Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Linear Poly(ethyleneimine) (L-PEI)
2.3. Synthesis of Poly(3-hydroxypropyl ethyleneimine) (P3HPEI)
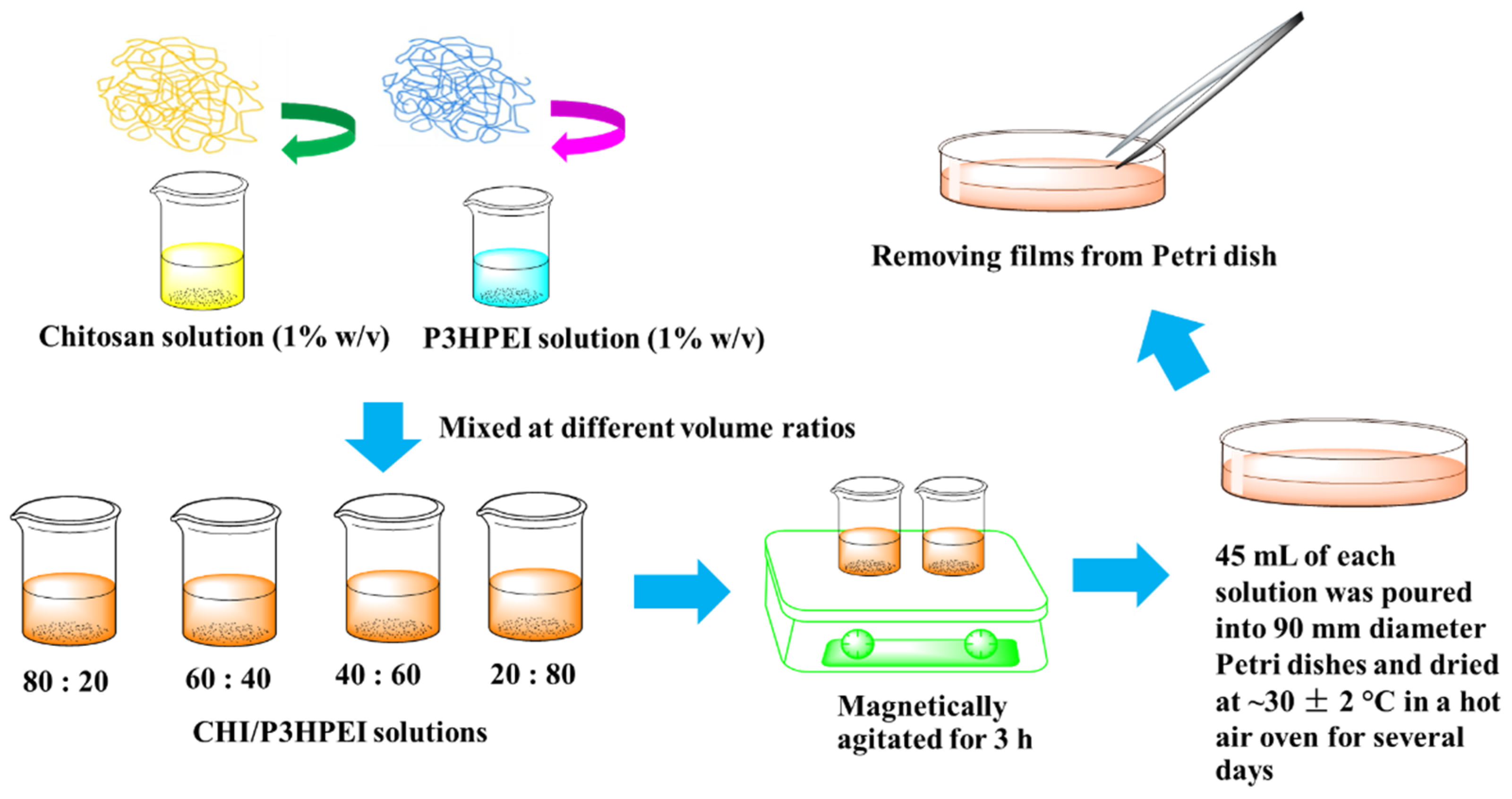
2.4. Preparation of Films
2.5. Preparation of Haloperidol-Loaded Films
2.6. Characterization of Polymers and Films
2.6.1. 1H-Nuclear Magnetic Resonance Spectroscopy (1H-NMR)
2.6.2. Fourier Transformed Infrared (FTIR) Spectroscopy
2.6.3. Differential Scanning Calorimetry
2.6.4. Thermogravimetric Analysis (TGA)
2.6.5. Powder X-ray Diffractrometry (PXRD)
2.6.6. Film Thickness
2.6.7. Scanning Electron Microscopy (SEM)
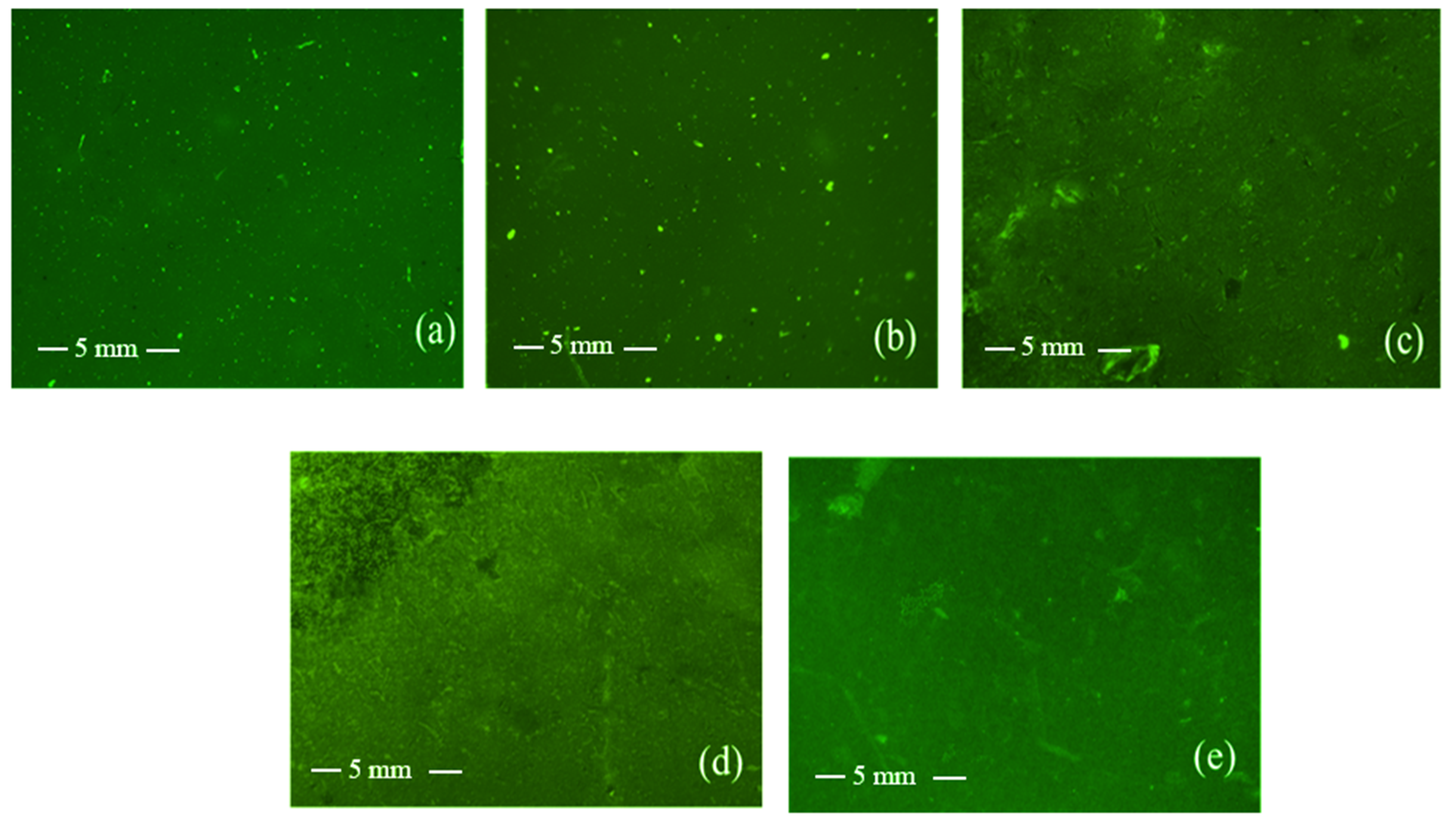
2.6.8. Fluorescence Microscopy
2.6.9. Polarized Light Microscopy
2.6.10. Mechanical Properties
2.7. In Vitro Drug Release Study
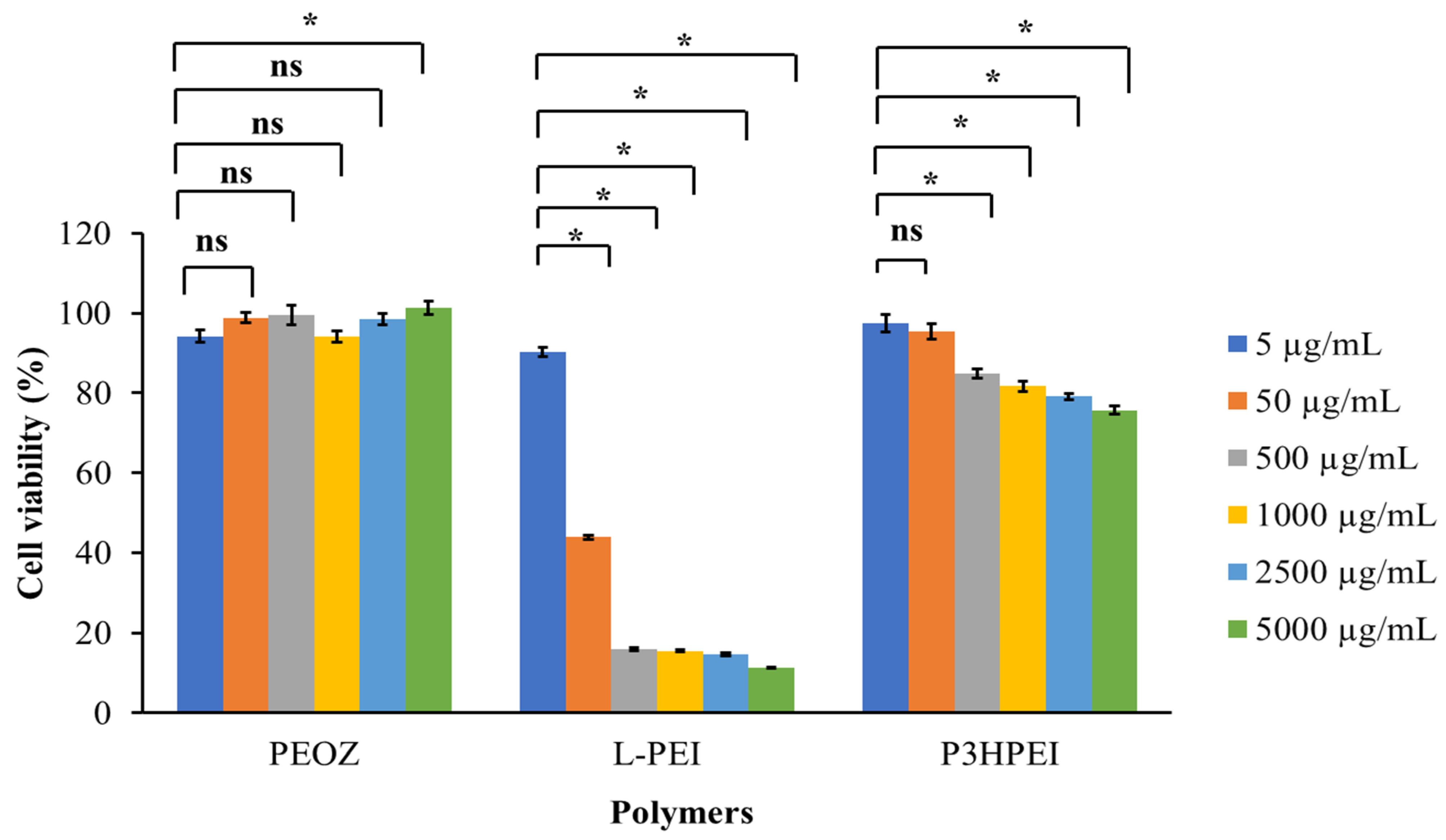
2.8. Cytotoxicity Test
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Evaluation of Poly(3-hydroxypropyl ethyleneimine)
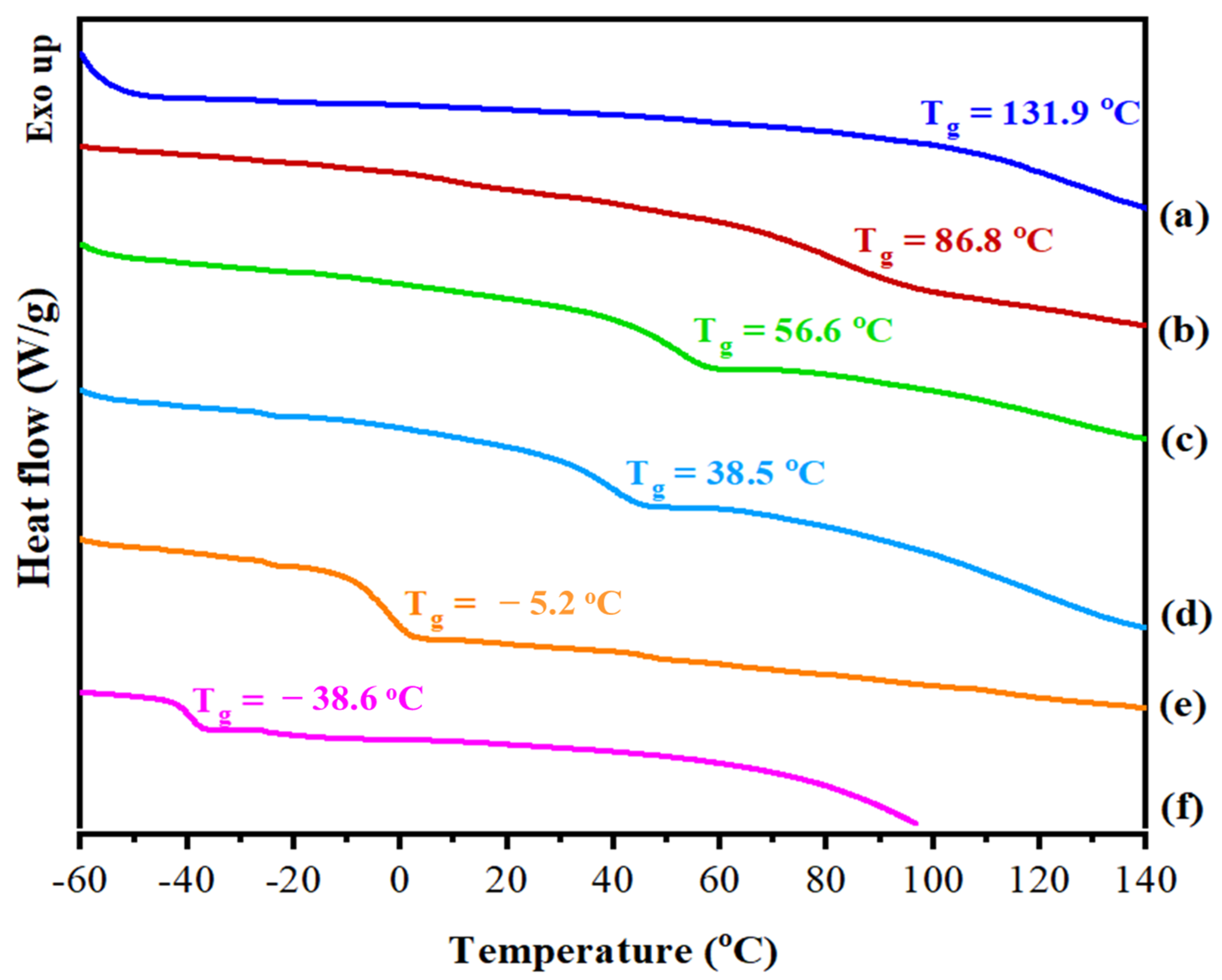
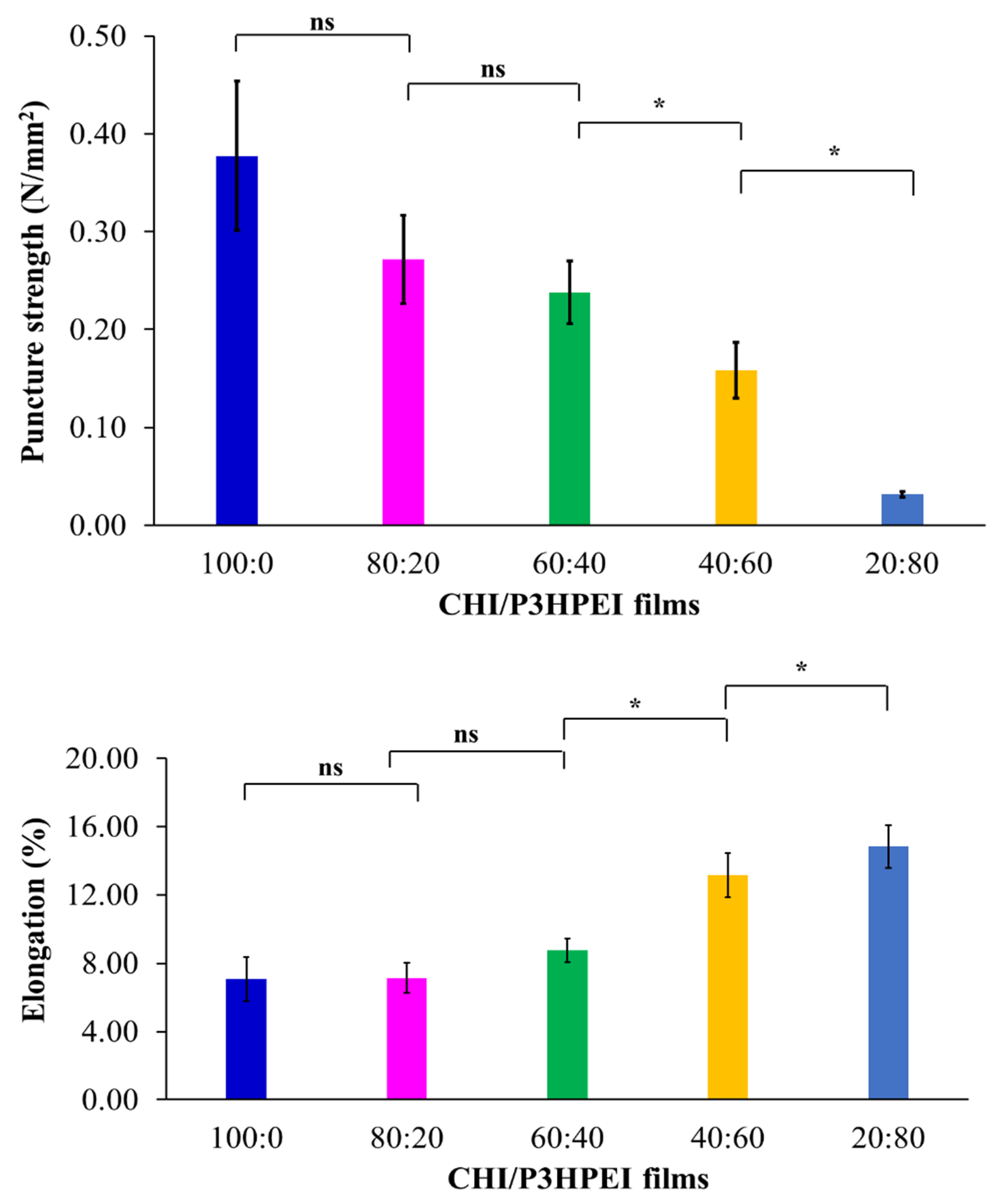
3.2. Novel Elastic Films based on Blends of Chitosan and Poly(3-hydroxypropyl ethyleneimine): Formulation, Miscibility, and Mechanical Properties
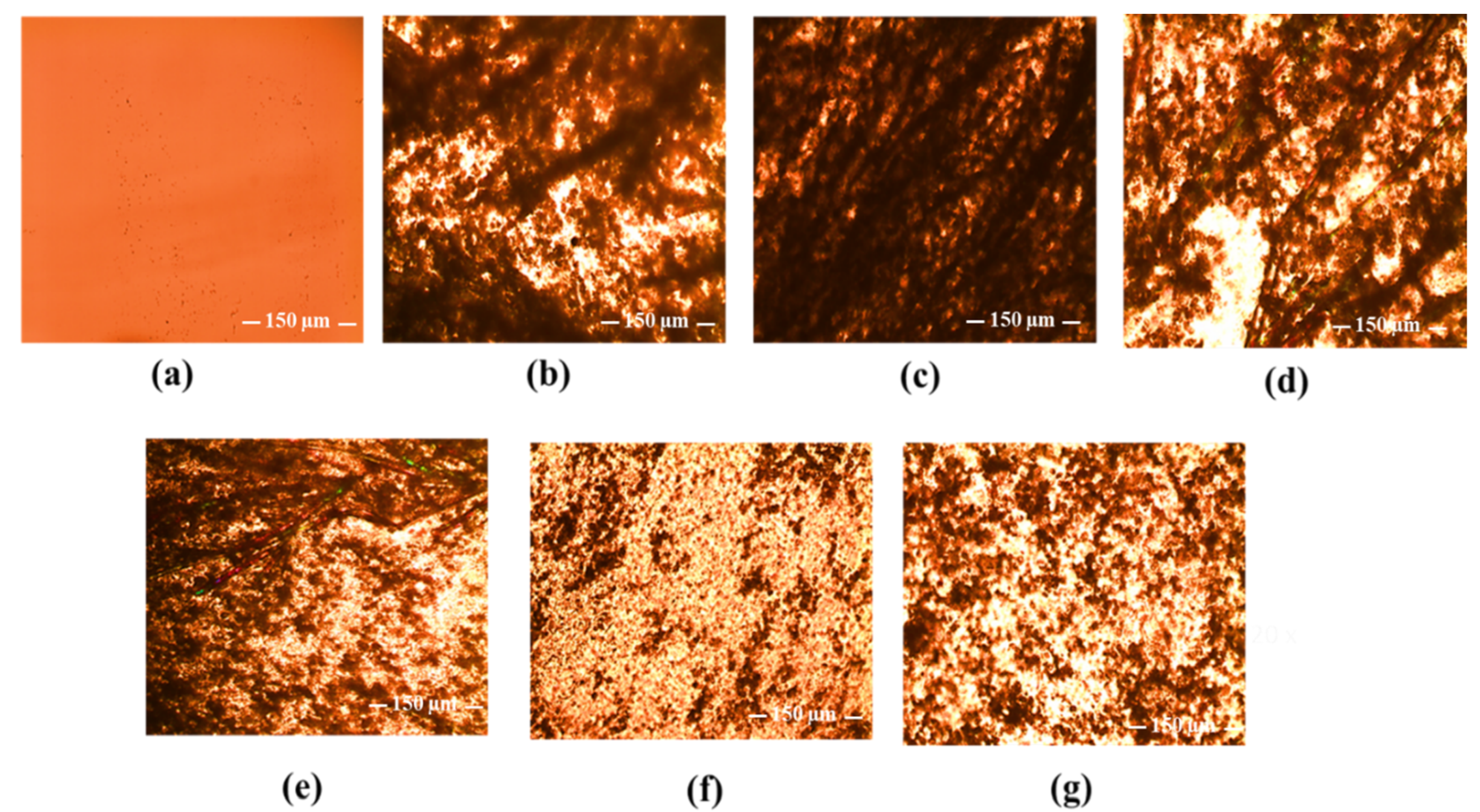
3.3. Chitosan/Poly(3-hydroxypropyl ethyleneimine) Film Formulations for Loading and Delivery of Haloperidol: X-ray, Microscopic, and Drug Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, P.G.; Ferreira, V.F.; da Silva, F.d.C.; Freitas, C.S.; Pereira, P.R.; Paschoalin, V.M.F. Chitosans and Nanochitosans: Recent Advances in Skin Protection, Regeneration, and Repair. Pharmaceutics 2022, 14, 1307. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-Loaded Chitosan Film Prepared via Facile Solution Casting and Air-Drying of Plain Water-Based Chitosan Solution for Ocular Drug Delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, J.; Zhang, Z.; Zhang, L. Study of Blend Films from Chitosan and Hydroxypropyl Guar Gum. J. Appl. Polym. Sci. 2003, 90, 1991–1995. [Google Scholar] [CrossRef]
- Irikura, K.; Ekapakul, N.; Choochottiros, C.; Chanthaset, N.; Yoshida, H.; Ajiro, H. Fabrication of Flexible Blend Films Using a Chitosan Derivative and Poly(Trimethylene Carbonate). Polym. J. 2021, 53, 823–833. [Google Scholar] [CrossRef]
- Hao, J.Y.; Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Schoung, J.Y.; Tsai, Y.H.; Huang, Y. Bin Control of Wound Infections Using a Bilayer Chitosan Wound Dressing with Sustainable Antibiotic Delivery. J. Biomed. Mater. Res. 2002, 59, 438–449. [Google Scholar] [CrossRef]
- Can, A.S.; Erdal, M.S.; Güngör, S.; Özsoy, Y. Optimization and Characterization of Chitosan Films for Transdermal Delivery of Ondansetron. Molecules 2013, 18, 5455–5471. [Google Scholar] [CrossRef]
- Michailid, G.; OuBikiaris, D.N. Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 1966. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why Is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Abilova, G.K.; Kaldybekov, D.B.; Ozhmukhametova, E.K.; Saimova, A.Z.; Kazybayeva, D.S.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Chitosan/Poly(2-Ethyl-2-Oxazoline) Films for Ocular Drug Delivery: Formulation, Miscibility, in Vitro and in Vivo Studies. Eur. Polym. J. 2019, 116, 311–320. [Google Scholar] [CrossRef]
- Lappe, S.; Mulac, D.; Langer, K. Polymeric Nanoparticles—Influence of the Glass Transition Temperature on Drug Release. Int. J. Pharm. 2017, 517, 338–347. [Google Scholar] [CrossRef]
- Akash, S.Z.; Lucky, F.Y.; Hossain, M.; Bepari, A.K.; Sayedur Rahman, G.M.; Reza, H.M.; Sharker, S.M. Remote Temperature-Responsive Parafilm Dermal Patch for on-Demand Topical Drug Delivery. Micromachines 2021, 12, 975. [Google Scholar] [CrossRef]
- Luo, K.; Yin, J.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Mucoadhesive and Elastic Films Based on Blends of Chitosan and Hydroxyethylcellulose. Macromol. Biosci. 2008, 8, 184–192. [Google Scholar] [CrossRef]
- Yin, J.; Luo, K.; Chen, X.; Khutoryanskiy, V.V. Miscibility Studies of the Blends of Chitosan with Some Cellulose Ethers. Carbohydr. Polym. 2006, 63, 238–244. [Google Scholar] [CrossRef]
- Marsano, E.; Vicini, S.; Skopińska, J.; Wisniewski, M.; Sionkowska, A. Chitosan and Poly(Vinyl Pyrrolidone): Compatibility and Miscibility of Blends. Macromol. Symp. 2004, 218, 251–260. [Google Scholar] [CrossRef]
- Mohd Nasir, N.F.; Zain, N.M.; Raha, M.G.; Kadri, N.A. Characterization of Chitosan-Poly (Ethylene Oxide) Blends as Haemodialysis Membrane. Am. J. Appl. Sci. 2005, 2, 1578–1583. [Google Scholar] [CrossRef]
- Yeh, J.T.; Chen, C.L.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, Characterization, and Application of PVP/Chitosan Blended Polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Soradech, S.; Williams, A.C.; Khutoryanskiy, V.V. Physically Cross-Linked Cryogels of Linear Polyethyleneimine: Influence of Cooling Temperature and Solvent Composition. Macromolecules 2022, 55, 9537–9546. [Google Scholar] [CrossRef]
- Shan, X.; Williams, A.C.; Khutoryanskiy, V.V. Polymer Structure and Property Effects on Solid Dispersions with Haloperidol: Poly(N-Vinyl Pyrrolidone) and Poly(2-Oxazolines) Studies. Int. J. Pharm. 2020, 590, 119884. [Google Scholar] [CrossRef]
- Mees, M.A.; Hoogenboom, R. Full and Partial Hydrolysis of Poly(2-Oxazoline)s and the Subsequent Post-Polymerization Modification of the Resulting Polyethylenimine (Co)Polymers. Polym. Chem. 2018, 9, 4968–4978. [Google Scholar] [CrossRef]
- Lungu, C.N.; Diudea, M.V.; Putz, M.V.; Grudziński, I.P. Linear and Branched PEIs (Polyethylenimines) and Their Property Space. Int. J. Mol. Sci. 2016, 17, 555. [Google Scholar] [CrossRef]
- Chatani, Y.; Tadokoro, H.; Saegusa, T.; Ikeda, H. Structural Studies of Poly (Ethylenimine). 1. Structures of Two Hydrates of Poly (Ethylenimine): Sesquihydrate and Dihydrate. Macromolecules 1981, 14, 315–321. [Google Scholar] [CrossRef]
- Van Kuringen, H.P.C.; Lenoir, J.; Adriaens, E.; Bender, J.; De Geest, B.G.; Hoogenboom, R. Partial Hydrolysis of Poly(2-Ethyl-2-Oxazoline) and Potential Implications for Biomedical Applications? Macromol. Biosci. 2012, 12, 1114–1123. [Google Scholar] [CrossRef]
- Yuan, J.J.; Jin, R.H. Fibrous Crystalline Hydrogels Formed from Polymers Possessing a Linear Poly(Ethyleneimine) Backbone. Langmuir 2005, 21, 3136–3145. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Taranejoo, S.; Liu, J.; Verma, P.; Hourigan, K. A Review of the Developments of Characteristics of PEI Derivatives for Gene Delivery Applications. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Patil, S.; Lalani, R.; Bhatt, P.; Vhora, I.; Patel, V.; Patel, H.; Misra, A. Hydroxyethyl Substituted Linear Polyethylenimine for Safe and Efficient Delivery of SiRNA Therapeutics. RSC Adv. 2018, 8, 35461–35473. [Google Scholar] [CrossRef]
- Samanta, M.K.; Dube, R.; Suresh, B. Transdermal Drug Delivery System of Haloperidol to Overcome Self-Induced Extrapyramidal Syndrome. Drug Dev. Ind. Pharm. 2003, 29, 405–415. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cerchiara, T.; Luppi, B.; Bigucci, F. Transdermal Delivery of Antipsychotics: Rationale and Current Status. CNS Drugs 2019, 33, 849–865. [Google Scholar] [CrossRef]
- Sushmita, G.; Maha Lakshmi, J.; Prathyusha, K.S.S.; Srinivasa Rao, Y. Formulation and Evaluation of Haloperdiol-Carrier Loaded Buccal Film. Int. J. Curr. Adv. Res. 2018, 7, 5–10. [Google Scholar] [CrossRef]
- Sedlacek, O.; Janouskova, O.; Verbraeken, B.; Richard, H. Straightforward Route to Superhydrophilic Poly(2-Oxazoline)s via Acylation of Well-Defined Polyethylenimine. Biomacromolecules 2018, 20, 222–230. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and Evaluation of Dry Alginate-Chitosan Microcapsules as an Enteric Delivery Vehicle for Probiotic Bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef]
- Soradech, S.; Limatvapirat, S.; Luangtana-anan, M. Stability Enhancement of Shellac by Formation of Composite Film: Effect of Gelatin and Plasticizers. J. Food Eng. 2013, 116, 572–580. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-Anan, M. An Approach for the Enhancement of the Mechanical Properties and Film Coating Efficiency of Shellac by the Formation of Composite Films Based on Shellac and Gelatin. J. Food Eng. 2012, 108, 94–102. [Google Scholar] [CrossRef]
- Shan, X.; Aspinall, S.; Kaldybekov, D.B.; Buang, F.; Williams, A.C.; Khutoryanskiy, V.V. Synthesis and Evaluation of Methacrylated Poly(2-Ethyl-2-Oxazoline) as a Mucoadhesive Polymer for Nasal Drug Delivery. ACS Appl. Polym. Mater. 2021, 3, 5882–5892. [Google Scholar] [CrossRef]
- Saegusa, T.; Ikeda, H.; Fujii, H. Crystalline Polyethylenimine. Macromolecules 1972, 5, 108. [Google Scholar] [CrossRef]
- Lorson, T.; Lübtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-Oxazoline)s Based Biomaterials: A Comprehensive and Critical Update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Gholami, L.; Sadeghnia, H.R.; Darroudi, M.; Kazemi Oskuee, R. Evaluation of Genotoxicity and Cytotoxicity Induced by Different Molecular Weights of Polyethylenimine/DNA Nanoparticles. Turkish J. Biol. 2014, 38, 380–387. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; De La Caba, K. Characterization and Antimicrobial Analysis of Chitosan-Based Films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- Silva, C.L.; Pereira, J.C.; Ramalho, A.; Pais, A.A.C.C.; Sousa, J.J.S. Films Based on Chitosan Polyelectrolyte Complexes for Skin Drug Delivery: Development and Characterization. J. Membr. Sci. 2008, 320, 268–279. [Google Scholar] [CrossRef]
- Matveev, Y.I.; Grinberg, V.Y.; Tolstoguzov, V.B. The Plasticizing Effect of Water on Proteins, Polysaccharides and Their Mixtures. Glassy State of Biopolymers, Food and Seeds. Food Hydrocoll. 2000, 14, 425–437. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Cascone, M.G.; Lazzeri, L.; Barbani, N.; Nurkeeva, Z.S.; Mun, G.A.; Bitekenova, A.B.; Dzhusupbekova, A.B. Hydrophilic Films Based on Blends of Poly(Acrylic Acid) and Poly(2-Hydroxyethyl Vinyl Ether): Thermal, Mechanical, and Morphological Characterization. Macromol. Biosci. 2003, 3, 117–122. [Google Scholar] [CrossRef]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass Transition Temperature of Chitosan and Miscibility of Chitosan/Poly(N-Vinyl Pyrrolidone) Blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of Glass Transition Temperatures: Binary Blends and Copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Seong, D.W.; Yeo, J.S.; Hwang, S.H. Fabrication of Polycarbonate Blends with Poly(Methyl Methacrylate-Co-Phenyl Methacrylate) Copolymer: Miscibility and Scratch Resistance Properties. J. Ind. Eng. Chem. 2016, 36, 251–254. [Google Scholar] [CrossRef]
- Rao, V.; Ashokan, P.V.; Shridhar, M.H. Studies on the Compatibility and Specific Interaction in Cellulose Acetate Hydrogen Phthalate (CAP) and Poly Methyl Methacrylate (PMMA) Blend. Polymer 1999, 40, 7167–7171. [Google Scholar] [CrossRef]
- Rao, V.; Ashokan, P.V.; Shridhar, M.H. Miscible Blends of Cellulose Acetate Hydrogen Phthalate and Poly(Vinyl Pyrollidone) Characterization by Viscometry, Ultrasound, and DSC. J. Appl. Polym. Sci. 2000, 76, 859–867. [Google Scholar] [CrossRef]
- Mujaheddin, J.R.; Rai, K.S.; Guru, G. Miscibility Studies of Agar-Agar/Starch Blends Using Various Techniques. Int. J. Res. Pharm. Chem. 2012, 2, 1049–1056. [Google Scholar]
- Shubha, A.; Manohara, S.R.; Gerward, L. Influence of Polyvinylpyrrolidone on Optical, Electrical, and Dielectric Properties of Poly(2-Ethyl-2-Oxazoline)-Polyvinylpyrrolidone Blends. J. Mol. Liq. 2017, 247, 328–336. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/Hydrophilic Plasticizer-Based Films: Preparation, Physicochemical and Antimicrobial Properties. J. Polym. Environ. 2014, 22, 41–51. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; Da Silva, M.A.; Dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and Limitations of Drug Delivery Systems Formulated as Eye Drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef]
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef]
- Brown, M.; Williams, A. The Art and Science of Dermal Formulation Development; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780429059872. [Google Scholar]
- Berillo, D.; Zharkinbekov, Z.; Kim, Y.; Raziyeva, K.; Temirkhanova, K.; Saparov, A. Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery. Pharmaceutics 2021, 13, 2050. [Google Scholar] [CrossRef]
- Williams, A. Controlled Drug Delivery into and Through Skin. In Fundamentals of Drug Delivery; Benson, H.A.E., Roberts, M.S., Williams, A.C., Liang, X., Eds.; Wiley: Noboken, NJ, USA, 2021; Chapter 20; pp. 507–534. ISBN 9781119769675. [Google Scholar]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.X.; Farooq, M.A.; Khan, D.H. An Overview of Hydrogels and Their Role in Transdermal Drug Delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Al Omari, M.M.; Zughul, M.B.; Davies, J.E.D.; Badwan, A.A. A Study of Haloperidol Inclusion Complexes with β-Cyclodextrin Using Phase Solubility, NMR Spectroscopy and Molecular Modeling Techniques. J. Solution Chem. 2009, 38, 669–683. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Luangtana-Anan, M.; Sriamornsak, P. Buccal Administration of Mucoadhesive Blend Films Saturated with Propranolol Loaded Nanoparticles. Asian J. Pharm. Sci. 2018, 13, 34–43. [Google Scholar] [CrossRef]
- Giovino, C.; Ayensu, I.; Tetteh, J.; Boateng, J.S. An Integrated Buccal Delivery System Combining Chitosan Films Impregnated with Peptide Loaded PEG-b-PLA Nanoparticles. Colloids Surf. B Biointerfaces 2013, 112, 9–15. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Controlling the in Vitro Release Profiles for a System of Haloperidol-Loaded PLGA Nanoparticles. Int. J. Pharm. 2008, 346, 151–159. [Google Scholar] [CrossRef]








| FTIR Absorption of Blends (cm−1) | Assignment | |||||
|---|---|---|---|---|---|---|
| 100:0 | 80:20 | 60:40 | 40:60 | 20:80 | 0:100 | |
| 3247 | 3248 | 3258 | 3259 | 3348 | 3291 | -OH and NH stretching |
| 2917 | 2928 | 2929 | 2928 | 2920 | 2940 | CH stretching |
| 2878 | 2879 | 2880 | 2880 | 2849 | 2827 | CH stretching |
| 1625 1,2 | 1624 1,2 | 1635 1,2 | 1631 1,2 | 1633 1,2 | 1675 2 | C=O stretching (amide I) 1, water region 2 |
| 1514 3,4 | 1514 3,4 | 1515 3,4 | 1515 3,4 | 1468 3,4 | 1464 4 | NH bending (amide II) 3, CH2 vibration 4 |
| 1412 | 1416 | 1418 | 1410 | 1422 | 1424 | CH and OH vibration |
| 1376 5,6 | 1375 5,6 | 1376 5,6 | 1376 5,6 | 1372 5,6 | 1371 6 | Acetamide groups 5, CH vibration 6 |
| 1311 | 1321 | 1317 | 1315 | 1302 | - | CN stretching (amide III) |
| 1152 | 1151 | 1152 | 1152 | 1150 | - | Anti-symmetric strechnig of the C-O-C bridge |
| 1060 7,8 | 1056 7,8 | 1055 7,8 | 1053 7,8 | 1053 7,8 | 1054 8 | Skeletal vibration involving the C-O stretching 7, C-C stretching 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soradech, S.; Kengkwasingh, P.; Williams, A.C.; Khutoryanskiy, V.V. Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol. Pharmaceutics 2022, 14, 2671. https://doi.org/10.3390/pharmaceutics14122671
Soradech S, Kengkwasingh P, Williams AC, Khutoryanskiy VV. Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol. Pharmaceutics. 2022; 14(12):2671. https://doi.org/10.3390/pharmaceutics14122671
Chicago/Turabian StyleSoradech, Sitthiphong, Pattarawadee Kengkwasingh, Adrian C. Williams, and Vitaliy V. Khutoryanskiy. 2022. "Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol" Pharmaceutics 14, no. 12: 2671. https://doi.org/10.3390/pharmaceutics14122671
APA StyleSoradech, S., Kengkwasingh, P., Williams, A. C., & Khutoryanskiy, V. V. (2022). Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol. Pharmaceutics, 14(12), 2671. https://doi.org/10.3390/pharmaceutics14122671







