Pyruvate Kinase M2 Promotes Hair Regeneration by Connecting Metabolic and Wnt/β-Catenin Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Maintenance
2.2. Depilation-Induced Hair Cycle
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. Immunohistochemistry (IHC)
2.5. Reverse Transcription and Quantitative Real-Time PCR
2.6. Pyruvate Kinase Activity Assay
2.7. In Vivo Hair Re-Growth Test
2.8. Wound-Induced Hair Follicle Neogenesis Assay
2.9. Whole-Mount Alkaline Phosphatase (ALP) Staining
2.10. Database
2.11. Mouse Vibrissa Ex Vivo Culture
2.12. Statistical Analyses
3. Results
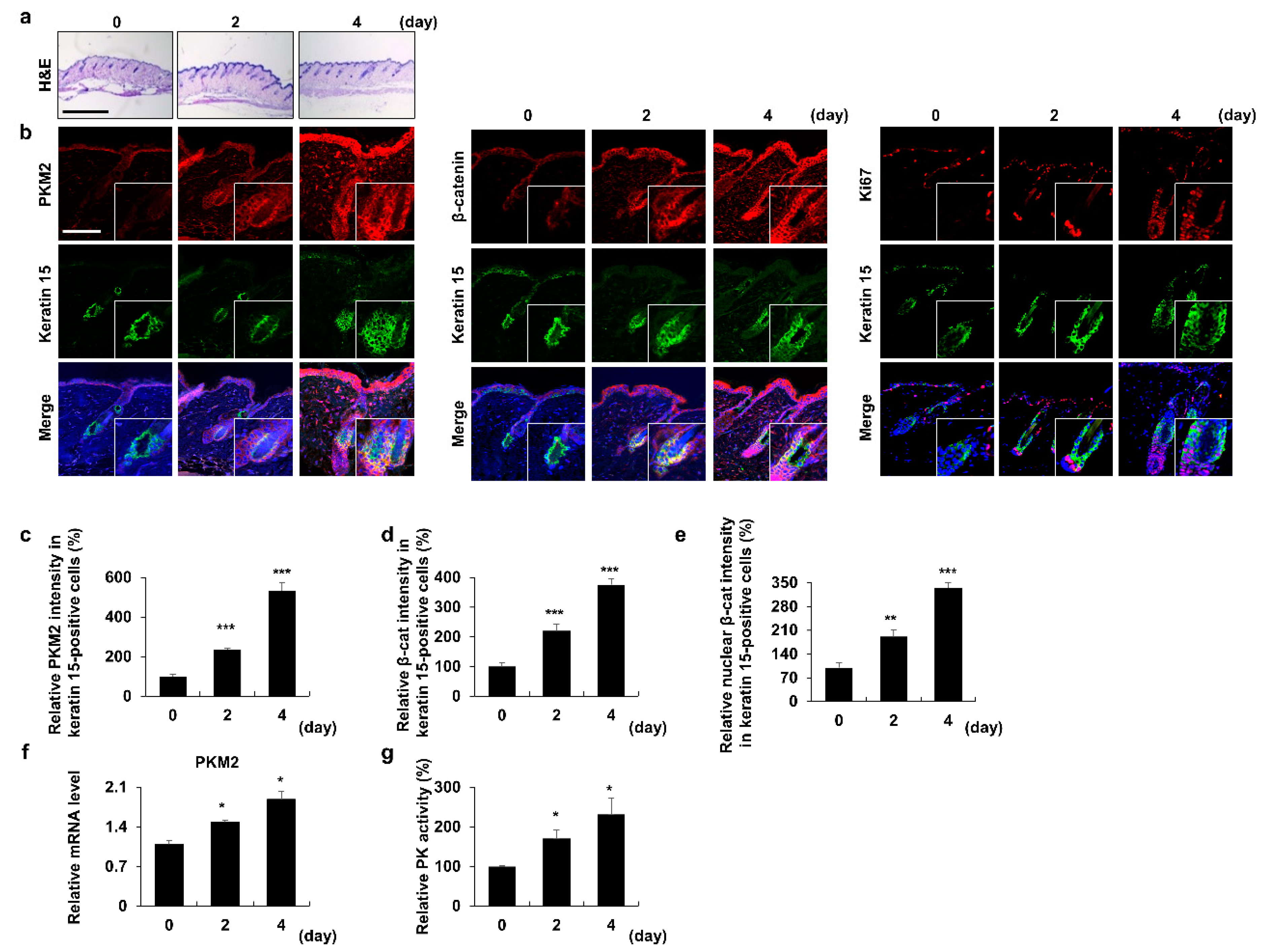
3.1. PKM2 Increases in HFSCs during the Anagen Induction
3.2. Activation of PKM2 Induces HFSC Proliferation and Hair Growth
3.3. PKM2 Expression Depends on Wnt/β-Catenin Signaling
3.4. Combined Treatment with Activators of PKM2 and Wnt/β-Catenin Signaling Promotes Hair Re-Growth
3.5. PKM2 Is Highly Expressed in the Neogenic Follicles during WIHN
3.6. Combined Treatment with TEPP-46 and KY19382 Enhances WIHN
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacón-Martínez, C.A.; Klose, M.; Niemann, C.; Glauche, I.; Wickström, S.A. Hair follicle stem cell cultures reveal self-organizing plasticity of stem cells and their progeny. EMBO J. 2017, 36, 151–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Fuchs, E. The tortoise and the hair: Slow-cycling cells in the stem cell race. Cell 2009, 137, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Philpott, M.P.; Kealey, T. Metabolism of freshly isolated human hair follicles capable of hair elongation: A glutaminolytic, aerobic glycolytic tissue. J. Investig. Dermatol. 1993, 100, 834–840. [Google Scholar] [CrossRef]
- Kealey, T.; Williams, R.; Philpott, M.P. The human hair follicle engages in glutaminolysis and aerobic glycolysis: Implications for skin, splanchnic and neoplastic metabolism. Ski. Pharmacol. Off. J. Ski. Pharmacol. Soc. 1994, 7, 41–46. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef] [Green Version]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Diabetes and Digestive and Kidney Diseases. Protein Kinase Inhibitors. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Jurica, M.S.; Mesecar, A.; Heath, P.J.; Shi, W.; Nowak, T.; Stoddard, B.L. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 1998, 6, 195–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, X.; Liu, Y.; Liu, Y.; Sun, L.; Chen, F. PKM2, function and expression and regulation. Cell Biosci. 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M.; Shamsi, A.; Elasbali, A.M.; Siddiqui, A.J.; Patel, M.; Alshammari, N.; Alharethi, S.H.; Alhassan, H.H.; Bardakci, F.; Hassan, M.I. Structure-Guided Approach to Discover Tuberosin as a Potent Activator of Pyruvate Kinase M2, Targeting Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 13172. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.J.; Jeong, J.K.; Kwon, Y.; Ryu, J.S.; Mun, S.J.; Kim, H.J.; Kim, S.W.; Yoo, S.; Kook, J.; Lee, H.; et al. A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming. Exp. Mol. Med. 2018, 50, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.Y.; Wang, X.; Wang, Z.Y.; Wang, Y.Z.; Chen, L.W.; Luo, Z.J. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J. Neurosci. Res. 2013, 91, 30–41. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.; He, B.; Yang, R.; Zhang, Y.; Shen, Z.; Chen, P.; Du, W. Geraniin promotes osteoblast proliferation and differentiation via the activation of Wnt/β-catenin pathway. Biomed. Pharmacother. 2018, 99, 319–324. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Cha, P.H.; Hwang, J.H.; Kwak, D.K.; Koh, E.; Kim, K.S.; Choi, K.Y. APC loss induces Warburg effect via increased PKM2 transcription in colorectal cancer. Br. J. Cancer 2021, 124, 634–644. [Google Scholar] [CrossRef]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Lee, D.H.; Shim, J.; Park, J.; Kim, Y.R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.H.; Choi, K.Y. KY19382, a novel activator of Wnt/β-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.S.; Min, D.S.; Kim, H.Y.; Choi, K.Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, P.J.; Moon, B.S.; Lee, S.H.; Kim, S.N.; Kim, A.R.; Kim, H.J.; Park, W.S.; Choi, K.Y.; Cho, E.G.; Lee, T.R. Hair growth-promoting effect of Aconiti Ciliare Tuber extract mediated by the activation of Wnt/β-catenin signaling. Life Sci. 2012, 91, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.K.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Kim, H.Y.; Cha, P.H.; Seo, S.H.; Lee, C.; Choi, Y.; Shin, W.; Heo, Y.; Han, G.; Lee, W.; et al. CXXC5 mediates growth plate senescence and is a target for enhancement of longitudinal bone growth. Life Sci. Alliance 2019, 2, e201800254. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Wu, N.; Asara, J.M.; Cantley, L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 2008, 452, 181–186. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lyle, S.; Christofidou-Solomidou, M.; Liu, Y.; Elder, D.E.; Albelda, S.; Cotsarelis, G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci. 1998, 111 Pt 21, 3179–3188. [Google Scholar] [CrossRef]
- Linas, S.L.; Nies, A.S. Minoxidil. Ann. Intern. Med. 1981, 94, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, J.; Jiang, Z.; Wang, B.; Wang, Y.; Hu, X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011, 30, 4297–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Luedtke, M.A.; Prouty, S.M.; Burrows, M.; Kollias, N.; Cotsarelis, G. Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy. Ski. Res. Technol. 2011, 17, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Premanand, A.; Reena Rajkumari, B. Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia. Arch. Dermatol. Res. 2018, 310, 391–399. [Google Scholar] [CrossRef]
- Dinh, Q.Q.; Sinclair, R. Female pattern hair loss: Current treatment concepts. Clin. Interv. Aging 2007, 2, 189–199. [Google Scholar]
- Sahin, G.; Pancar, G.S.; Kalkan, G. New pattern hair loss in young Turkish women; What's wrong in their daily life? Ski. Res. Technol. 2019, 25, 367–374. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Krajewska-Włodarczyk, M.; Kruszewska, A.; Banasiak, Ł.; Placek, W.; Maksymowicz, W.; Wojtkiewicz, J. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018, 2018, 1049641. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, C.; Brahmbhatt, A.; Deng, S.X.; Zheng, J.J. Wnt signaling activation: Targets and therapeutic opportunities for stem cell therapy and regenerative medicine. RSC Chem. Biol. 2021, 2, 1144–1157. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Wang, S.; Zheng, L.; Sun, Y.; Wang, G.; Song, Z.; Bao, Y. TSP50 promotes the Warburg effect and hepatocyte proliferation via regulating PKM2 acetylation. Cell Death Dis. 2021, 12, 517. [Google Scholar] [CrossRef]
- Qin, S.; Yang, D.; Chen, K.; Li, H.; Zhang, L.; Li, Y.; Le, R.; Li, X.; Gao, S.; Kang, L. Pkm2 can enhance pluripotency in ESCs and promote somatic cell reprogramming to iPSCs. Oncotarget 2017, 8, 84276–84284. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Israelsen, W.J.; Lee, D.; Yu, V.W.C.; Jeanson, N.T.; Clish, C.B.; Cantley, L.C.; Vander Heiden, M.G.; Scadden, D.T. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell 2014, 158, 1309–1323. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, Y.C.; Kim, Y.-R.; Park, J.; Choi, S.; Ryu, W.-J.; Kim, G.-U.; Kim, E.; Hwang, Y.; Kim, H.; Han, G.; et al. Pyruvate Kinase M2 Promotes Hair Regeneration by Connecting Metabolic and Wnt/β-Catenin Signaling. Pharmaceutics 2022, 14, 2774. https://doi.org/10.3390/pharmaceutics14122774
Ryu YC, Kim Y-R, Park J, Choi S, Ryu W-J, Kim G-U, Kim E, Hwang Y, Kim H, Han G, et al. Pyruvate Kinase M2 Promotes Hair Regeneration by Connecting Metabolic and Wnt/β-Catenin Signaling. Pharmaceutics. 2022; 14(12):2774. https://doi.org/10.3390/pharmaceutics14122774
Chicago/Turabian StyleRyu, Yeong Chan, You-Rin Kim, Jiyeon Park, Sehee Choi, Won-Ji Ryu, Geon-Uk Kim, Eunhwan Kim, Yumi Hwang, Heejene Kim, Gyoonhee Han, and et al. 2022. "Pyruvate Kinase M2 Promotes Hair Regeneration by Connecting Metabolic and Wnt/β-Catenin Signaling" Pharmaceutics 14, no. 12: 2774. https://doi.org/10.3390/pharmaceutics14122774






