Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host–Pathogen Interface Hypothesis †
Abstract
:1. Introduction
2. Speculation from New Perspectives on SARS-CoV-2 Vaccines
2.1. The Hypothesis
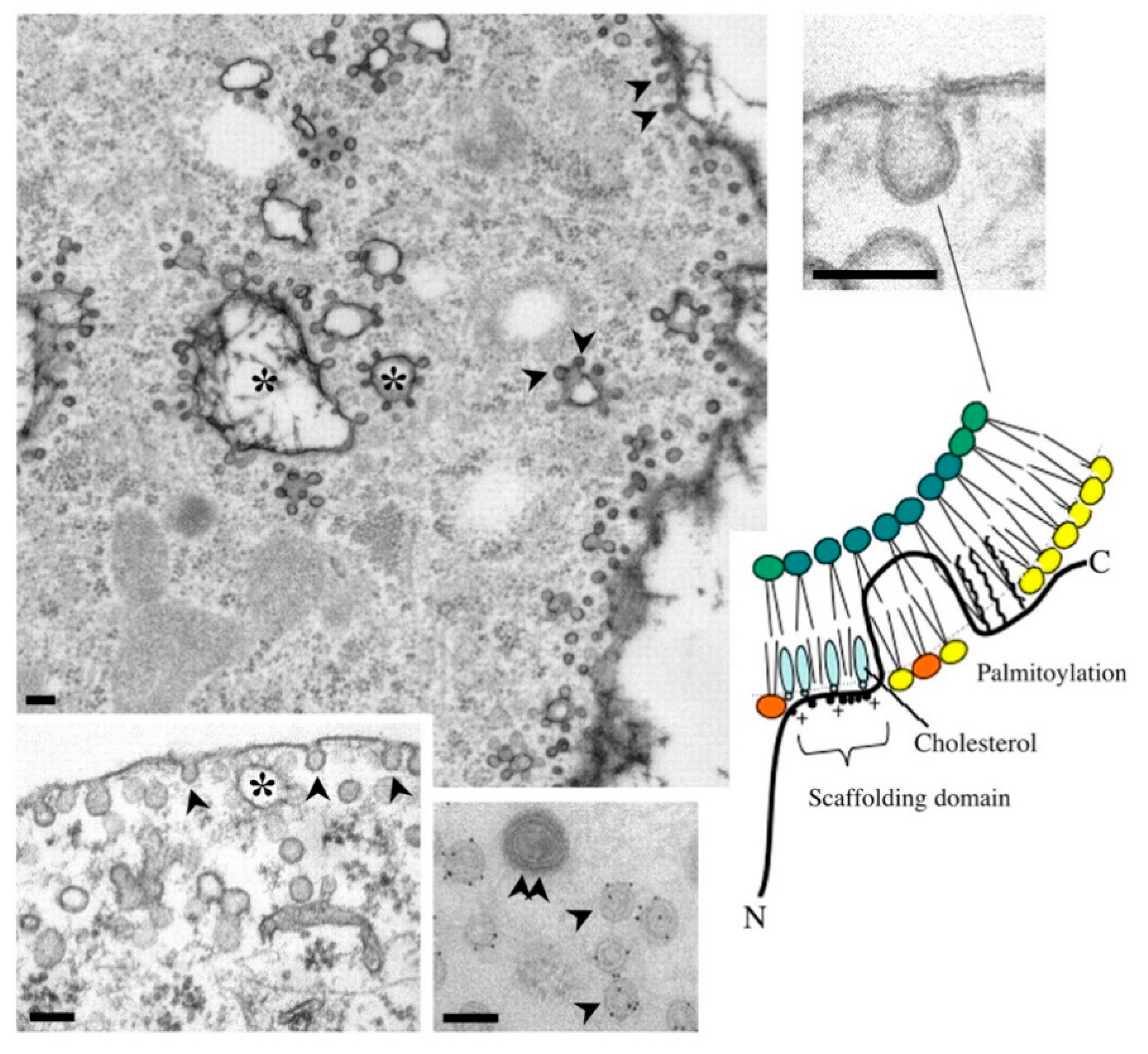
2.2. Cav-1/Caveolae-Budded EVs as Potential Hijackable Gates in Cell Communication of Caveolin-Related Diseases Named Caveolinopathies
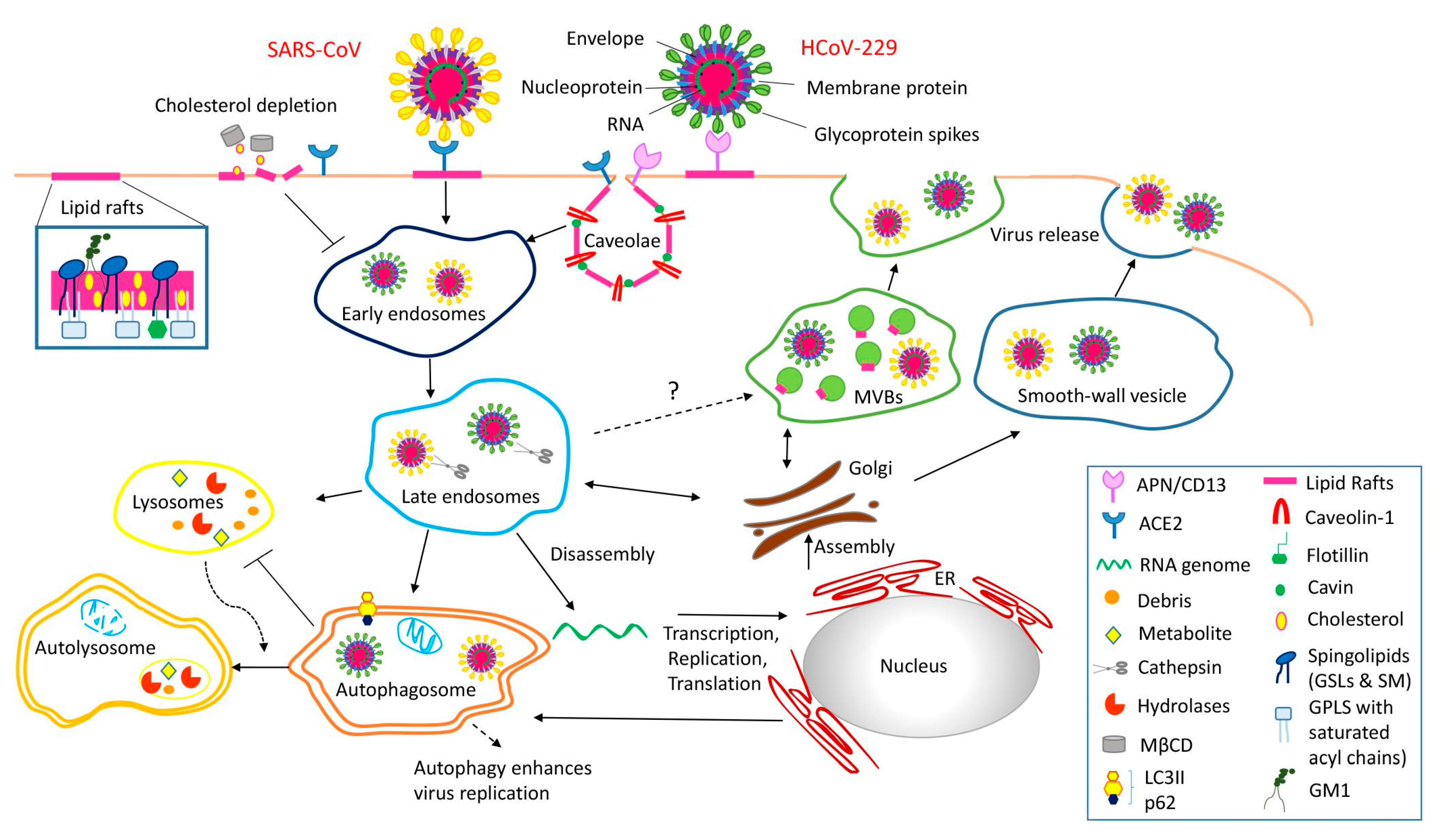
2.3. Supporting Background: A Lesson from the COVID-19 Pandemic
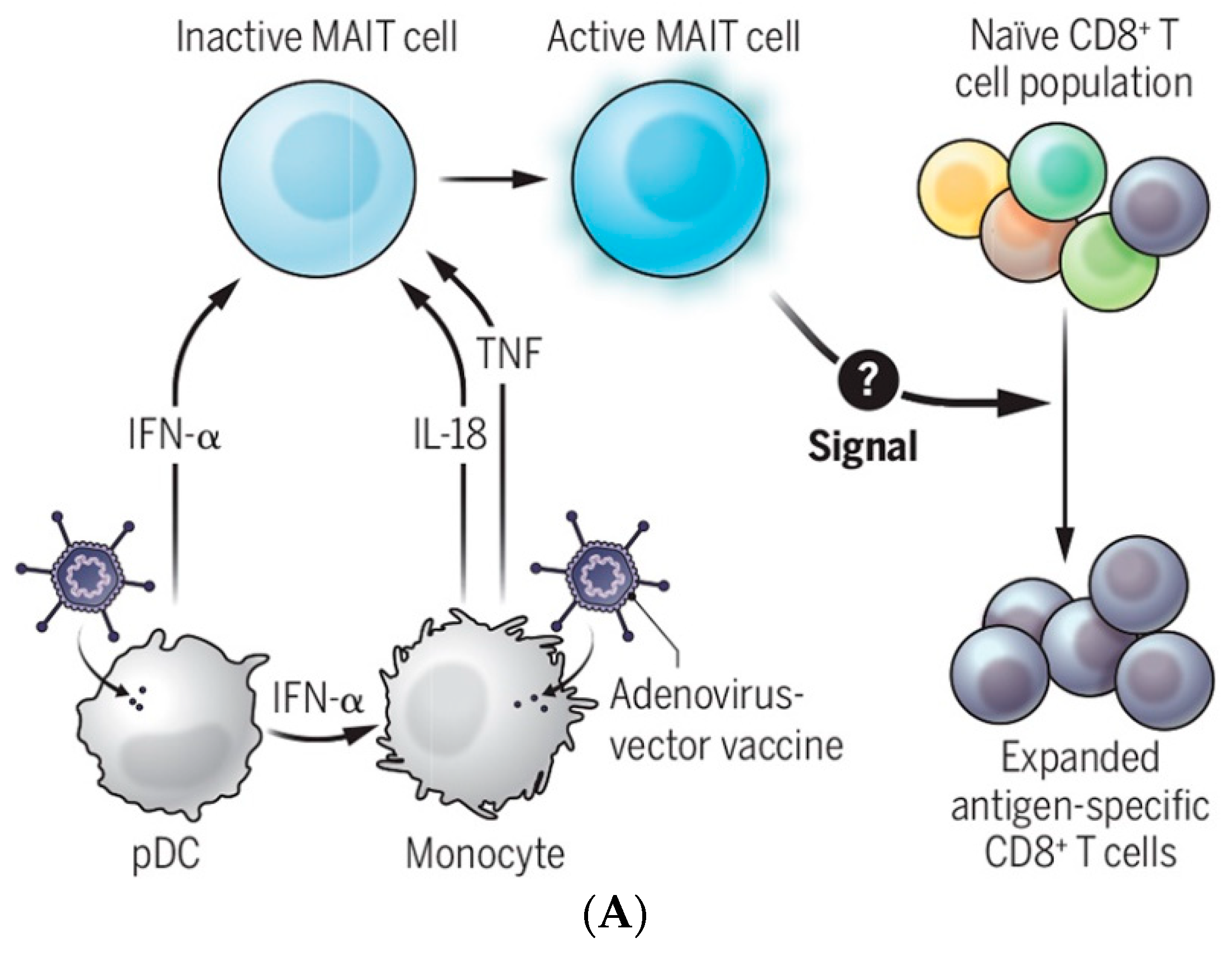
Lessons Learned from COVID-19-Related Vaccination
3. Evidence for Caveolae-Mediated PPS Signaling in Vaccination and Immunotherapy: Cav-1 Exalts Dual Roles of Pro-Inflammation and Anti-Inflammation upon Bacterial Internalization
3.1. Caveolae Act as an Entry-Port of Infectious Agents and as a Signaling Organelle
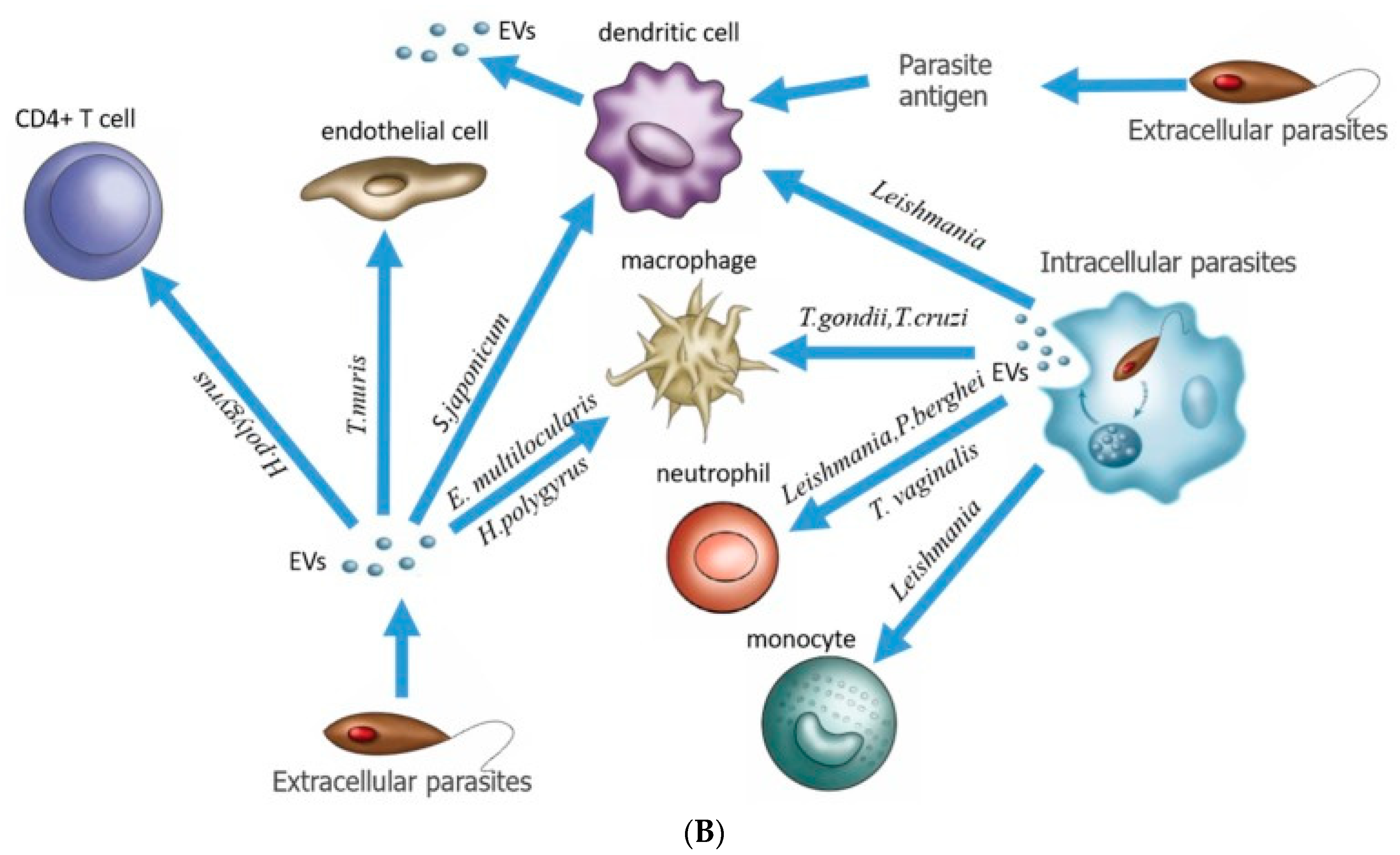
3.2. Caveolae Bud out Extracellular Vesicles (EVs)
3.3. Cav-1 Mediated the Anti-Inflammatory Impact
3.4. Cav-1 Mediated the Pro-Inflammatory Impact
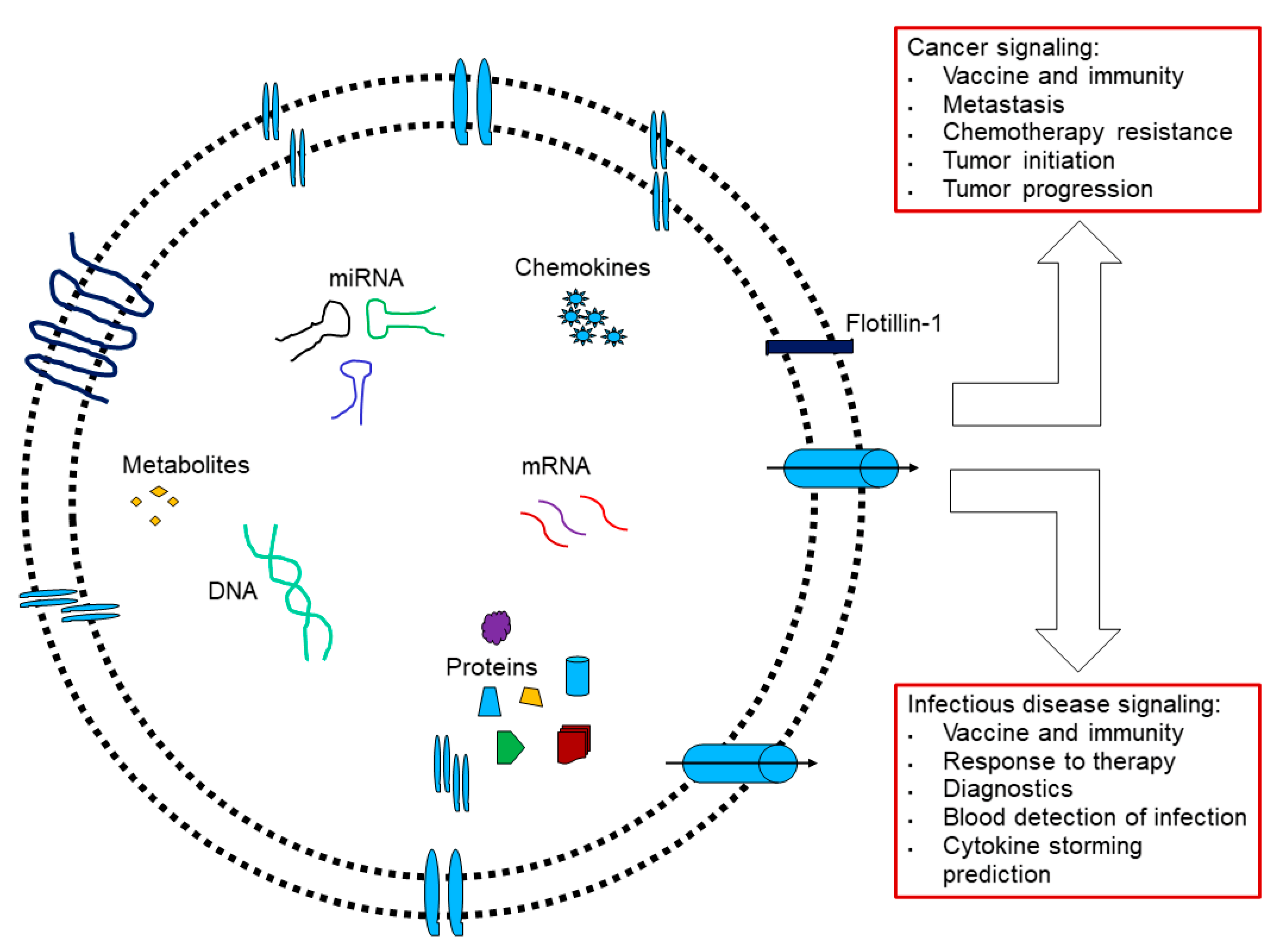
3.5. Beyond Caveolae: Engineered-EVs
4. Therapeutic Intervention Involving Caveolae-Mediated Vehicle of Therapeutic Delivery Approaches to Target the CXCL12/CXCR4/CXCR7 Axes
5. Perspective: Beyond PPSV 23-Valent Unconjugated Pneumococcal Vaccine to Venture to Cancer Vaccine
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD14 | cluster of differentiation-14 |
| CSD | Caveolin-1 Scaffolding Domain |
| DC-SIGN | DC-Specific intercellular adhesion molecule-3-Grabbing Non-integrin (a.k.a., CD209) |
| eNOS | endothelial NO synthase |
| EVs | extracellular vesicles |
| Fas-L | Fas ligand |
| HSP | Heat Shock Proteins HSP70 and HSP90 |
| IFITM3 | interferon-induced transmembrane protein 3 |
| IFN-α | cytokines interferon-α |
| IL-18 | interleukin-18 |
| ISKNV | infectious spleen and kidney necrosis virus |
| ISCT | International Society for Cellular and Gene Therapies () |
| ISEV | International Society for Extracellular Vesicles |
| JAK2 | Janus kinase 2 |
| LAPF | lysosome-associated and apoptosis-inducing protein containing PH and FYVE domains |
| MAIT | mucosal-associated invariant T cells |
| MD-2 | myeloid differentiation-2 |
| MSCs | mesenchymal stem (stromal) cells |
| ncRNA | non-coding RNA, including transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), as well as small RNAs, such as microRNAs, siRNAs, piRNAs, snoRNAs, snRNAs, exRNAs, and scaRNAs, and long ncRNAs, such as Xist and HOTAIR |
| PAH | pulmonary arterial hypertension |
| pDCs | plasmacytoid dendritic cells |
| PGE2 | Prostaglandin E2 |
| PPSV23 | Pneumovax® 23 (23-valent polysaccharides, Polyvalent polysaccharide vaccine) |
| PPV | pneumococcal polysaccharide vaccine |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| TGF-β | transforming growth factor Beta |
| TiO2 NPs | titanium dioxide nanoparticles |
| TLR4 | Toll-like receptor (TLR) 4 |
| TNF | tumor necrosis factor |
References
- MacLennan, I.C.; de Vinuesa, C.G.; Casamayor-Palleja, M. B-cell memory and the persistence of antibody responses. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, K.S.; Koh, S.S.; Kikuchi, A.; Lisanti, P.M. Baculovirus-based expression of mammalian caveolin in Sf21 insect cells. A model system for the biochemical and morphological study of caveolae biogenesis. J. Biol. Chem. 1996, 271, 28647–28654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jais, X.; Ioos, V.; Jardim, C.; Sitbon, O.; Parent, F.; Hamid, A.; Fadel, E.; Dartevelle, P.; Simonneau, G.; Humbert, M. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax 2005, 60, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2-Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Denis, K.J.S.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef]
- Obukhanych, T.V.; Nussenzweig, M.C. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006, 203, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef]
- Kocks, C.; Rajewsky, K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu. Rev. Immunol. 1989, 7, 537–559. [Google Scholar] [CrossRef]
- Clutterbuck, E.A.; Lazarus, R.; Yu, L.-M.; Bowman, J.; Bateman, E.A.L.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.; Mant, D.; et al. Pneumococcal Conjugate and Plain Polysaccharide Vaccines Have Divergent Effects on Antigen-Specific B Cells. J. Infect. Dis. 2012, 205, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- van Westen, E.; Knol, M.J.; Wijmenga-Monsuur, A.J.; Tcherniaeva, I.; Schouls, L.M.; Sanders, E.A.M.; Van Els, C.A.C.M.; Berbers, G.A.M.; Rots, N.Y. Serotype-Specific IgG Antibody Waning after Pneumococcal Conjugate Primary Series Vaccinations with either the 10-Valent or the 13-Valent Vaccine. Vaccines 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
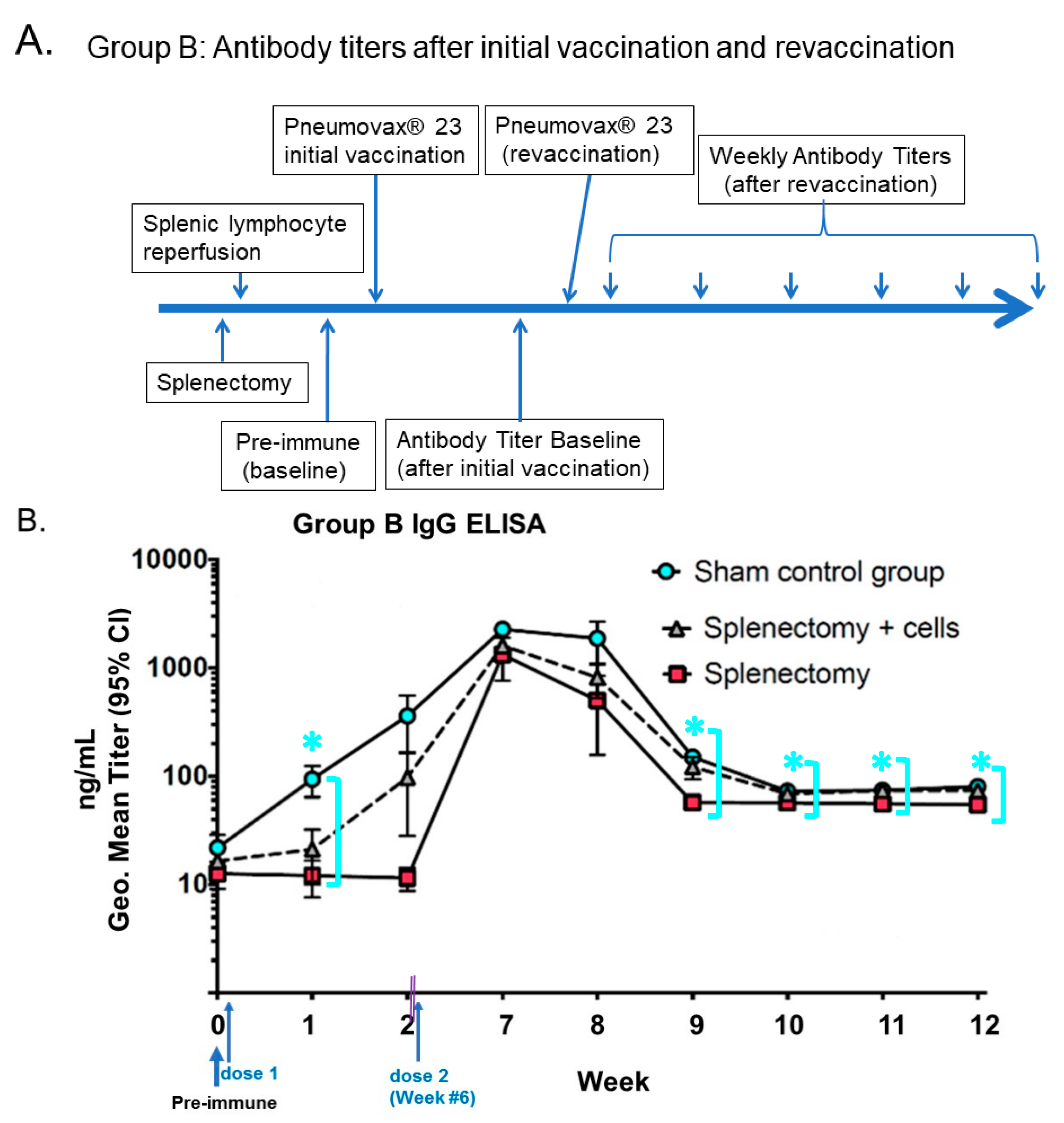
- Li, S.C.; Kabeer, M.H. Autologous Splenocyte Reinfusion Improves Antibody-Mediated Immune Response to the 23-Valent Pneumococcal Polysaccharide-Based Vaccine in Splenectomized Mice. Biomolecules 2020, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Nygard, N.R.; Bono, C.; Brown, L.R.; Gorka, J.; Giacoletto, K.S.; Schaiff, W.T.; Graham, M.B.; McCourt, D.W.; Kabeer, M.; Braciale, V.L. Antibody recognition of an immunogenic influenza hemagglutinin-human leukocyte antigen class II complex. J. Exp. Med. 1991, 174, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Granoff, D.; Sheetz, K.; Pandey, J.P.; Nahm, M.H.; Rambeck, J.H.; Jacobs, J.L.; Musser, J.; Selander, R.K.; Kabeer, M.; Murphy, T.V.; et al. Host and bacterial factors associated with Haemophilus influenzae type b disease in Minnesota children vaccinated with type b polysaccharide vaccine. J. Infect. 1989, 159, 908–916. [Google Scholar] [CrossRef]
- Munson, R.S.; Kabeer, M.H.; Lenoir, A.A.; Granoff, D.M. Epidemiology and Prospects for Prevention of Disease Due to Haemophilus influenzae in Developing Countries. Clin. Infect. Dis. 1989, 11, S588–S597. [Google Scholar] [CrossRef]
- Ledford, H. Six months of COVID vaccines: What 1.7 billion doses have taught scientists. Nature 2021, 594, 164–167. [Google Scholar] [CrossRef]
- Marshall, M. The four most urgent questions about long COVID. Nature 2021, 594, 168–170. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Z.; Bao, L.; Wang, L.; Cao, L.; Chi, H.; Hu, Y.; Li, Q.; Zhou, Y.; Jiang, Y.; et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature 2022, 603, 919–925. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Wang, L.; Li, J.; Wang, L.; Wu, Z.; Sun, X. Extracellular Vesicle-Mediated Communication Within Host-Parasite Interactions. Front. Immunol. 2018, 9, 3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coakley, G.; McCaskill, J.L.; Borger, J.G.; Simbari, F.; Robertson, E.; Millar, M.; Harcus, Y.; McSorley, H.J.; Maizels, R.M.; Buck, A.H. Extracellular Vesicles from a Helminth Parasite Suppress Macrophage Activation and Constitute an Effective Vaccine for Protective Immunity. Cell Rep. 2017, 19, 1545–1557. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, R.; Ghazvini, K.; Farsiani, H.; Soleimanpour, S. Mycobacterium tuberculosis extracellular vesicles: Exploitation for vaccine technology and diagnostic methods. Crit. Rev. Microbiol. 2020, 47, 13–33. [Google Scholar] [CrossRef]
- Bastiani, M.; Parton, R.G. Caveolae at a glance. J. Cell Sci. 2010, 123, 3831–3836. [Google Scholar] [CrossRef] [Green Version]
- Browman, D.T.; Resek, M.E.; Zajchowski, L.D.; Robbins, S. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J. Cell Sci. 2006, 119, 3149–3160. [Google Scholar] [CrossRef] [Green Version]
- Eberle, H.B.; Serrano, R.L.; Füllekrug, J.; Schlosser, A.; Lehmann, W.D.; Lottspeich, F.; Kaloyanova, D.; Wieland, F.; Helms, J. Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J. Cell Sci. 2002, 115, 827–838. [Google Scholar] [CrossRef]
- Sobo, K.; Chevallier, J.; Parton, R.G.; Gruenberg, J.; van der Goot, F.G. Diversity of Raft-Like Domains in Late Endosomes. PLoS ONE 2007, 2, e391. [Google Scholar] [CrossRef] [Green Version]
- Dermine, J.-F.; Duclos, S.; Garin, J.; St-Louis, F.; Rea, S.; Parton, R.; Desjardins, M. Flotillin-1-enriched Lipid Raft Domains Accumulate on Maturing Phagosomes. J. Biol. Chem. 2001, 276, 18507–18512. [Google Scholar] [CrossRef]
- Sargiacomo, M.; Sudol, M.; Tang, Z.; Lisanti, M.P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J. Cell Biol. 1993, 122, 789–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtun, O.; Tillu, V.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.; Howard, G.; Nichols, B.J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J. Cell Sci. 2011, 124, 2777–2785. [Google Scholar] [CrossRef] [Green Version]
- Parton, R.G.; Tillu, V.; McMahon, K.-A.; Collins, B.M. Key phases in the formation of caveolae. Curr. Opin. Cell Biol. 2021, 71, 7–14. [Google Scholar] [CrossRef]
- Detampel, P.; Tehranian, S.; Mukherjee, P.; Foret, M.; Fuerstenhaupt, T.; Darbandi, A.; Bogari, N.; Hlasny, M.; Jeje, A.; Olszewski, M.A.; et al. Caveolin-initiated macropinocytosis is required for efficient silica nanoparticles’ transcytosis across the alveolar epithelial barrier. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Ran, Q.; Jin, F.; Xiang, Y.; Xiang, L.; Wang, Q.; Li, F.; Chen, L.; Zhang, Y.; Wu, C.; Zhou, L.; et al. CRIF1 as a potential target to improve the radiosensitivity of osteosarcoma. Proc. Natl. Acad. Sci. USA. 2019, 116, 20511–20516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, C.; Carnino, J.; Jin, Y. The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles. Med. Sci. 2020, 8, 46. [Google Scholar] [CrossRef]
- Li, S.; Couet, J.; Lisanti, M.P. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J. Biol. Chem. 1996, 271, 29182–29190. [Google Scholar] [CrossRef] [Green Version]
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of Peptide and Protein Ligands for the Caveolin-scaffolding Domain. J. Biol. Chem. 1997, 272, 6525–6533. [Google Scholar] [CrossRef]
- Fecchi, K.; Anticoli, S.; Peruzzu, D.; Iessi, E.; Gagliardi, M.C.; Matarrese, P.; Ruggieri, A. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front. Microbiol. 2020, 11, 1821. [Google Scholar] [CrossRef]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Dudãu, M.; Codrici, E.; Tanase, C.; Gherghiceanu, M.; Enciu, A.-M.; Hinescu, M.E. Caveolae as Potential Hijackable Gates in Cell Communication. Front. Cell Dev. Biol. 2020, 8, 581732. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yin, X.; Meng, X.; Chan, J.F.; Ye, Z.W.; Riva, L.; Pache, L.; Chan, C.C.; Lai, P.M.; Chan, C.C.; et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature 2021, 593, 418–423. [Google Scholar] [CrossRef]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus aureus extracellular vesicles and their ap-plication as a vaccine platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gasparo, R.; Pedotti, M.; Simonelli, L.; Nickl, P.; Muecksch, F.; Cassaniti, I.; Percivalle, E.; Lorenzi, J.C.C.; Mazzola, F.; Magri, D.; et al. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature 2021, 593, 424–428. [Google Scholar] [CrossRef]
- Hope, J.L.; Bradley, L.M. Lessons in antiviral immunity. Science 2021, 371, 464–465. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Mammadova, R.; Ramos Juarez, A.P.; Bokka, R.; Trepiccione, F.; Capasso, G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press. Res. 2020, 45, 661–670. [Google Scholar] [CrossRef]
- Jiang, H.W.; Li, Y.; Zhang, H.N.; Wang, W.; Yang, X.; Qi, H.; Li, H.; Men, D.; Zhou, J.; Tao, S.C. SARS-CoV-2 proteome microarray for global profiling of COVID-19 specific IgG and IgM responses. Nat. Commun. 2020, 11, 3581. [Google Scholar] [CrossRef]
- Kreutmair, S.; Unger, S.; Nunez, N.G.; Ingelfinger, F.; Alberti, C.; de Feo, D.; Krishnarajah, S.; Kauffmann, M.; Friebel, E.; Babaei, S.; et al. Distinct immunological signatures discriminate severe COVID-19 from non-SARS-CoV-2-driven critical pneumonia. Immunity 2021, 54, 1578–1593. [Google Scholar] [CrossRef]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Tanfous, T.B.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kousathanas, A.; Pairo-Castineira, E.; Rawlik, K.; Stuckey, A.; Odhams, C.A.; Walker, S.; Russell, C.D.; Malinauskas, T.; Wu, Y.; Millar, J.; et al. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature 2022, 607, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sowa, G. Caveolae, caveolins, cavins, and endothelial cell function: New insights. Front. Physiol. 2012, 2, 120. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ozawa, T.; Kurata, T.; Nakajima, N.; Zamponi, G.W.; Giles, W.R.; Imaizumi, Y.; Yamamura, H. A molecular complex of Cav1.2/CaMKK2/CaMK1a in caveolae is responsible for vascular remodeling via excitation-transcription coupling. Proc. Natl. Acad. Sci. USA 2022, 119, e2117435119. [Google Scholar] [CrossRef]
- Sancho, M.; Fletcher, J.; Welsh, D.G. Inward Rectifier Potassium Channels: Membrane Lipid-Dependent Mechanosensitive Gates in Brain Vascular Cells. Front. Cardiovasc. Med. 2022, 9, 869481. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.P.; Lim, Y.-W.; Xiong, Z.; Martel, N.; Ferguson, C.; Ariotti, N.; Giacomotto, J.; Rae, J.; Floetenmeyer, M.; Moradi, S.V.; et al. Cavin4 interacts with Bin1 to promote T-tubule formation and stability in developing skeletal muscle. J. Cell Biol. 2021, 220, e201905065. [Google Scholar] [CrossRef] [PubMed]
- Kushner, J.S.; Liu, G.; Eisert, R.J.; Bradshaw, G.A.; Pitt, G.S.; Hinson, J.T.; Kalocsay, M.; Marx, S.O. Detecting Cardiovascular Protein-Protein Interactions by Proximity Proteomics. Circ. Res. 2022, 130, 273–287. [Google Scholar] [CrossRef]
- Gonzalez-Mora, A.M.; Garcia-Lopez, P. Estrogen Receptors as Molecular Targets of Endocrine Therapy for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 12404. [Google Scholar] [CrossRef]
- McEnery, T.; White, M.M.; Gogoi, D.; Coleman, O.; Bergin, D.; Jundi, B.; Flannery, R.; Alsaif, F.A.T.; Landers, S.A.; Casey, M.; et al. Alpha-1 Antitrypsin Therapy Modifies Neutrophil Adhesion in Patients with Obstructive Lung Disease. Am. J. Respir. Cell Mol. Biol. 2022, 67, 76–88. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. Skin aging: Dermal adipocytes metabolically reprogram dermal fibroblasts. BioEssays 2021, 44, e2100207. [Google Scholar] [CrossRef]
- Feng, H.; Guo, W.; Han, J.; Li, X.-A. Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci. 2013, 93, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.Y.; Kitchens, R.L.; Munford, R.S. Bacterial lipopolysaccharide binds to CD14 in low-density domains of the monocyte-macrophage plasma membrane. J. Inflamm. 1995, 47, 126–137. [Google Scholar] [PubMed]
- Wang, H.; Haas, M.; Liang, M.; Cai, T.; Tian, J.; Li, S.; Xie, Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004, 279, 17250–17259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poussin, C.; Foti, M.; Carpentier, J.-L.; Pugin, J. CD14-dependent Endotoxin Internalization via a Macropinocytic Pathway. J. Biol. Chem. 1998, 273, 20285–20291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, M.G.; Morrison, D.C. Differential Expression of Caveolin-1 in Lipopolysaccharide-Activated Murine Macrophages. Infect. Immun. 2000, 68, 5084–5089. [Google Scholar] [CrossRef] [Green Version]
- Rohde, M.; Muller, E.; Chhatwal, G.S.; Talay, S.R. Host cell caveolae act as an entry-port for Group A streptococci. Cell Microbiol. 2003, 5, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, G.; Mayer, R.M.; Dellafiora, L.; Janker, L.; Niederstaetter, L.; Dall’Asta, C.; Gerner, C.; Marko, D. Structural Similarity with Cholesterol Reveals Crucial Insights into Mechanisms Sustaining the Immunomodulatory Activity of the Mycotoxin Alternariol. Cells 2020, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Qin, K.; Li, N.; Han, C.; Cao, X. An endosomal LAPF is required for macrophage endocytosis and elimination of bacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 12958–12963. [Google Scholar] [CrossRef] [Green Version]
- Asmat, T.M.; Agarwal, V.; Saleh, M.; Hammerschmidt, S. Endocytosis of Streptococcus pneumoniae via the polymeric immunoglobulin receptor of epithelial cells relies on clathrin and caveolin dependent mechanisms. Int. J. Med. Microbiol. 2014, 304, 1233–1246. [Google Scholar] [CrossRef]
- Li, S.; Lisanti, M.; Puszkin, S. Purification and molecular characterization of NP185, a neuronal-specific and synapse-enriched clathrin assembly polypeptide. Bioquim. Patol. Clin. 1998, 62, 5–17. [Google Scholar]
- Haleem, K.S.; Ali, Y.M.; Yesilkaya, H.; Kohler, T.; Hammerschmidt, S.; Andrew, P.W.; Schwaeble, W.J.; Lynch, N.J. The Pneumococcal Surface Proteins PspA and PspC Sequester Host C4-Binding Protein To Inactivate Complement C4b on the Bacterial Surface. Infect. Immun. 2019, 87, e00742-18. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kong, Q.; Xia, Z.; Zhan, L.; Duan, W.; Song, X. Penehyclidine hydrochloride alleviates lipopolysaccharide-induced acute lung injury in rats: Potential role of caveolin-1 expression upregulation. Int. J. Mol. Med. 2019, 43, 2064–2074. [Google Scholar] [CrossRef] [Green Version]
- Juno, J.A.; O’Connor, S.L. Translating viral vaccines into immunity. Science 2021, 371, 460–461. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, M.; Nohtomi, K.; Mori, Y.; Kataoka, H.; Sugano, M.; Ohnuma, K.; Kuwata, H.; Hirano, T. Caveolin-1, a binding protein of CD26, is essential for the anti-inflammatory effects of dipeptidyl peptidase-4 inhibitors on human and mouse macrophages. Biochem. Biophys. Res. Commun. 2018, 495, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. How do oxidized phospholipids inhibit LPS signaling? Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Shuto, T.; Kato, K.; Mori, Y.; Viriyakosol, S.; Oba, M.; Furuta, T.; Okiyoneda, T.; Arima, H.; Suico, M.A.; Kai, H. Membrane-anchored CD14 is required for LPS-induced TLR4 endocytosis in TLR4/MD-2/CD14 overexpressing CHO cells. Biochem. Biophys. Res. Commun. 2005, 338, 1402–1409. [Google Scholar] [CrossRef]
- Li, S.; Song, K.S.; Lisanti, M.P. Expression and characterization of recombinant caveolin: Purification by poly-histidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J. Biol. Chem. 1996, 271, 568–573. [Google Scholar] [CrossRef] [Green Version]
- Medina, F.A.; Williams, T.M.; Sotgia, F.; Tanowitz, H.B.; Lisanti, M.P. A Novel Role for Caveolin-1 in B Lymphocyte Function and the Development of Thymus-Independent Immune Responses. Cell Cycle 2006, 5, 1865–1871. [Google Scholar] [CrossRef]
- Medina, F.A.; de Almeida, C.J.; Dew, E.; Li, J.; Bonuccelli, G.; Williams, T.M.; Cohen, A.W.; Pestell, R.G.; Frank, P.G.; Tanowitz, H.B.; et al. Caveolin-1-Deficient Mice Show Defects in Innate Immunity and Inflammatory Immune Response during Salmonella enterica Serovar Typhimurium Infection. Infect. Immun. 2006, 74, 6665–6674. [Google Scholar] [CrossRef] [Green Version]
- Garrean, S.; Gao, X.P.; Brovkovych, V.; Shimizu, J.; Zhao, Y.Y.; Vogel, S.M.; Malik, A.B. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. 2006, 177, 4853–4860. [Google Scholar] [CrossRef] [Green Version]
- García-Cardeña, G.; Martasek, P.; Masters, B.S.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the Interaction between Nitric Oxide Synthase (NOS) and Caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440. [Google Scholar] [CrossRef]
- Elsasser, T.H.; Kahl, S.; Li, C.-J.; Sartin, J.L.; Garrett, W.M.; Rodrigo, J. Caveolae Nitration of Janus Kinase-2 at the 1007Y-1008Y Site: Coordinating Inflammatory Response and Metabolic Hormone Readjustment within the Somatotropic Axis. Endocrinology 2007, 148, 3803–3813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruyra, A.; Cano-Sarabia, M.; Mackenzie, S.A.; Maspoch, D.; Roher, N. A novel liposome-based nanocarrier loaded with an LPS-dsRNA cocktail for fish innate immune system stimulation. PloS ONE 2013, 8, e76338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-C.; Seitz, R.; Lisanti, M.P. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J. Biol. Chem. 1996, 271, 3863–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, H.; Zhang, Y.; Yan, Z.; Wang, Z.G.; Liu, G.; Minshall, R.D.; Malik, A.B.; Hu, G. Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J. Immunol. 2013, 191, 6191–6199. [Google Scholar] [CrossRef] [Green Version]
- Savio, L.E.B.; Mello, P.d.; Santos, S.; de Sousa, J.C.; Oliveira, S.D.S.; Minshall, R.D.; Kurtenbach, E.; Wu, Y.; Longhi, M.S.; Robson, S.C.; et al. P2X7 receptor activation increases expression of caveolin-1 and formation of macrophage lipid rafts, thereby boosting CD39 activity. J. Cell Sci. 2020, 133, jcs237560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuvaev, V.V.; Khoshnejad, M.; Pulsipher, K.W.; Kiseleva, R.Y.; Arguiri, E.; Cheung-Lau, J.C.; LeFort, K.M.; Christofidou-Solomidou, M.; Stan, R.V.; Dmochowski, I.J.; et al. Spatially controlled assembly of affinity ligand and enzyme cargo enables targeting ferritin nanocarriers to caveolae. Biomaterials 2018, 185, 348–359. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lai, T.-Y.; Tsai, M.-K.; Chang, Y.-C.; Ho, Y.-H.; Yu, I.-S.; Yeh, T.-W.; Chou, C.-C.; Lin, Y.-S.; Lawrence, T.; et al. The ubiquitin ligase ZNRF1 promotes caveolin-1 ubiquitination and degradation to modulate inflammation. Nat. Commun. 2017, 8, 15502. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Yuan, M.; Zhang, T.; Zheng, N.; Wu, Z. EVs Containing Host Restriction Factor IFITM3 Inhibited ZIKV Infection of Fetuses in Pregnant Mice through Trans-placenta Delivery. Mol. Ther. 2021, 29, 176–190. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Nazimek, K.; Bryniarski, K. Perspectives in Manipulating EVs for Therapeutic Applications: Focus on Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 4623. [Google Scholar] [CrossRef] [PubMed]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Chen, N.-N.; Li, Z.-M.; Wang, Y.-Y.; Weng, S.-P.; Guo, C.-J.; He, J.-G. Evidence for a Novel Antiviral Mechanism of Teleost Fish: Serum-Derived Exosomes Inhibit Virus Replication through Incorporating Mx1 Protein. Int. J. Mol. Sci. 2021, 22, 10346. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Jiang, S. Immune Cell-Derived Exosomes in the Cancer-Immunity Cycle. Trends Cancer 2020, 6, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Taghikhani, A.; Farzaneh, F.; Sharifzad, F.; Mardpour, S.; Ebrahimi, M.; Hassan, Z.M. Engineered Tumor-Derived Extracellular Vesicles: Potentials in Cancer Immunotherapy. Front. Immunol. 2020, 11, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börger, V.; Weiss, D.J.; Anderson, J.D.; Borràs, F.E.; Bussolati, B.; Carter, D.R.F.; Dominici, M.; Falcón-Pérez, J.M.; Gimona, M.; Hill, A.F.; et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: Considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy 2020, 22, 482–485. [Google Scholar]
- Torres, M.; Rosselló, C.A.; Fernández-García, P.; Lladó, V.; Kakhlon, O.; Escribá, P.V. The Implications for Cells of the Lipid Switches Driven by Protein–Membrane Interactions and the Development of Membrane Lipid Therapy. Int. J. Mol. Sci. 2020, 21, 2322. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Okamoto, T.; Chun, M.; Sargiacomo, M.; Casanova, J.E.; Hansen, S.H.; Nishimoto, I.; Lisanti, M.P. Evidence for a Regulated Interaction between Heterotrimeric G Proteins and Caveolin. J. Biol. Chem. 1995, 270, 15693–15701. [Google Scholar] [CrossRef] [Green Version]
- Potje, S.R.; Grando, M.D.; Chignalia, A.Z.; Antoniali, C.; Bendhack, L.M. Reduced caveolae density in arteries of SHR contributes to endothelial dysfunction and ROS production. Sci. Rep. 2019, 9, 6696. [Google Scholar] [CrossRef] [Green Version]
- Rathinasabapathy, A.; Copeland, C.; Crabtree, A.; Carrier, E.J.; Moore, C.; Shay, S.; Gladson, S.; Austin, E.D.; Kenworthy, A.K.; Loyd, J.E.; et al. Expression of a Human Caveolin-1 Mutation in Mice Drives Inflammatory and Metabolic Defect-Associated Pulmonary Arterial Hypertension. Front. Med. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Kiseleva, R.Y.; Arguiri, E.; Villa, C.H.; Muro, S.; Christofidou-Solomidou, M.; Stan, R.; Muzykantov, V.R. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J. Control. Release 2018, 272, 1–8. [Google Scholar] [CrossRef]
- Piperno, A.; Mazzaglia, A.; Scala, A.; Pennisi, R.; Zagami, R.; Neri, G.; Torcasio, S.M.; Rosmini, C.; Mineo, P.G.; Potara, M.; et al. Casting Light on Intracellular Tracking of a New Functional Graphene-Based MicroRNA Delivery System by FLIM and Raman Imaging. ACS Appl. Mater. Interfaces 2019, 11, 46101–46111. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nan, L.; Xiao, C.; Ji, Q.; Li, K.; Wei, Q.; Liu, Y.; Bao, G. Optimum Preparation Method for Self-Assembled PEGylation Nano-Adjuvant Based on Rehmannia glutinosa Polysaccharide and Its Immunological Effect on Macrophages. Int. J. Nanomed. 2019, 14, 9361–9375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrie, Y.; Crofts, F.; Devitt, A.; Griffiths, H.R.; Kastner, E.; Nadella, V. Designing liposomal adjuvants for the next generation of vaccines. Adv. Drug Deliv. Rev. 2016, 99, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divine, R.; Dang, H.V.; Ueda, G.; Fallas, J.A.; Vulovic, I.; Sheffler, W.; Saini, S.; Zhao, Y.T.; Raj, I.X.; Morawski, P.A.; et al. Designed proteins assemble antibodies into modular nanocages. Science 2021, 372, eabd9994. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Attia, J.; Li, S.C.; Newby, D. Pneumococcal polysaccharide vaccine is a cost saving strategy for prevention of acute coronary syndrome. Vaccine 2021, 39, 1721–1726. [Google Scholar] [CrossRef]
- Xiang, Y.; Ran, Q.; Wu, C.; Zhou, L.; Zhang, W.; Li, J.; Xiang, L.; Xiao, Y.; Chen, L.; Chen, Y.; et al. Single-cell transcriptomics uncovers the impacts of titanium dioxide nanoparticles on human bone marrow stromal cells. Chem. Eng. J. 2022, 440, 135814. [Google Scholar] [CrossRef]
- Kaplonek, P.; Cizmeci, D.; Fischinger, S.; Collier, A.-R.; Suscovich, T.; Linde, C.; Broge, T.; Mann, C.; Amanat, F.; Dayal, D.; et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci. Transl. Med. 2022, 14, eabm2311. [Google Scholar] [CrossRef]
- Low, J.-Y.; Laiho, M. Caveolae-Associated Molecules, Tumor Stroma, and Cancer Drug Resistance: Current Findings and Future Perspectives. Cancers 2022, 14, 589. [Google Scholar] [CrossRef]
- Kalaora, S.; Nagler, A.; Nejman, D.; Alon, M.; Barbolin, C.; Barnea, E.; Ketelaars, S.L.C.; Cheng, K.; Vervier, K.; Shental, N.; et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 2021, 592, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Riemer, A.B. Bacterial peptides presented on tumour cells could be immunotherapy targets. Nature 2021, 592, 28–29. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.C.; Kabeer, M.H. Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host–Pathogen Interface Hypothesis. Pharmaceutics 2022, 14, 2653. https://doi.org/10.3390/pharmaceutics14122653
Li SC, Kabeer MH. Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host–Pathogen Interface Hypothesis. Pharmaceutics. 2022; 14(12):2653. https://doi.org/10.3390/pharmaceutics14122653
Chicago/Turabian StyleLi, Shengwen Calvin, and Mustafa H. Kabeer. 2022. "Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host–Pathogen Interface Hypothesis" Pharmaceutics 14, no. 12: 2653. https://doi.org/10.3390/pharmaceutics14122653
APA StyleLi, S. C., & Kabeer, M. H. (2022). Caveolae-Mediated Extracellular Vesicle (CMEV) Signaling of Polyvalent Polysaccharide Vaccination: A Host–Pathogen Interface Hypothesis. Pharmaceutics, 14(12), 2653. https://doi.org/10.3390/pharmaceutics14122653






