PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases
Abstract
1. Introduction
2. Poly(lactide-co-glycolide) (PLGA) Chemistry and History in Drug Delivery
2.1. History of PLGA Utilization in Drug Delivery
2.2. Chemistry of PLGA
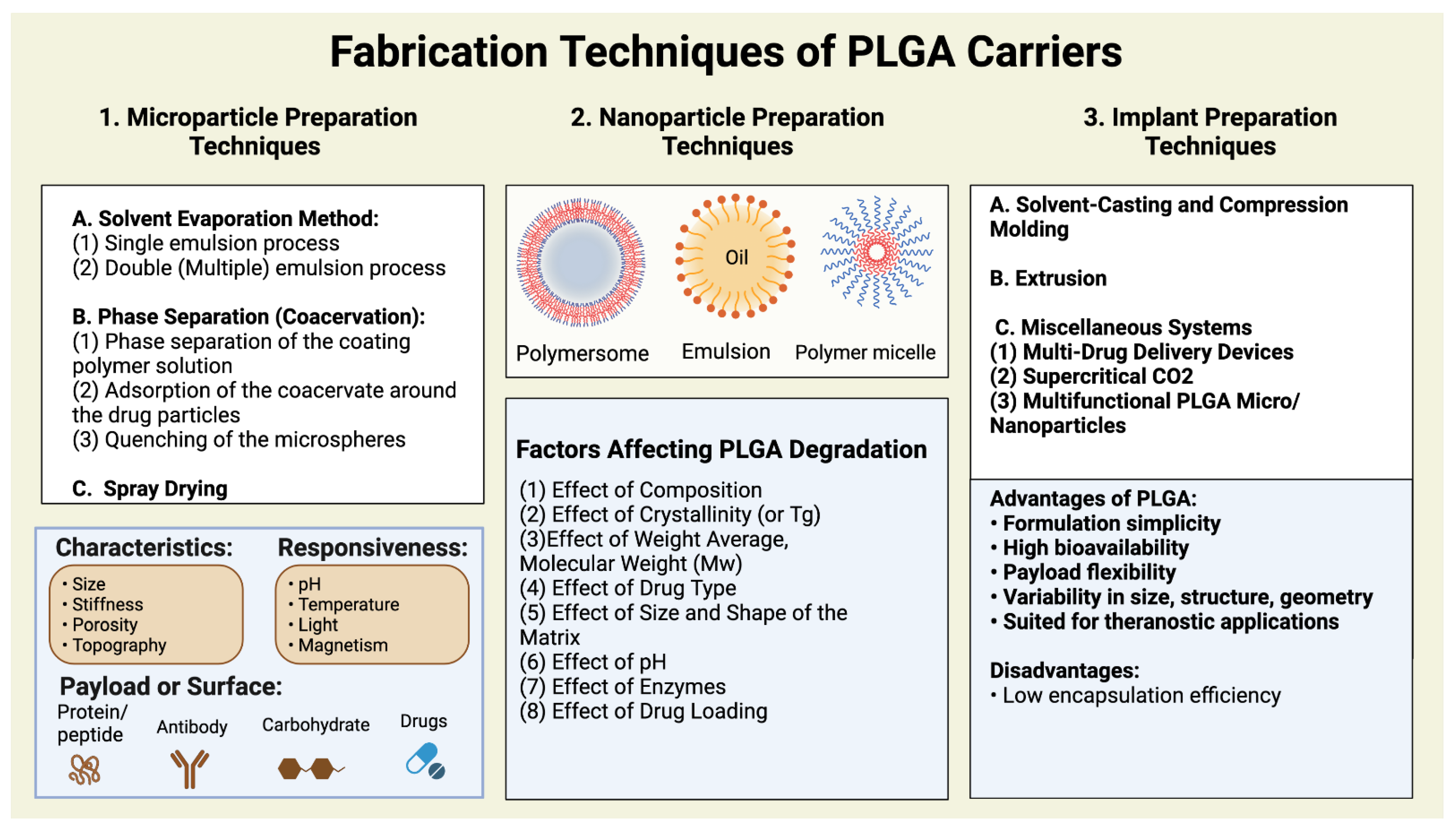
3. PLGA-Based Nanoparticle Types and Their Scale-Up Issues
3.1. Types of PLGA Nanomaterials
3.1.1. Polymeric Micelles
3.1.2. Polymersomes
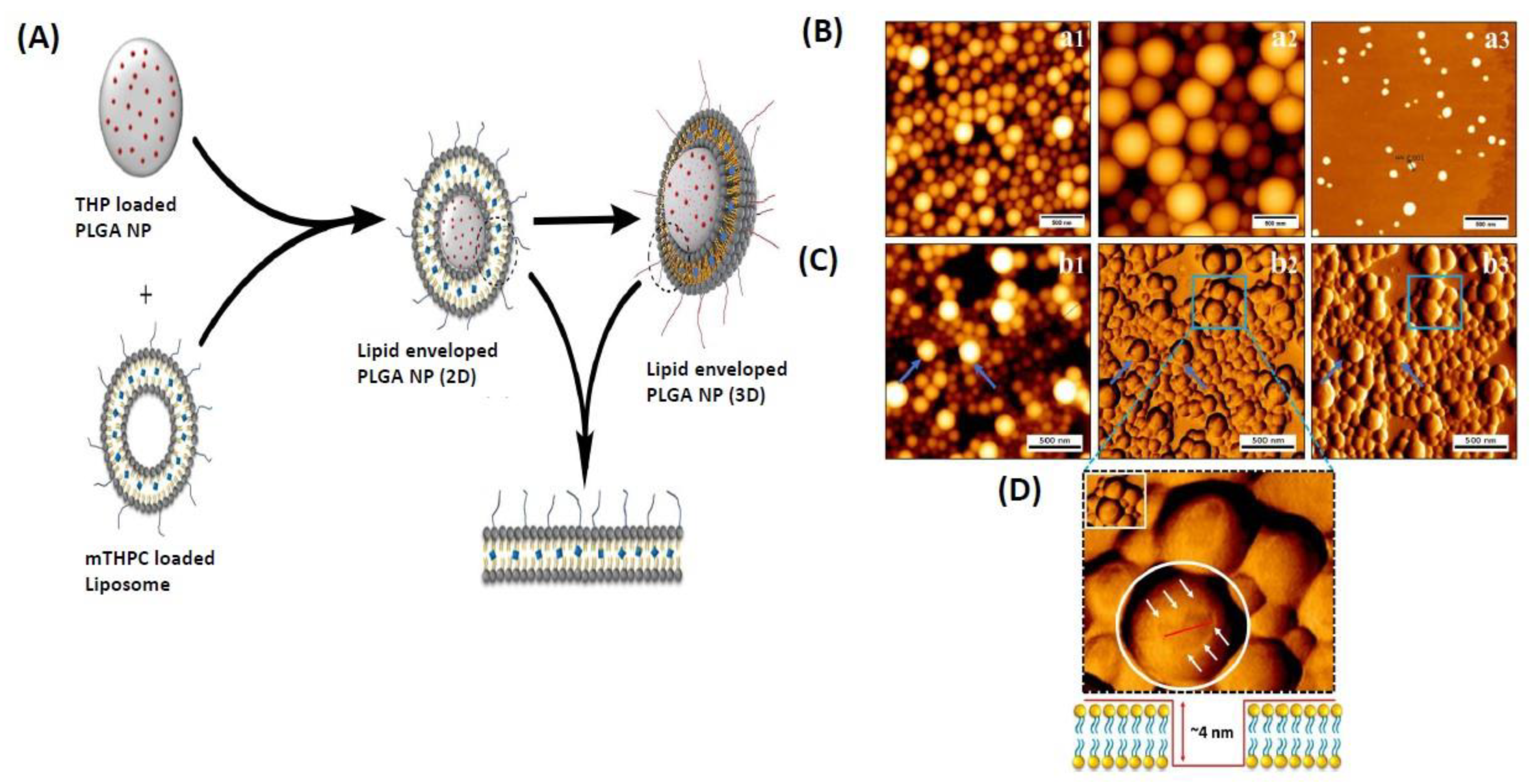
3.1.3. Lipid Nanoparticles
3.2. PLGA Systems Scale-Up Productions: Challenges and Efforts
3.3. Efficacy and Safety Assessment: Ways of Clinical Translations
3.4. Biodistribution Studies of PLGA Nanomedicine Formulations
4. PLGA as a Platform for Drug Delivery Systems
5. PLGA Nanomedicine Formulations as a Platform for Theranostic: Imaging and Biodistribution Studies
6. PLGA Nanomedicine Platforms for Different Diseases
6.1. Cancers
6.2. Neurological Diseases
6.3. Cardiovascular Diseases
6.4. Infectious Diseases
6.5. Other Diseases
6.5.1. Ophthalmic Delivery Systems
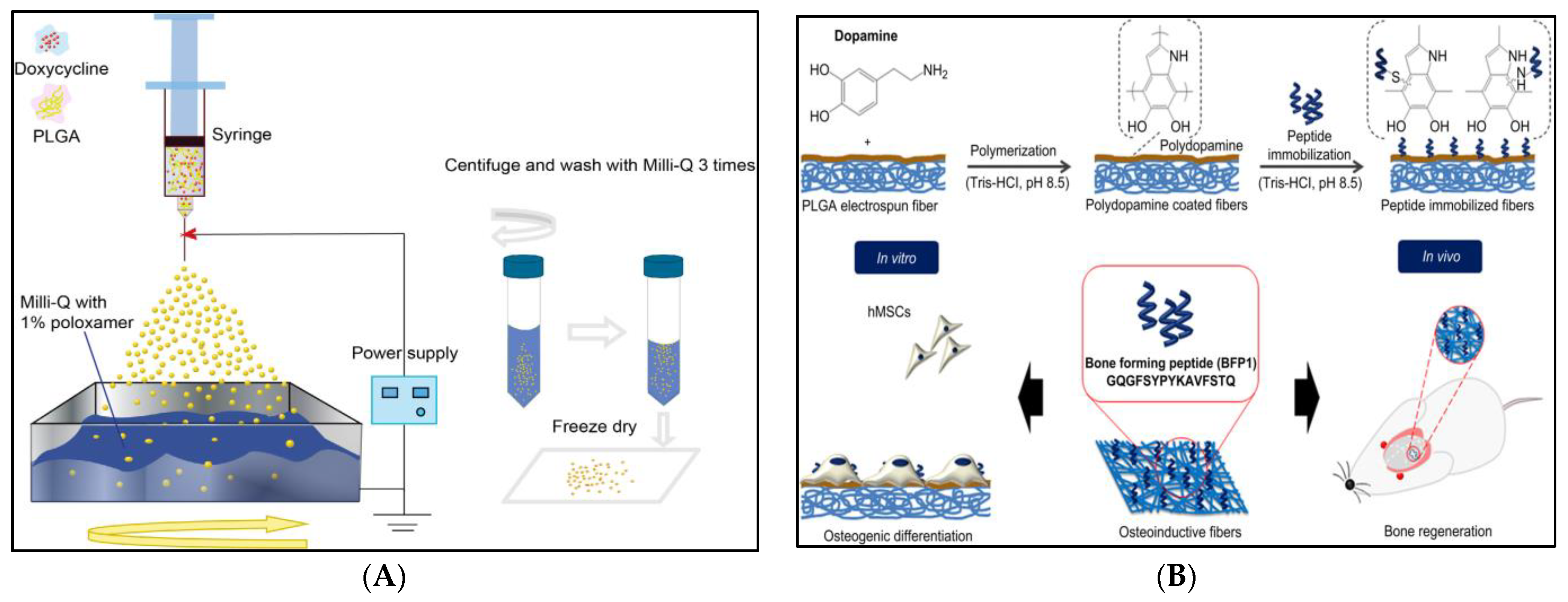
6.5.2. Periodontal Regeneration
6.5.3. Chronic Obstructive Pulmonary Diseases (COPD)
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Zhou, J.; Zhai, Y.; Xu, J.; Zhou, T.; Cen, L. Microfluidic preparation of PLGA composite microspheres with mesoporous silica nanoparticles for finely manipulated drug release. Int. J. Pharm. 2021, 593, 120173. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a “PEG-g-PEI/DNA nanoparticle-in-PLGA microsphere” hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2020, 106, 110294. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.G.; Zhou, Y.L.; Yang, Q.Q.; Ju, F.; Zhang, L.; Tang, J.B. Effects of nanoparticle-mediated growth factor gene transfer to the injured microenvironment on the tendon-to-bone healing strength. Biomater. Sci. 2020, 8, 6611–6624. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Moraveji, M.K. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019, 9, 2055–2072. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-based drug delivery systems for remotely triggered cancer therapeutic and diagnostic applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111334. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp composite scaffold for osteochondral tissue engineering and tissue regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Kostka, K.; Shalumon, K.; Prymak, O.; Chen, J.-P.; Epple, M. Synthesis and characterization of PLGA/HAP scaffolds with DNA-functionalised calcium phosphate nanoparticles for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.-Y.; Hu, H.; Xu, L.; Yang, S.-P.; Li, F.-H. Preparation and imaging investigation of dual-targeted C3F8-filled PLGA nanobubbles as a novel ultrasound contrast agent for breast cancer. Sci. Rep. 2018, 8, 3887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; García-Gabilondo, M.; Grayston, A.; Feiner, I.V.; Anton-Sales, I.; Loiola, R.A.; Llop, J.; Ramos-Cabrer, P.; Barba, I.; Garcia-Dorado, D. PLGA protein nanocarriers with tailor-made fluorescence/MRI/PET imaging modalities. Nanoscale 2020, 12, 4988–5002. [Google Scholar] [CrossRef]
- Chatterjee, M.; Chanda, N. Formulation of PLGA Nano-carrier: Specialized modification for cancer therapeutic applications. Mater. Adv. 2022, 3, 837–858. [Google Scholar] [CrossRef]
- Fornaguera, C.; Feiner-Gracia, N.; Calderó, G.; García-Celma, M.; Solans, C. PLGA nanoparticles from nano-emulsion templating as imaging agents: Versatile technology to obtain nanoparticles loaded with fluorescent dyes. Colloids Surf. B Biointerfaces 2016, 147, 201–209. [Google Scholar] [CrossRef]
- Akhavan Farid, E.; Davachi, S.M.; Pezeshki-Modaress, M.; Taranejoo, S.; Seyfi, J.; Hejazi, I.; Tabatabaei Hakim, M.; Najafi, F.; D’Amico, C.; Abbaspourrad, A. Preparation and characterization of polylactic-co-glycolic acid/insulin nanoparticles encapsulated in methacrylate coated gelatin with sustained release for specific medical applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 910–937. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Parumasivam, T.; Chan, J.G.Y.; Lin, L.C.; Flórido, M.; West, N.P.; Chan, H.-K.; Britton, W.J. PLGA particulate subunit tuberculosis vaccines promote humoral and Th17 responses but do not enhance control of Mycobacterium tuberculosis infection. PLoS ONE 2018, 13, e0194620. [Google Scholar] [CrossRef]
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-layered electrospun nanofibrous membrane with osteogenic and antibacterial properties for guided bone regeneration. Colloids Surf. B Biointerfaces 2019, 176, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Resende, A.; Pereira, A.; Moreira, T.; Patrício, P.; Fialho, S.; Cunha, G.; Silva-Cunha, A.; Magalhães, J.; Silva, G. PLGA Implants containing vancomycin and dexamethasone: Development, characterization and bactericidal effects. Die Pharm. Int. J. Pharm. Sci. 2016, 71, 439–446. [Google Scholar]
- Zhang, C.; Yang, L.; Wan, F.; Bera, H.; Cun, D.; Rantanen, J.; Yang, M. Quality by design thinking in the development of long-acting injectable PLGA/PLA-based microspheres for peptide and protein drug delivery. Int. J. Pharm. 2020, 585, 119441. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, W.; Chen, Y.; Chang, D.; Wang, T.; Zhang, X.; Mei, L.; Zeng, X.; Huang, L. Doxorubicin-loaded star-shaped copolymer PLGA-vitamin E TPGS nanoparticles for lung cancer therapy. J. Mater. Sci. Mater. Med. 2015, 26, 165. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.Y.; Cui, B.; Song, R.; Liu, X.; Xu, X.; Yao, S. Scalable production of monodisperse functional microspheres by multilayer parallelization of high aspect ratio microfluidic channels. Micromachines 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Villa, J.D.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; Triana-Chávez, O. Encapsulation of proteins from Leishmania panamensis into PLGA particles by a single emulsion-solvent evaporation method. J. Microbiol. Methods 2019, 162, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Joo, J.-R.; Hong, A.; Park, J.-S. Development of drug-loaded PLGA microparticles with different release patterns for prolonged drug delivery. Bull. Korean Chem. Soc. 2011, 32, 867–872. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers 2019, 11, 419. [Google Scholar] [CrossRef]
- Hussain, M.; Xie, J.; Hou, Z.; Shezad, K.; Xu, J.; Wang, K.; Gao, Y.; Shen, L.; Zhu, J. Regulation of drug release by tuning surface textures of biodegradable polymer microparticles. ACS Appl. Mater. Interfaces 2017, 9, 14391–14400. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, B.-Z.; Gao, Q. Effect of molecular weight of starch on the properties of cassava starch microspheres prepared in aqueous two-phase system. Carbohydr. Polym. 2017, 177, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Helder, L.; Shao, J.; Jansen, J.A.; Yang, M.; Yang, F. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: Effect of polymer end groups. Int. J. Pharm. 2019, 564, 1–9. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.; Tapeinos, C.; Bilalis, P.; Kordas, G. New approach in synthesis, characterization and release study of pH-sensitive polymeric micelles, based on PLA-Lys-b-PEGm, conjugated with doxorubicin. J. Nanopart. Res. 2011, 13, 6725–6736. [Google Scholar] [CrossRef]
- Park, J.; Mattessich, T.; Jay, S.M.; Agawu, A.; Saltzman, W.M.; Fahmy, T.M. Enhancement of surface ligand display on PLGA nanoparticles with amphiphilic ligand conjugates. J. Control. Release 2011, 156, 109–115. [Google Scholar] [CrossRef]
- Van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly (ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kagawa, A.; Makino, K. Skin permeability and transdermal delivery route of 30-nm cyclosporin A-loaded nanoparticles using PLGA-PEG-PLGA triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124866. [Google Scholar] [CrossRef]
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 79, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Bravo-Díaz, C.; Sova, M.; Kristl, J.; Saso, L. Nanotechnology-Based Drug Delivery to Improve the Therapeutic Benefits of NRF2 Modulators in Cancer Therapy. Antioxidants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Mishra, V.; Singh, S.K.; Gulati, M.; Kapoor, B.; Chellappan, D.K.; Gupta, G.; Dureja, H.; Anand, K.; Dua, K. Harnessing amphiphilic polymeric micelles for diagnostic and therapeutic applications: Breakthroughs and bottlenecks. J. Control. Release 2021, 334, 64–95. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, J.; Yang, M.; Du, R.; Pontrelli, G.; McGinty, S.; Wang, G.; Yin, T.; Wang, Y. Penetration of the blood–brain barrier and the anti-tumour effect of a novel PLGA-lysoGM1/DOX micelle drug delivery system. Nanoscale 2020, 12, 2946–2960. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán, Ó.; Rendueles, C.; Suárez, P.; Rey, V.; Murillo, D.; Morís, F.; Gutiérrez, G.; Blanco-López, M.d.C.; Matos, M.; Rodríguez, R. Nano-Encapsulation of Mithramycin in Transfersomes and Polymeric Micelles for the Treatment of Sarcomas. J. Clin. Med. 2021, 10, 1358. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef]
- Sharma, A.K.; Prasher, P.; Aljabali, A.A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.K.; Tambuwala, M.M.; Dua, K. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef]
- Neerooa, B.N.H.M.; Ooi, L.-T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.; Pushpamalar, J.; Teow, S.-Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy: An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for therapeutic delivery of protein and nucleic acid macromolecules: From design to therapeutic applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular design and preparation of polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Changalvaie, B.; Han, S.; Moaseri, E.; Scaletti, F.; Truong, L.; Caplan, R.; Cao, A.; Bouchard, R.; Truskett, T.M.; Sokolov, K.V. Indocyanine green J aggregates in polymersomes for near-infrared photoacoustic imaging. ACS Appl. Mater. Interfaces 2019, 11, 46437–46450. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, L.; Lu, S.; Wang, H.; Fan, W.; Yang, C. Three core-shell polymersomes for targeted doxorubicin delivery: Sustained and acidic release. J. Drug Deliv. Sci. Technol. 2021, 61, 102293. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Y. Fabrication strategies and supramolecular interactions of polymer-lipid complex nanoparticles as oral delivery systems. Nano Res. 2021, 14, 4487–4501. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Tahir, N.; Madni, A.; Li, W.; Correia, A.; Khan, M.M.; Rahim, M.A.; Santos, H.A. Microfluidic fabrication and characterization of Sorafenib-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery. Int. J. Pharm. 2020, 581, 119275. [Google Scholar] [CrossRef]
- Bose, R.J.; Lee, S.-H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, P.; Briffault, E.; Landin, M.; Evora, C.; Diaz-Rodriguez, P.; Delgado, A. Tailor-made oligonucleotide-loaded lipid-polymer nanosystems designed for bone gene therapy. Drug Deliv. Transl. Res. 2021, 11, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Godara, S.; Lather, V.; Kirthanashri, S.; Awasthi, R.; Pandita, D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C 2020, 109, 110576. [Google Scholar] [CrossRef] [PubMed]
- Maghrebi, S.; Joyce, P.; Jambhrunkar, M.; Thomas, N.; Prestidge, C.A. Poly (lactic-co-glycolic) Acid–Lipid Hybrid Microparticles Enhance the Intracellular Uptake and Antibacterial Activity of Rifampicin. ACS Appl. Mater. Interfaces 2020, 12, 8030–8039. [Google Scholar] [CrossRef]
- Operti, M.C.; Fecher, D.; van Dinther, E.A.; Grimm, S.; Jaber, R.; Figdor, C.G.; Tagit, O. A comparative assessment of continuous production techniques to generate sub-micron size PLGA particles. Int. J. Pharm. 2018, 550, 140–148. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X. Microfluidics for producing poly (lactic-co-glycolic acid)-based pharmaceutical nanoparticles. Adv. Drug Deliv. Rev. 2018, 128, 101–114. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Li, H.; Ma, G. Recent research and development of PLGA/PLA microspheres/nanoparticles: A review in scientific and industrial aspects. Front. Chem. Sci. Eng. 2019, 13, 14–27. [Google Scholar] [CrossRef]
- Hänggi, D.; Etminan, N.; Mayer, S.A.; Aldrich, E.F.; Diringer, M.N.; Schmutzhard, E.; Faleck, H.J.; Ng, D.; Saville, B.R.; Macdonald, R.L. Clinical trial protocol: Phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy, and safety study comparing EG-1962 to standard of care oral nimodipine in adults with aneurysmal subarachnoid hemorrhage [NEWTON-2 (nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage)]. Neurocrit. Care 2019, 30, 88–97. [Google Scholar]
- Caminal, M.; Peris, D.; Fonseca, C.; Barrachina, J.; Codina, D.; Rabanal, R.; Moll, X.; Morist, A.; García, F.; Cairó, J. Cartilage resurfacing potential of PLGA scaffolds loaded with autologous cells from cartilage, fat, and bone marrow in an ovine model of osteochondral focal defect. Cytotechnology 2016, 68, 907–919. [Google Scholar] [CrossRef]
- Dimchevska, S.; Geskovski, N.; Koliqi, R.; Matevska-Geskovska, N.; Vallejo, V.G.; Szczupak, B.; San Sebastian, E.; Llop, J.; Hristov, D.R.; Monopoli, M.P. Efficacy assessment of self-assembled PLGA-PEG-PLGA nanoparticles: Correlation of nano-bio interface interactions, biodistribution, internalization and gene expression studies. Int. J. Pharm. 2017, 533, 389–401. [Google Scholar] [CrossRef]
- Ali, S.; Amin, M.U.; Tariq, I.; Sohail, M.F.; Ali, M.Y.; Preis, E.; Ambreen, G.; Pinnapireddy, S.R.; Jedelská, J.; Schäfer, J. Lipoparticles for Synergistic Chemo-Photodynamic Therapy to Ovarian Carcinoma Cells: In vitro and in vivo Assessments. Int. J. Nanomed. 2021, 16, 951. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef]
- Zhang, R.; Billingsley, M.M.; Mitchell, M.J. Biomaterials for vaccine-based cancer immunotherapy. J. Control. Release 2018, 292, 256–276. [Google Scholar]
- Dinakaran, D.; Sengupta, J.; Pink, D.; Raturi, A.; Chen, H.; Usmani, N.; Kumar, P.; Lewis, J.D.; Narain, R.; Moore, R.B. PEG-PLGA nanospheres loaded with nanoscintillators and photosensitizers for radiation-activated photodynamic therapy. Acta Biomater. 2020, 117, 335–348. [Google Scholar] [CrossRef]
- López-Cano, J.J.; Sigen, A.; Andrés-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martínez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-responsive PLGA-PEG-PLGA hydrogels as novel injectable platforms for neuroprotective combined therapies in the treatment of retinal degenerative diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Thanki, K.; van Eetvelde, D.; Geyer, A.; Fraire, J.; Hendrix, R.; Van Eygen, H.; Putteman, E.; Sami, H.; de Souza Carvalho-Wodarz, C.; Franzyk, H. Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration. J. Control. Release 2019, 310, 82–93. [Google Scholar] [CrossRef]
- Morelli, L.; Gimondi, S.; Sevieri, M.; Salvioni, L.; Guizzetti, M.; Colzani, B.; Palugan, L.; Foppoli, A.; Talamini, L.; Morosi, L. Monitoring the fate of orally administered PLGA nanoformulation for local delivery of therapeutic drugs. Pharmaceutics 2019, 11, 658. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef]
- Wu, J.; Deng, C.; Meng, F.; Zhang, J.; Sun, H.; Zhong, Z. Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J. Control. Release 2017, 259, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.-U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-T.; Lin, H.; Wang, C.-S.; Chang, C.-H.; Lin, A.M.-Y.; Yang, J.C.-H.; Lo, Y.-L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Zhang, X.; He, C.; Zhao, P.; Li, M.; Fan, T.; Yan, R.; Lu, Y.; Lee, R.J. Platinum complexes of curcumin delivered by dual-responsive polymeric nanoparticles improve chemotherapeutic efficacy based on the enhanced anti-metastasis activity and reduce side effects. Acta Pharm. Sin. B 2020, 10, 1106–1121. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Santos, J.F.; Mattos, R.R.; Barros, E.G.; Nasciutti, L.E.; Cabral, L.M.; Sousa, V.P. Characterization and in vitro antitumor activity of polymeric nanoparticles loaded with Uncaria tomentosa extract. An. Acad. Bras. Ciênc. 2020, 92, e20190336. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic therapy of ovarian carcinoma cells with curcumin-loaded biodegradable polymeric nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-Fluorouracil and Chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib loaded inhalable polymeric nanocarriers against non-small cell lung cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef]
- Takada, N.; Kawabe, H. Leuprorelin (leuplin, lupron, viadur). In Drug Discovery in Japan; Springer: Berlin/Heidelberg, Germany, 2019; pp. 65–84. [Google Scholar]
- Asem, H.; Malmström, E. Polymeric nanoparticles explored for drug-delivery applications. In Gels and Other Soft Amorphous Solids; ACS Publications: Washington, DC, USA, 2018; pp. 315–331. [Google Scholar]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.-L. In vitro and in vivo comparison of curcumin-encapsulated chitosan-coated poly (lactic-co-glycolic acid) nanoparticles and curcumin/hydroxypropyl-β-Cyclodextrin inclusion complexes administered intranasally as therapeutic strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.-H.; Cho, H.-J.; Kim, H.K.; Yoon, T.R.; Shin, H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials 2013, 34, 5059–5069. [Google Scholar] [CrossRef]
- Upadhyay, P.; Bhattacharjee, M.; Bhattacharya, S.; Ahir, M.; Adhikary, A.; Patra, P. Silymarin-Loaded, Lactobionic Acid-Conjugated Porous PLGA Nanoparticles Induce Apoptosis in Liver Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 7178–7192. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, R.; Guo, W.; Yan, T.; He, X.; Hu, F.; Ren, F.; Ma, X.; Lei, J.; Zheng, W. PEGylated-PLGA Nanoparticles Coated with pH-Responsive Tannic Acid–Fe (III) Complexes for Reduced Premature Doxorubicin Release and Enhanced Targeting in Breast Cancer. Mol. Pharm. 2020, 18, 2161–2173. [Google Scholar] [CrossRef]
- Brauner, B.; Semmler, J.; Rauch, D.; Nokaj, M.; Haiss, P.; Schwarz, P.; Wirth, M.; Gabor, F. Trimethoprim-loaded PLGA nanoparticles grafted with WGA as potential intravesical therapy of urinary tract infections—Studies on adhesion to SV-HUCs under varying time, pH, and drug-loading conditions. ACS Omega 2020, 5, 17377–17384. [Google Scholar] [CrossRef]
- Far, J.; Abdel-Haq, M.; Gruber, M.; Abu Ammar, A. Developing biodegradable nanoparticles loaded with Mometasone Furoate for potential nasal drug delivery. ACS Omega 2020, 5, 7432–7439. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Chen, Z.; Xie, X.; Zhang, H.; Feng, Y.; Li, S.; Qin, X.; Yang, H.; Wu, C. NIR-light-triggered anticancer strategy for dual-modality imaging-guided combination therapy via a bioinspired hybrid PLGA nanoplatform. Mol. Pharm. 2019, 16, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ju, Y.; Ali, Z.; Yin, H.; Sheng, F.; Lin, J.; Wang, B.; Hou, Y. Near-infrared light and tumor microenvironment dual responsive size-switchable nanocapsules for multimodal tumor theranostics. Nat. Commun. 2019, 10, 4418. [Google Scholar] [CrossRef]
- Varani, M.; Galli, F.; Capriotti, G.; Mattei, M.; Cicconi, R.; Campagna, G.; Panzuto, F.; Signore, A. Theranostic Designed Near-Infrared Fluorescent Poly (Lactic-co-Glycolic Acid) Nanoparticles and Preliminary Studies with Functionalized VEGF-Nanoparticles. J. Clin. Med. 2020, 9, 1750. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Jiang, P.; Li, F.; Yu, B.; Yan, F. Lipid/PLGA hybrid microbubbles as a versatile platform for noninvasive image-guided targeted drug delivery. ACS Appl. Mater. Interfaces 2019, 11, 41842–41852. [Google Scholar] [CrossRef]
- Allavena, P.; Palmioli, A.; Avigni, R.; Sironi, M.; La Ferla, B.; Maeda, A. PLGA based nanoparticles for the monocyte-mediated anti-tumor drug delivery system. J. Biomed. Nanotechnol. 2020, 16, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei Mirakabad, F.S.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Caban-Toktas, S.; Sahin, A.; Lule, S.; Esendagli, G.; Vural, I.; Oguz, K.K.; Soylemezoglu, F.; Mut, M.; Dalkara, T.; Khan, M. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int. J. Pharm. 2020, 578, 119076. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Shamshiri, A.; Saeedi, M.; Tayebi, L.; Yazdian-Robati, R. Aptamer-conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs. Arch. Biochem. Biophys. 2020, 691, 108485. [Google Scholar] [CrossRef]
- Handali, S.; Moghimipour, E.; Rezaei, M.; Ramezani, Z.; Dorkoosh, F.A. PHBV/PLGA nanoparticles for enhanced delivery of 5-fluorouracil as promising treatment of colon cancer. Pharm. Dev. Technol. 2020, 25, 206–218. [Google Scholar] [CrossRef]
- Upadhaya, P.G.; Pulakkat, S.; Patravale, V.B. Nose-to-brain delivery: Exploring newer domains for glioblastoma multiforme management. Drug Deliv. Transl. Res. 2020, 10, 1044–1056. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Grapa, C.M.; Mocan, T.; Gonciar, D.; Zdrehus, C.; Mosteanu, O.; Pop, T.; Mocan, L. Epidermal growth factor receptor and its role in pancreatic cancer treatment mediated by nanoparticles. Int. J. Nanomed. 2019, 14, 9693. [Google Scholar] [CrossRef]
- Lee, D.H. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacol. Ther. 2017, 174, 1–21. [Google Scholar] [CrossRef]
- Bao, S.; Zheng, H.; Ye, J.; Huang, H.; Zhou, B.; Yao, Q.; Lin, G.; Zhang, H.; Kou, L.; Chen, R. Dual targeting EGFR and STAT3 with Erlotinib and Alantolactone co-loaded PLGA nanoparticles for pancreatic cancer treatment. Front. Pharmacol. 2021, 12, 625084. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, 13, 3679. [Google Scholar] [CrossRef] [PubMed]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Rehman, U.; Parveen, N.; Sheikh, A.; Abourehab, M.A.; Sahebkar, A.; Kesharwani, P. Polymeric nanoparticles-siRNA as an emerging nano-polyplexes against ovarian cancer. Colloids Surf. B Biointerfaces 2022, 218, 112766. [Google Scholar] [CrossRef]
- Sidhu, H.S.; Sadhotra, A. Current status of the new antiepileptic drugs in chronic pain. Front. Pharmacol. 2016, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Nigam, K.; Kaur, A.; Tyagi, A.; Nematullah, M.; Khan, F.; Gabrani, R.; Dang, S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 879–890. [Google Scholar] [CrossRef]
- Nigam, K.; Kaur, A.; Tyagi, A.; Manda, K.; Gabrani, R.; Dang, S. Baclofen-loaded poly (d, l-lactide-co-glycolic acid) nanoparticles for neuropathic pain management: In vitro and in vivo evaluation. Rejuvenation Res. 2019, 22, 235–245. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- Handa, M.; Tiwari, S.; Yadav, A.K.; Almalki, W.H.; Alghamdi, S.; Alharbi, K.S.; Shukla, R.; Beg, S. Therapeutic potential of nanoemulsions as feasible wagons for targeting Alzheimer’s disease. Drug Discov. Today 2021, 26, 2881–2888. [Google Scholar] [CrossRef]
- McAloon, C.J.; Boylan, L.M.; Hamborg, T.; Stallard, N.; Osman, F.; Lim, P.B.; Hayat, S.A. The changing face of cardiovascular disease 2000–2012: An analysis of the world health organisation global health estimates data. Int. J. Cardiol. 2016, 224, 256–264. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr. Diabetes Rep. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Xie, L.; Zhang, Y.; Hanyu, M.; Zhang, M.-R. Stabilin-2-specific peptide-based radiotracers for atherosclerosis plaque PET imaging. J. Nuclear Med. 2022, 63, 2899. [Google Scholar]
- Esfandyari-Manesh, M.; Abdi, M.; Talasaz, A.H.; Ebrahimi, S.M.; Atyabi, F.; Dinarvand, R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. DARU J. Pharm. Sci. 2020, 28, 131–138. [Google Scholar] [CrossRef]
- Foroughi-Nia, B.; Barar, J.; Memar, M.Y.; Aghanejad, A.; Davaran, S. Progresses in polymeric nanoparticles for delivery of tyrosine kinase inhibitors. Life Sci. 2021, 278, 119642. [Google Scholar] [CrossRef]
- Pala, R.; Anju, V.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int. J. Nanomed. 2020, 15, 3741. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.-S.; Brott, B.C.; Jun, H.-W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins reduce epicardial adipose tissue attenuation independent of lipid lowering: A potential pleiotropic effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
- Yokoyama, R.; Li, M.; Tabata, Y.; Hoshiga, M.; Ishizaka, N.; Asahi, M. Cardiac regeneration by statin-polymer nanoparticle-loaded adipose-derived stem cell therapy in myocardial infarction. Stem Cells Transl. Med. 2019, 8, 1055–1067. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Fan, B.; Wang, Y.; Mao, Z.; Wang, W.; Wu, J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 2020, 321, 463–474. [Google Scholar] [CrossRef]
- Afzal, I.; Sarwar, H.S.; Sohail, M.F.; Varikuti, S.; Jahan, S.; Akhtar, S.; Yasinzai, M.; Satoskar, A.R.; Shahnaz, G. Mannosylated thiolated paromomycin-loaded PLGA nanoparticles for the oral therapy of visceral leishmaniasis. Nanomedicine 2019, 14, 387–406. [Google Scholar] [CrossRef]
- Cumagun, C.J.R.; Manalo, J.O.; Salcedo-Bacalangco, N.A.; Ilag, L.L. Cellulose decomposing ability of Trichoderma in relation to their saprophytic survival. Arch. Phytopathol. Plant Prot. 2009, 42, 698–704. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly (lactic-co-glycolic acid): Applications and future prospects for periodontal tissue regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior eye segment drug delivery systems: Current treatments and future challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105. [Google Scholar] [CrossRef]
- Shivhare, R.; Pathak, A.; Shrivastava, N.; Singh, C.; Tiwari, G.; Goyal, R. An update review on novel advanced ocular drug delivery system. World J. Pharm. Pharm. Sci. 2012, 1, 545–568. [Google Scholar]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Hadizadeh, F.; Tekie, F.S.M.; Jalali, A.; Mohajeri, S.A.; Ganji, F. Preparation and analysis of a sustained drug delivery system by PLGA–PEG–PLGA triblock copolymers. Polym. Bull. 2012, 69, 429–438. [Google Scholar] [CrossRef]
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA–PEG–PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J. Mater. Chem. B 2017, 5, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Z.; Ding, J. Influence of LA and GA sequence in the PLGA block on the properties of thermogelling PLGA-PEG-PLGA block copolymers. Biomacromolecules 2011, 12, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chang, G.T.; Zhang, H.; Ding, J.D. Injectable block copolymer hydrogels for sustained release of a PEGylated drug. Int. J. Pharm. 2008, 348, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled release and targeted drug delivery with poly (lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- D’Avanzo, N.; Bruno, M.C.; Giudice, A.; Mancuso, A.; Gaetano, F.D.; Cristiano, M.C.; Paolino, D.; Fresta, M. Influence of materials properties on bio-physical features and effectiveness of 3D-scaffolds for periodontal regeneration. Molecules 2021, 26, 1643. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.M.; Gritsch, K.; Salles, V.; Attik, G.N.; Grosgogeat, B. Surface entrapment of fibronectin on electrospun PLGA scaffolds for periodontal tissue engineering. BioRes. Open Access 2014, 3, 117–126. [Google Scholar] [CrossRef]
- Agossa, K.; Lizambard, M.; Rongthong, T.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F. Physical key properties of antibiotic-free, PLGA/HPMC-based in-situ forming implants for local periodontitis treatment. Int. J. Pharm. 2017, 521, 282–293. [Google Scholar] [CrossRef]
- Reis, E.C.C.; Borges, A.P.; Araújo, M.V.; Mendes, V.C.; Guan, L.; Davies, J.E. Periodontal regeneration using a bilayered PLGA/calcium phosphate construct. Biomaterials 2011, 32, 9244–9253. [Google Scholar] [CrossRef]
- Ruvuna, L.; Sood, A. Epidemiology of chronic obstructive pulmonary disease. Clin. Chest Med. 2020, 41, 315–327. [Google Scholar] [CrossRef]
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; Agusti, A.; Ash, S.Y.; Bafadhel, M.; Bai, C.; Chalmers, J.D.; Criner, G.J.; Dharmage, S.C. Towards the elimination of chronic obstructive pulmonary disease: A Lancet Commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Caramori, G.; Cari, L.; Chung, K.F.; Diamant, Z.; Eguiluz-Gracia, I. Comparing biologicals and small molecule drug therapies for chronic respiratory diseases: An EAACI Taskforce on Immunopharmacology position paper. Allergy 2019, 74, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Mei, L.; Vishwasrao, H.D.; Jacobson, O.; Wang, Z.; Liu, Y.; Yung, B.C.; Fu, X.; Jin, A.; Niu, G. Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat. Commun. 2017, 8, 1482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Bisen, M.; Misra, A.; Srivastava, V.K.; Kaushik, S.; Siddiqui, A.J.; Mishra, N.; Singh, A.; Jyoti, A. Targeting COPD with PLGA-Based Nanoparticles: Current Status and Prospects. BioMed Res. Int. 2022, 2022, 5058121. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Morjaria, J.B. Emerging therapeutic strategies in COPD. Drug Discov. Today 2015, 20, 371–379. [Google Scholar] [CrossRef]
- Lococo, F.; Cesario, A.; Bufalo, A.D.; Ciarrocchi, A.; Prinzi, G.; Mina, M.; Bonassi, S.; Russo, P. Novel therapeutic strategy in the management of COPD: A systems medicine approach. Curr. Med. Chem. 2015, 22, 3655–3675. [Google Scholar] [CrossRef]
- Vij, N.; Min, T.; Bodas, M.; Gorde, A.; Roy, I. Neutrophil targeted nano-drug delivery system for chronic obstructive lung diseases. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2415–2427. [Google Scholar] [CrossRef]
- Chikuma, K.; Arima, K.; Asaba, Y.; Kubota, R.; Asayama, S.; Sato, K.; Kawakami, H. The potential of lipid-polymer nanoparticles as epigenetic and ROS control approaches for COPD. Free Radic. Res. 2020, 54, 829–840. [Google Scholar] [CrossRef]
- Tulbah, A.S.; Pisano, E.; Scalia, S.; Young, P.M.; Traini, D.; Ong, H.X. Inhaled simvastatin nanoparticles for inflammatory lung disease. Nanomedicine 2017, 12, 2471–2485. [Google Scholar] [CrossRef] [PubMed]





| Method | Main Idea | Main Advantage | Negative Aspects |
|---|---|---|---|
| 1-Nanoprecipitation method | The nanoprecipitation method produces lipid-polymer particles with a high yield of less than 100 nanometers. | Nanoparticles with a higher production rate and better size homogeneity. | Organic solvents have the potential to damage biomolecules (protein nuclei acids). |
| 2-Microfluidic method | This method can control immiscible liquids in small quantities in a precise capillary network with microscale fluid channels. | Small size distribution (lower PDI), higher encapsulation and loading efficiencies, improved batch-to-batch uniformity, and simple scale-up possibilities all contribute to cost-effective nanocarrier development. | The yield Is relatively poor. |
| 3-One-step method | This approach uses covalent conjugation to combine various lipid and polymer precursors. | Cost-effectiveness, scalability, and a traditional method of preparation. | Instability of biomacromolecules. |
| 4-Two-step method | Monolayer, bilayer, and multilayer shells are usually made with it. Cationic lipid vesicles are coupled with anionic polymeric nanoparticles using electrostatic interactions in this process. | Nanoparticles produced easily cross the membrane barrier and circulate in the bloodstream, allowing them to deliver drugs for long periods of time. | Separate preparation of polymeric nanoparticles and lipid vesicles, which takes a long time and consumes a lot of resources. |
| 5-Spray-drying method | Polymers are used to make nanoparticles with sizes ranging from 400 to 500 nm, which are then dispersed in an organic solvent containing various lipids. To complete the product, the lipoidal polymeric suspension is spray dried. | Fast and effective. Ideally suited to commercial scale-up. Protein parameters that are more appropriate. | Small lots with a moderate yield. |
| Nanoparticle (NPs) | Polymer and Additives | Function of Polymer | Drug | Type of Cancer | Type of Cell Line | Target Action | Reference |
|---|---|---|---|---|---|---|---|
| Afatinib-loaded PLGA NPs) | PLGA | Protect Afatinib, improve drug delivery | Afatinib | Colon Cancer | Caco-2 cells | pH-responsive characteristics to increase the sensitivity of colon cancer cells to afatinib. | 2019 [84] |
| Platinum–curcumin complexes loaded into pH and redox dual-responsive nanoparticles (PteCUR@ PSPPN) | mPEG-SS-PBAE-PLGA | Control intracellular release, synergistic anticancer effects | Platinum–curcumin | Lung Cancer | A549 cells | Synergistic anticancer effects, enhanced anti-metastatic activity | 2019 [85] |
| Uncaria tomentosa extract (UT)-PLGA & UTPCL | PCL and PLGA | Better drug delivery—UT-PLGA nanoparticles showed higher drug loading | Uncaria tomentosa extract | Prostate Cancer | LNCaP, DU145 cells | UT-PLGA showed higher cytotoxicity towards DU145 cells, UTPCL showed higher cytotoxicity against LNCaP cells | 2019 [86] |
| Curcumin (Cur)-loaded polymeric poly (lactic-co-glycolic acid) (PLGA) nanoparticles (Cur-PLGA NPs) | PLGA | Stabilize curcumin in the presence of light, improved serum stability compared with free curcumin | Curcumin | Ovarian Cancer | SKOV3 cells | Cytotoxic effects on tumor cells upon irradiation at a low intensity inhibits tumor growth | 2019 [87] |
| 5-FU-Chrysin-loaded PLGA-PEG-PLGA nanoparticles (5FU-Chrysin-PLGA-PEG-PLGA NPs) | PLGA-PEG-PLGA | Improve the functional delivery efficacy of 5-FU and Chrysin in cancer | 5-FU, Chrysin | Colon Cancer | HT-29 cells | Apoptosis, growth inhibitory effects | 2020 [88] |
| Sorafenib (SF)-loaded catatonically-modified polymeric nanoparticles (NPs) | PLGA | Aerosolization efficiency for pulmonary delivery | Sorafenib | Lung Cancer | A549 cells | Enhanced cell migration inhibition, reduction in cell survival, inhibition in the formation of colonies | 2020 [89] |
| Brand Name | Indication | Clinicaltrials.Gov Identifier |
|---|---|---|
| Somatuline® LA | Acromegaly | NCT03066245 |
| Sandostatin® LAR | Acromegaly and carcinoid | NCT03060655 |
| Nutropin Depot ® | Growth deficiency | NCT02568527 |
| Zoladex® | Breast cancer. Prostate cancer | NCT03474900 |
| Arestin | Periodontal disease | NCT02726646 |
| TrelstarTM Depot | Advanced Prostatic Cancer | NCT01681381 |
| Suprecur® MP | Prostate cancer | NCT0 1753 089 |
| Pamorelin® | Prostate cancer | NCT03045913 |
| Lupron Depot | Prostate cancer | NCT02578069 |
| Eligard | Advanced Prostatic Cancer | NCT03401216 |
| Atridox® | Chronic adult periodontitis | NCT03429803 |
| Risperidal® Consta | Antipsychotic | NA |
| Decapepty | Prostate cancer | NA |
| Clinical Trial No. (Status) | Study Title | Conditions | Interventions |
|---|---|---|---|
| NCT05475444 (Completed) | PLGA Nanoparticles Entrapping Ciprofloxacin to Treat E-Fecalis Infections in Endodontics | Bacterial Infections Oral | Device: Chitosan-coated PLGA nanoparticles entrapping Ciprofloxacin incorporated in smart gels Device: Ciprofloxacin paste and solution |
| NCT03060655 (Unknown status) | Study of PLGA-Mg Material in Clinical Orthopedics | Fracture Dislocation | Biological: PLGA-Mg material Biological: titanium alloy |
| NCT04735601 (Recruiting) | Ahmed Valve Implantation Coated With Poly Lactic-Co-glycolic Acid (PLGA) Saturated With Mitomycin-C in the Management of Adult Onset Glaucoma in Sturge Weber Syndrome | Glaucoma | Procedure: Ahmed Valve |
| NCT03066245 (Unknown status) | Use of Stem Cells Cultured on a Scaffold for the Treatment of Aneurysmal Bone Cysts (ABC) | Aneurysmal Bone Cyst | Biological: MSC-PLGA |
| NCT03474627 (Completed) | PLGA-coated Biphasic Calcium Phosphate in Sinus Lift | Sinus Floor Augmentation | Device: Non-coated HA/TCP particles Device: PLGA-coated HA/TCP particles |
| NCT02487186 (Completed) | Locally Delivered Doxycycline Adjunct to Nonsurgical Periodontal Therapy. | Periodontal Disease | Drug: Doxycycline Procedure: Full-mouth debridement |
| NCT02568527 (Completed) | Biodegradable Synthetic Scaffold as a Substitute for hAM in Limbal Epithelial Cells Transplant in LSCD Patients | Limbal Stem Cell Deficiency | Device: PLGA scaffold |
| NCT00836797 (Completed) | Radiographic Assessment of Bone Regeneration in Alveolar Sockets with PLGA Scaffold | Preservation of Alveolar Bone Height With PLGA Bioscaffold | NA |
| NCT04619056 (Recruiting) | First-in-man Clinical Trial of CEB-01 PLGA Membrane in Recurrent or Locally Advanced Retroperitoneal Soft Tissue Sarcoma | Locally Advanced Soft Tissue Sarcoma Recurrent Soft Tissue Sarcoma | Combination Product: CEB-01 membrane loaded with SN-38 |
| NCT04848818 (Recruiting) | Comparative Trial of Operative Treatment of Distal Pediatric Forearm Fractures With Biodegradable Nails and K-wires | Fracture Wrist | Procedure: Distal radial metaphyseal fracture fixation with percutaneous K-wires Procedure: Distal radial and/or ulnar metaphyseal fracture fixation with biodegradable PLGA-based (Activa Im-Nail) implants |
| NCT05442736 (Completed) | Modified Surface of PLGA Nanoparticles in Smart Hydrogel | Antibiotic Resistant Infection | Drug: Ciprofloxacin |
| NCT03474900 (Completed) | Biodegradable Versus Titanium Nailing in Forearm Shaft Fractures in Children | Forearm Fracture | Device: PLGA implant, Bioretec ltd. Finland Device: Titanium elastic stable nail |
| NCT05456022 (Not yet recruiting) | Therapeutic Efficacy of Quercetin Versus Its Encapsulated Nanoparticle on Tongue Squamous Cell Carcinoma Cell Line | Oral Cancer | Drug: Quercetin 3,3’,4’,5,6-Pentahydroxyflavone, 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one Drug: Quercetin-encapsulated PLGA-PEG nanoparticles (Nano-QUT) Drug: Doxorubicin chemotherapeutic drug as a positive control |
| NCT01729195 (Completed) | Ankle Syndesmosis Fixation by Antibiotic Releasing Bioabsorbable Screw | Ankle Fracture | Procedure: A ciprofloxacin containing bioabsorbable PLGA bone screw |
| NCT02726646 (Completed) | Evaluation of Local Doxycycline in Smokers With Chronic Periodontitis | Chronic Periodontitis | Drug: Doxycycline Procedure: Mechanical debridement Drug: Placebo |
| NCT04339764 (Recruiting) | Autologous Transplantation of Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium for Geographic Atrophy Associated With Age-Related Macular Degeneration | Age-Related Macular Degeneration | Drug: iPSC-derived RPE/PLGA transplantation |
| NCT01681381 (Unknown status) | Evaluate Safety and Effectiveness of the Tivoli® DES and The Firebird2® DES for Treatment Coronary Revascularization | Ischemic Heart Disease Myocardial Ischemia Coronary Artery Lesions, Primary Coronary Disease Acute Coronary Syndrome Furcation Lesion of Coronary Artery | Device: Tivoli® DES Device: Firebird2® DES |
| NCT02017275 (Completed) | Comparison of BuMA eG Based Biodegradable Polymer Stent with EXCEL Biodegradable Polymer Sirolimus-eluting Stent in “Real-World” Practice | Coronary Artery Disease | Device: BuMA Device: EXCEL |
| NCT01753089 (Active, not recruiting) | Dendritic Cell Activating Scaffold in Melanoma | Melanoma | Biological: WDVAX |
| NCT04751786 (Recruiting) | Dose Escalation Study of Immunomodulatory Nanoparticles | Advanced Solid Tumor | Drug: PRECIOUS-01 |
| NCT04941612 (Recruiting) | Use of the Bioabsorbable Activa IM-Nail™ in Pediatric Diaphyseal Forearm Fractures | Fracture Fixation, Intramedullary Forearm Fracture Fracture Healing Child, Only Implant Complication | Device: Activa IM-Nail |
| NCT04385745 (Unknown status) | Treatment of Children’s Forearm Shaft Fractures With Biodegradable Intramedullary Nailing, Compared With Elastic Stable Intramedullary Nailing | Fractures, Bone Injury Arm | Procedure: Biodegradable Intramedullary Nailing (BIN) |
| NCT02255188 (Completed) | Experimental Study of the Vascular Prosthesis Manufactured by Electrospinning | Arterial Occlusive Disease | Procedure: blood sampling procedure |
| NCT03707769 (Recruiting) | TIPS Microspheres for Perianal Fistula | Perianal Fistula | Device: TIPS microspheres |
| NCT05448625 (Recruiting) | DES in Patients With a High Risk of Ischemic Events | Drug-eluting Stent Coronary Artery Disease | Device: Genoss DES |
| NCT03045913 (Active, not recruiting) | Genoss DES Prospective Multicenter Registry | Coronary Artery Disease Myocardial Ischemia Myocardial Infarction | Device: Genoss DES |
| NCT04082962 (Recruiting) | Dexamethasone Implant for Retinal Detachment in Uveal Melanoma | Exudative Retinal Detachment Uveal Melanoma | Drug: Dexamethasone intravitreal implant |
| NCT03762655 (Active, not recruiting) | Study of Probable Benefit of the Neuro-Spinal Scaffold™ in Subjects with Complete Thoracic AIS A Spinal Cord Injury as Compared to Standard of Care | Injury, Spinal Cord | Device: Neuro-Spinal Scaffold |
| NCT04094298 (Recruiting) | Use of Extended Release Triamcinolone in the Treatment of Rotator Cuff Disease | Rotator Cuff Tears Rotator Cuff Tendinitis Rotator Cuff Impingement | Drug: FX006 Injection Injections, Glucocorticoids |
| NCT05104853 (Recruiting) | Study to Evaluate the Safety, Tolerability, PDs, and Efficacy of CNP-104 in Subjects With Primary Biliary Cholangitis | Primary Biliary Cholangitis | Drug: CNP-104 Drug: Placebo |
| NCT05250856 (Recruiting) | CNP-201 in Subjects with Peanut Allergy | Peanut Allergy | Drug: CNP-201 Drug: Placebo |
| NCT02578069 (Completed) | First-in-man Trial Evaluating the Safety and Efficacy of the NOVA Intracranial Stent (NOVA Trial) | Ischemic Stroke | Device: NOVA Intracranial Sirolimus Eluting Stent System Device: Apollo Intracranial Stent System |
| NCT04012567 (Active, not recruiting) | Safety and Effectiveness of BIOSURE RG in Cruciate Ligaments Reconstruction in Chinese | Cruciate Ligament Reconstruction, Knee | Investigational device: Biosure Regenesorb Interference Screw Control device: Biosure HA Interference Screw |
| NCT02982889 (Completed) | Single Ascending Dose Study of Two Liquidia Bupivacaine Formulations | Acute Pain | Drug: LIQ865A bupivacaine formulation Drug: LIQ865B bupivacaine formulation Drug: Diluent for LIQ865 Drug: 0.5% bupivacaine hydrochloride |
| NCT03401216 (Unknown status) | Stent Coverage and Neointimal Tissue Characterization After Extra Long Everolimus—Eluting Stent Implantation | Ischemic Heart Disease Coronary Artery Disease Coronary Atherosclerosis | Device: SYNERGY 48 mm Procedure: PCI Procedure: 3-month OCT follow-up Procedure: 6-month OCT follow-up |
| NCT01734512 (Active, not recruiting) | Phase II Study of Everolimus for Recurrent or Progressive Low-grade Gliomas in Children | Pediatric Recurrent Progressive Low-grade Gliomas Pediatric Progressive Low-grade Gliomas | Drug: Everolimus |
| NCT03429803 (Active, not recruiting) | DAY101 In Gliomas and Other Tumors | Low-grade Glioma | Drug: DAY101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. https://doi.org/10.3390/pharmaceutics14122728
Alsaab HO, Alharbi FD, Alhibs AS, Alanazi NB, Alshehri BY, Saleh MA, Alshehri FS, Algarni MA, Almugaiteeb T, Uddin MN, et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics. 2022; 14(12):2728. https://doi.org/10.3390/pharmaceutics14122728
Chicago/Turabian StyleAlsaab, Hashem O., Fatima D. Alharbi, Alanoud S. Alhibs, Nouf B. Alanazi, Bayan Y. Alshehri, Marwa A. Saleh, Fahad S. Alshehri, Majed A. Algarni, Turki Almugaiteeb, Mohammad N. Uddin, and et al. 2022. "PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases" Pharmaceutics 14, no. 12: 2728. https://doi.org/10.3390/pharmaceutics14122728
APA StyleAlsaab, H. O., Alharbi, F. D., Alhibs, A. S., Alanazi, N. B., Alshehri, B. Y., Saleh, M. A., Alshehri, F. S., Algarni, M. A., Almugaiteeb, T., Uddin, M. N., & Alzhrani, R. M. (2022). PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics, 14(12), 2728. https://doi.org/10.3390/pharmaceutics14122728








