre-osteogenic cells. | [73] | |
| PDA@BPNs-PLLA | Improved the stability of the BPN and promoted osteogenic differentiation. | [74] | |
| L-NH-BP-PLCL | Promoted the proliferation and osteogenic differentiation of mesenchymal. | [72] | |
| GO@BP-PPF | Promoted cell adhesion and osteogenic differentiation. | [75] | |
| Vascularized Osteogenesis | Deferoxamine/BP-GelMA | Promoted local expression of CD31 and positively affected a vascular repair. | [76] |
| VEGF@BPNs-DNA hydrogel | Continuous release of VEGF. Accelerated vascular regeneration and bone regeneration. | [77] | |
| BP@Mg double-layer scaffold | Promoted early nerve regeneration and angiogenesis in the process of bone regeneration. | [78] | |
| Photothermal Osteogenesis | BP/IBU@SA-PLLA | Significantly high photothermal conversion efficiency and photothermal-responsive intelligent drug release performance. | [79] |
| SrCl2/BPNs-PLGA | Remarkable cell compatibility and degradation capability. Remarkably controlled release of strontium ions. | [18] | |
| BP@HA/SiO2-PLLA | The photothermal effect promoted the release of elements, thereby achieving accelerated osteogenesis. | [80] | |
| Antibacterial | BPNs-GelMA | The hydrogel could be heated up to 55.3 ℃ and showed efficient antibacterial and antitumor effects. | [81] |
| BPNs/HA coating | Remarkable photothermal conversion capability and good anti-biofilm properties. | [82] | |
| BP@Mg-GelMA | Remarkable antibacterial capability and induced innerved bone regeneration. | [83] | |
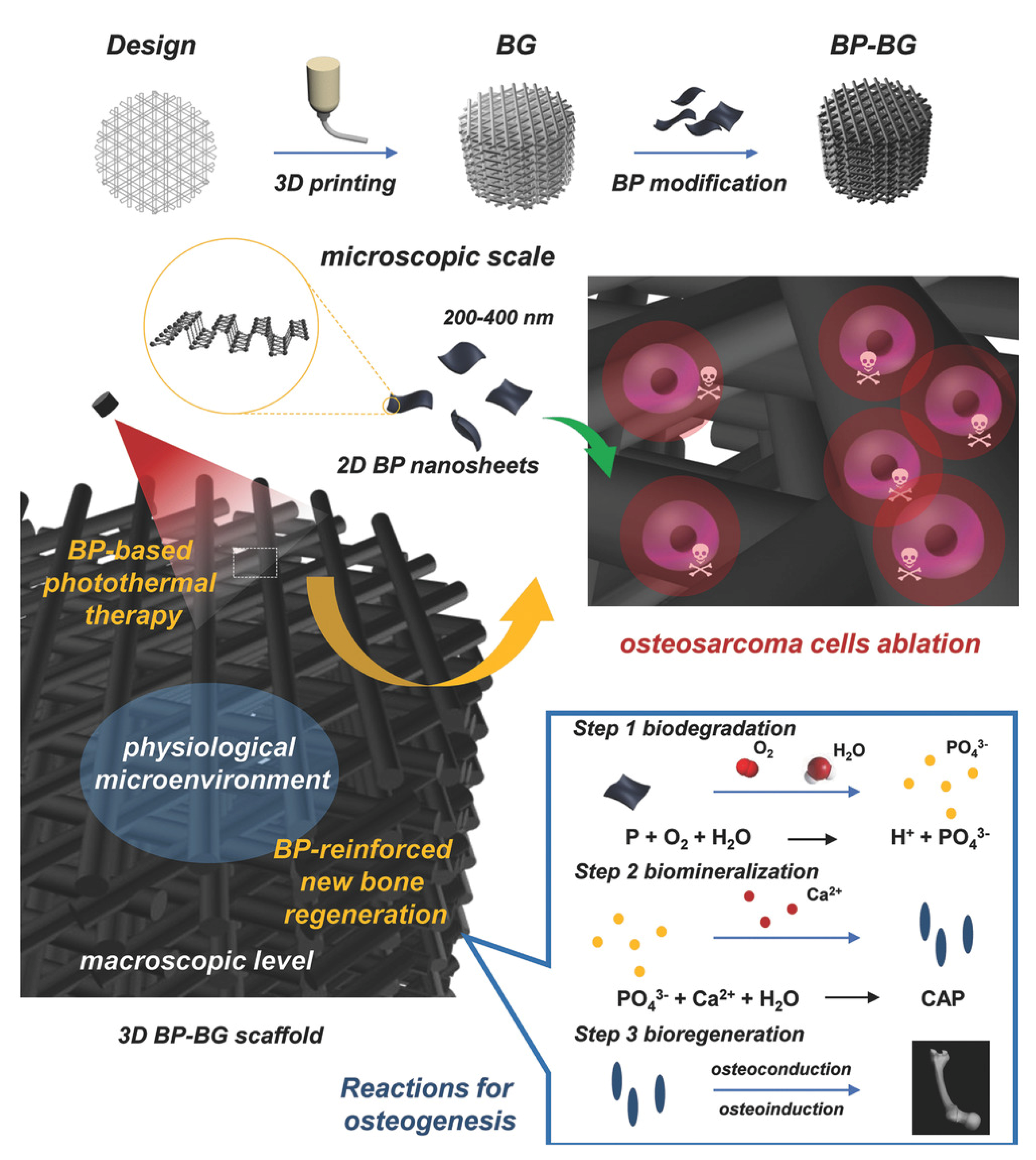
| Antitumor | BPNs -BG scaffold | Remarkable photothermal antitumor activity and osteogenic induction ability. | [84] |
| BP/doxorubicin hydrochloride/PDA coating | NIR/pH dual controlled antitumor drug release and high antibacterial activity. | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, X.; Xiong, Z.; Lin, Z.; Sun, T. The Application of Black Phosphorus Nanomaterials in Bone Tissue Engineering. Pharmaceutics 2022, 14, 2634. https://doi.org/10.3390/pharmaceutics14122634
Jing X, Xiong Z, Lin Z, Sun T. The Application of Black Phosphorus Nanomaterials in Bone Tissue Engineering. Pharmaceutics. 2022; 14(12):2634. https://doi.org/10.3390/pharmaceutics14122634
Chicago/Turabian StyleJing, Xirui, Zekang Xiong, Zian Lin, and Tingfang Sun. 2022. "The Application of Black Phosphorus Nanomaterials in Bone Tissue Engineering" Pharmaceutics 14, no. 12: 2634. https://doi.org/10.3390/pharmaceutics14122634
APA StyleJing, X., Xiong, Z., Lin, Z., & Sun, T. (2022). The Application of Black Phosphorus Nanomaterials in Bone Tissue Engineering. Pharmaceutics, 14(12), 2634. https://doi.org/10.3390/pharmaceutics14122634







