Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease
Abstract
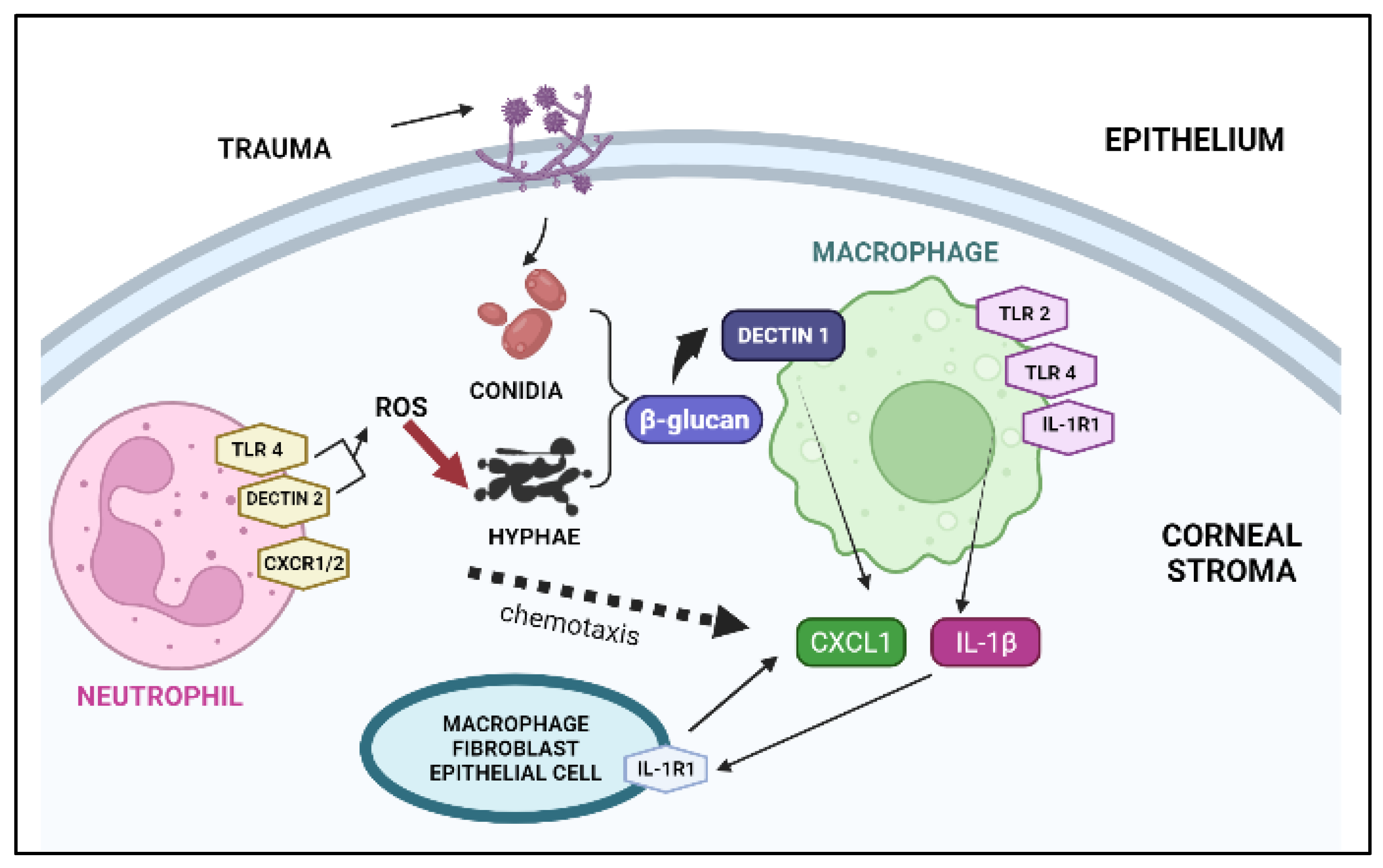
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method Development by RP-HPLC
2.3. Preparation and Optimization of Blank (BC-NPs) and VCZ-Loaded Chitosan Mucoadhesive Nanoparticles (VCZ-MA-NPs)
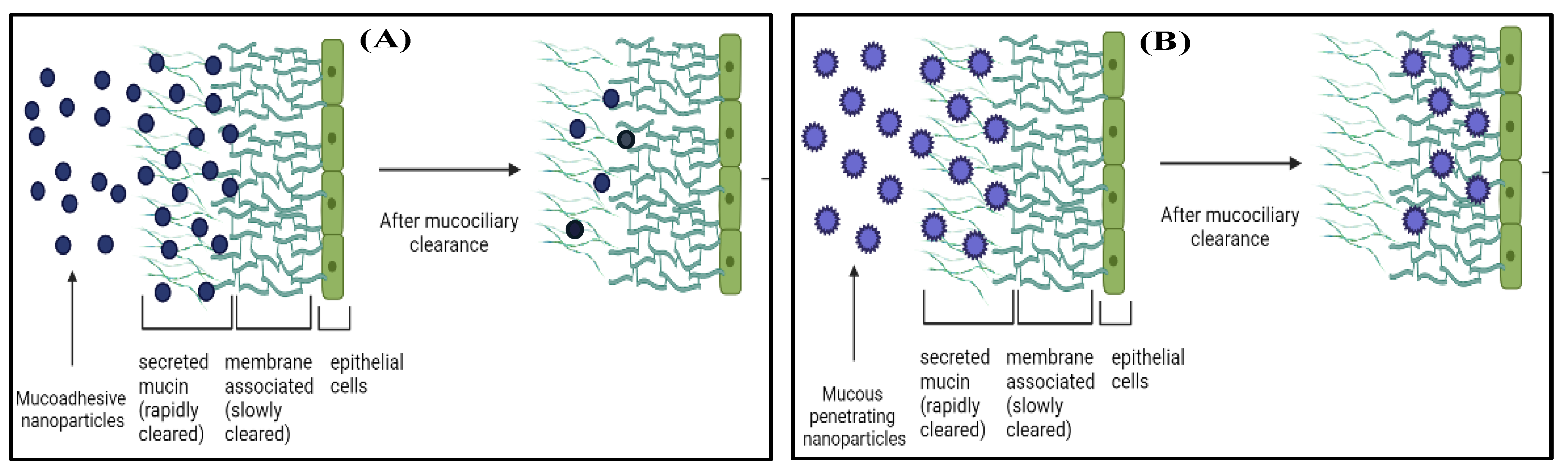
2.4. Preparation and Optimization of VCZ Loaded Mucous Penetrating Nanoparticles (VCZ-MP-NPs)
2.5. In Vitro Characterization of VCZ-MA-NPs and VCZ-MP-NPs
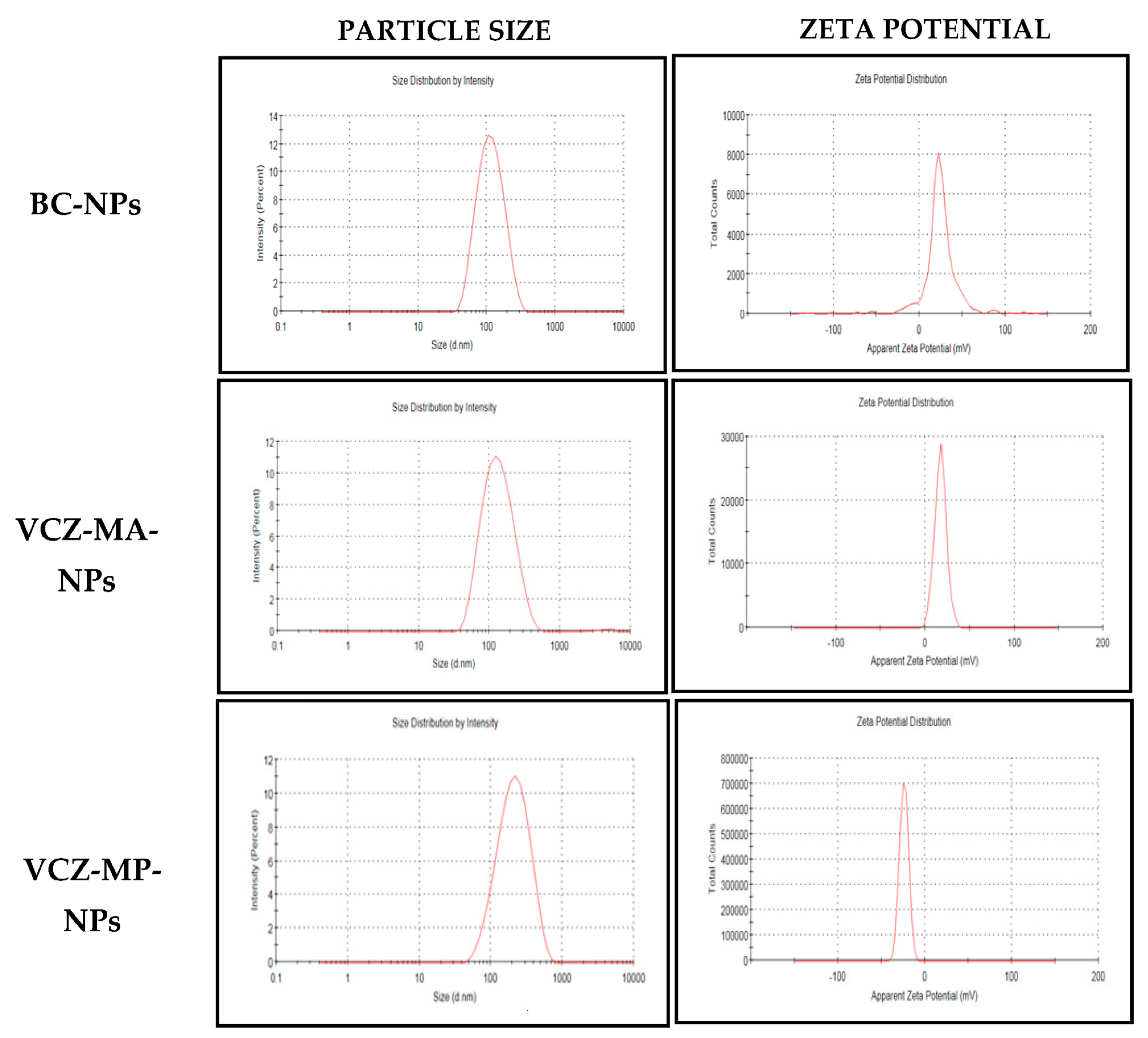
2.5.1. Particle Size, Polydispersity Index (PDI) and ζ Potential Measurement
2.5.2. Entrapment Efficiency and Drug Loading
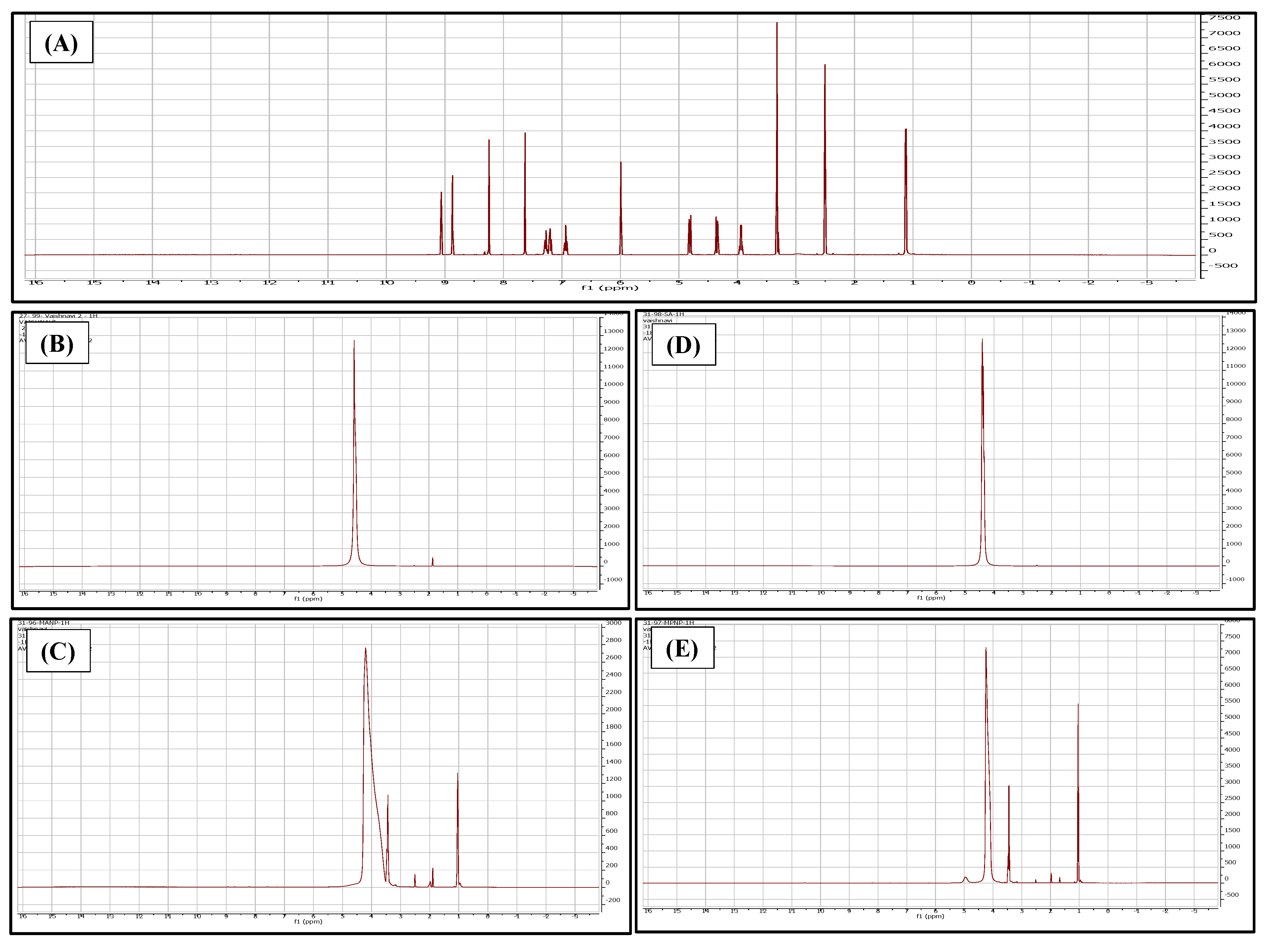
2.5.3. Compatibility Study
2.5.4. Nuclear Magnetic Resonance (NMR)
2.5.5. pH and Osmolarity Measurement
2.5.6. Electron Microscopy
Scanning Electron Microscopy (SEM)
Transmission Electron Microscopy (TEM)
2.5.7. Physical Stability Study
2.5.8. In Vitro Drug Release Study
2.5.9. Ocular Irritancy Test
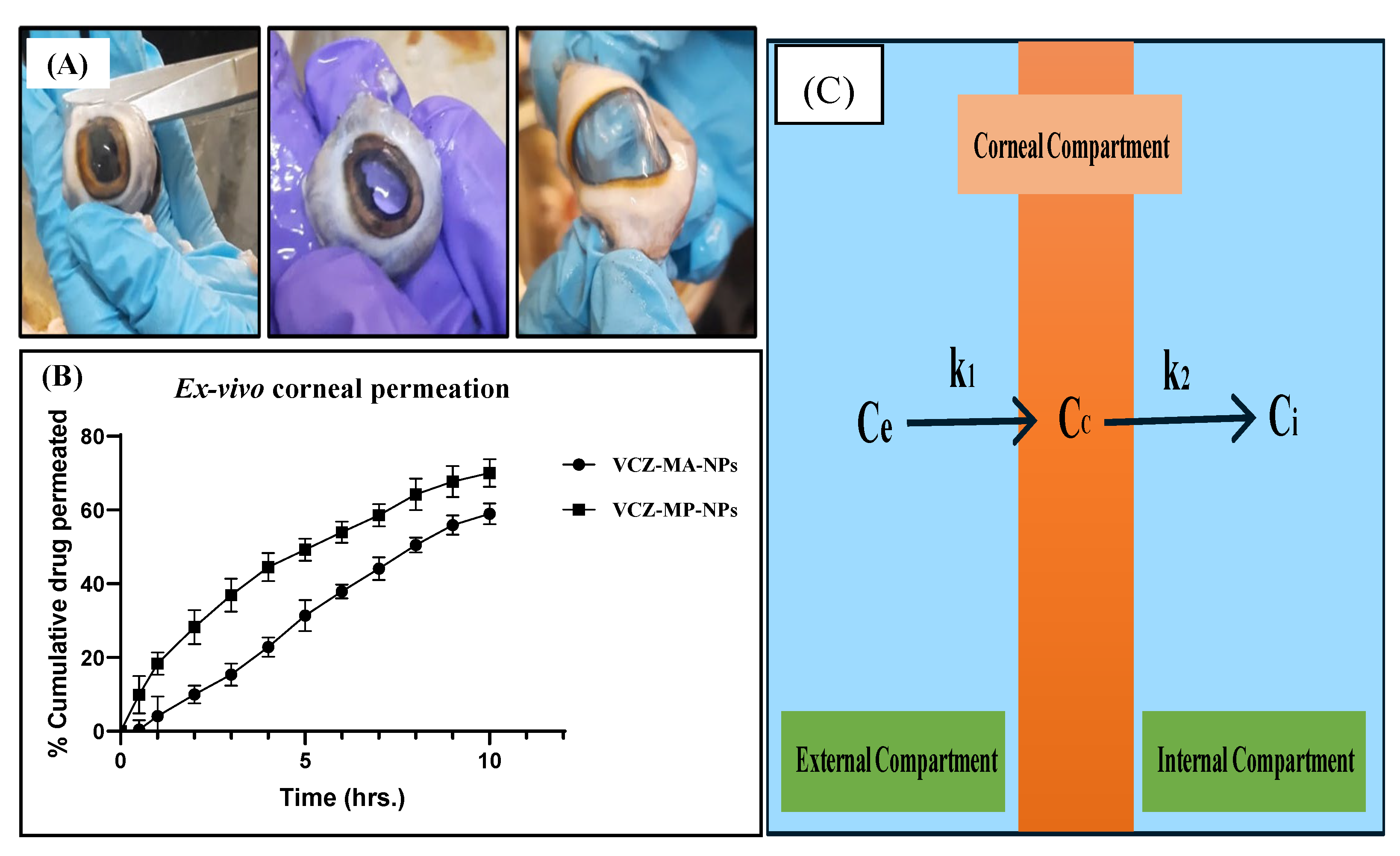
2.5.10. Ex Vivo Corneal Permeation Study
2.6. Statistical Analysis
3. Results and Discussion
Preparation and Optimization of Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, J.J.; Burton, M.J.; Leck, A. Mycotic keratitis—A global threat from the Filamentous fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Yoon, K.; Ryu, S.; Cho, N.-C.; You, I. Clinical aspects and prognosis of mixed microbial (bacterial and fungal) keratitis. Cornea 2011, 30, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2020, 21, E49–E57. [Google Scholar] [CrossRef] [PubMed]
- Ansari, Z.; Miller, D.; Galor, A. Current thoughts in fungal keratitis: Diagnosis and treatment. Curr. Fungal Infect. Rep. 2014, 7, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Gopinathan, U.; Sharma, S.; Garg, P.; Rao, G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthlamology 2009, 57, 273–279. [Google Scholar] [CrossRef]
- Qiao, G.L.; Ling, J.; Wong, T.; Yeung, S.N.; Iovieno, A. Candida keratitis: Epidemiology, management, and clinical outcomes. Clin. Sci. 2020, 39, 801–805. [Google Scholar] [CrossRef]
- Ahearn, D.G.; Zhang, S.; Stulting, R.D.; Schwam, B.L.; Simmons, R.B.; Ward, M.A.; Pierce, G.E.; Crow, S.A.; Practice, P. Fusarium keratitis and contact lens wear: Facts and speculations. Med. Mycol. 2008, 46, 397–410. [Google Scholar] [CrossRef] [Green Version]
- Acharya, Y.; Acharya, B.; Karki, P. Fungal keratitis: Study of increasing trend and common determinants. Nepal J. Epidemiol. 2017, 7, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Castano, G.; Elnahry, A.G.; Mada, P.K. Fungal Keratitis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Knutsson, K.A.; Iovieno, A.; Matuska, S.; Fontana, L.; Rama, P. Topical corticosteroids and fungal keratitis: A review of the literature and case series. J. Clin. Med. 2021, 10, 1178. [Google Scholar] [CrossRef]
- Santacruz, C.; Linares, M.; Garfias, Y.; Loustaunau, L.M. Expression of IL-8, IL-6 and IL-1 β in tears as a main characteristic of the immune response in human microbial keratitis. Int. J. Mol. Sci. 2015, 16, 4850–4864. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2019, 19, 103802. [Google Scholar] [CrossRef]
- Donovan, C.; Arenas, E.; Ayyala, R.S.; Margo, C.E.; Espana, E.M. Fungal keratitis: Mechanisms of infection and management strategies. Surv. Ophthlmol. 2022, 67, 758–769. [Google Scholar] [CrossRef]
- Tollemar, J.; Klingspor, L.; Ringdén, O. Liposomal amphotericin B (AmBisome) for fungal infections in immunocompromised adults and children. Clin. Microbiol. Infect. 2001, 7, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Delma, F.Z.; Al-Hatmi, A.M.S.; Brüggemann, R.J.M.; Melchers, W.J.G.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular mechanisms of 5-fluorocytosine resistance in yeasts and filamentous fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef]
- Greer, N.D. Voriconazole: The newest triazole antifungal agent. BUMC Proc. 2003, 16, 241–248. [Google Scholar] [CrossRef]
- Bunya, V.Y.; Hammersmith, K.M.; Rapuano, C.J.; Ayres, B.D.; Cohen, E.J. Topical and oral voriconazole in the treatment of fungal keratitis. Br. Rep. 2006, 143, 151–153. [Google Scholar] [CrossRef]
- Mehra, N.K.; Cai, D.; Kuo, L.; Hein, T.; Palakurthi, S. Safety and toxicity of nanomaterials for ocular drug delivery applications. Nanotoxicology 2016, 10, 836–860. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and cornealwound healing with chitosan-based delivery systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Feczkó, T. Polymeric nanotherapeutics acting at special regions of body. J. Drug Deliv. Sci. Technol. 2021, 64, 102597. [Google Scholar] [CrossRef]
- Valamla, B.; Thakor, P.; Phuse, R.; Dalvi, M.; Kharat, P. Engineering drug delivery systems to overcome the vaginal mucosal barrier: Current understanding and research agenda of mucoadhesive formulations of vaginal delivery. J. Drug Deliv. Sci. Technol. 2022, 70, 103162. [Google Scholar] [CrossRef]
- Cordeiro, S.; Silva, B.; Martins, A.M.; Ribeiro, H.M.; Gonçalves, L.; Marto, J. Antioxidant-loaded mucoadhesive nanoparticles for eye drug delivery: A new strategy to reduce oxidative stress. Processes 2021, 9, 379. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, J.; Gonçalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochizuki, H.; Yamada, M.; Hatou, S.; Tsubota, K. Turnover rate of tear-film lipid layer determined by fluorophotometry. Br. J. Ophthlamology. 2009, 93, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Popov, A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J. Ocul. Pharmacol. Ther. 2020, 36, 366–375. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin sulfate: Emerging biomaterial for biopharmaceutical purpose and tissue engineering. Carbohydr. Polym. 2022, 286, 119305–119334. [Google Scholar] [CrossRef]
- Schattling, P.; Taipaleenmäki, E.; Zhang, Y.; Städler, B. A polymer chemistry point of view on mucoadhesion and mucopenetration. Macromol. Biosci. 2017, 17, 1700060. [Google Scholar] [CrossRef]
- Ojha, B.; Jain, V.K.; Mehra, N.K.; Jain, K. Nanotechnology: Introduction and basic concepts. In Dendrimers in Nanomedicine; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–17. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L.; Barbosa, S.; Ferreira, D.S. Mucoadhesive and mucus-penetrating polymers for drug delivery. In Nanotechnology for Oral Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 77–141. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple roles of chitosan in mucosal drug delivery: An updated review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef]
- Shilpi, S.; Dhakad, U.; Lodhi, R.; Dixit, S.; Khatri, K.; Mehra, N.K.; Gulbake, A. Role of polymers in formulation design and drug delivery. In Micro-Nanotechnologies-Based Product Development, 1st ed.; Mehra, N.K., Gulbake, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 243–255. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. J. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [Green Version]
- Srinubabu, G.; Raju, C.A.I.; Sarath, N.; Kumar, P.K.; Rao, J.V.L.N.S. Development and validation of a HPLC method for the determination of voriconazole in pharmaceutical formulation using an experimental design. Talanta 2007, 71, 1424–1429. [Google Scholar] [CrossRef]
- Abruzzo, A.; Giordani, B.; Miti, A.; Vitali, B.; Zuccheri, G.; Cerchiara, T.; Luppi, B. Mucoadhesive and mucopenetrating chitosan nanoparticles for glycopeptide antibiotic administration. Int. J. Pharm. 2021, 606, 120874. [Google Scholar] [CrossRef]
- Fabregas, A.; Minarro, M.; Garcia-Montoyo, E.; Perez-Lozano, P.; Sarrate, R.; Sánchez, N.; Ticó, J.R.; Su, J.M. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan-tripolyphosphate nanoparticles. Int. J. Pharm. 2013, 446, 199–204. [Google Scholar] [CrossRef]
- Rathore, P.; Mahor, A.; Jain, S. Formulation development, in vitro and in vivo evaluation of chitosan engineered nanoparticles for ocular delivery of insulin. RSC Adv. 2020, 10, 43629–43639. [Google Scholar] [CrossRef]
- Kumar, A.; Valamla, B.; Thakor, P.; Chary, P.S.; Rajana, N.; Mehra, N.K. Development and evaluation of nanocrystals loaded hydrogel for topical application. J. Drug Deliv. Sci. Technol. 2022, 74, 103503. [Google Scholar] [CrossRef]
- Teba, H.E.; Khalil, I.A.; el Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef]
- Dubey, V.; Mohan, P.; Dangi, J.S.; Kesavan, K. Brinzolamide loaded chitosan-pectin mucoadhesive nanocapsules for management of glaucoma: Formulation, characterization and pharmacodynamic study. Int. J. Biol. Macromol. 2019, 152, 1224–1232. [Google Scholar] [CrossRef]
- Bin-jumah, M.; Gilani, S.J.; Kala, C. Clarithromycin-loaded ocular chitosan nanoparticle: Formulation, optimization, characterization, ocular irritation, and antimicrobial activity. Int. J. Nanomed. 2020, 15, 7861–7875. [Google Scholar] [CrossRef]
- ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method. 2010; pp. 3–30. Available online: https://ntp.niehs.nih.gov/iccvam/docs/ocutox_docs/invitro-2010/tmer-vol1.pdf (accessed on 8 July 2022).
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Dadou, S.M.; El-Barghaouthi, M.I.; Alabdallah, S.K.; Badwan, A.A.; Antonijevic, M.D.; Chowdhry, B.Z. Effect of protonation state and N-acetylation of chitosan on its interaction with xanthan gum: A Molecular Dynamics Simulation Study. Mar. Drugs 2017, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Masarudin, M.J.; Cutts, S.M.; Evison, B.J.; Phillips, D.R.; Pigram, P.J. Factors determining the stability, size distribution, and cellular accumulation of small, monodisperse chitosan nanoparticles as candidate vectors for anticancer drug delivery: Application to the passive encapsulation of 14C.-doxorubicin. Nanotechnol. Sci. App. 2015, 8, 67–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate nanoparticles as ocular drug delivery carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Feng, Y.; Jinyong, Z.; Du, Y.; Jiang, Z.; van Ongeval, C.; Ni, Y.; Li, Y. Utilisation of chick embryo chorioallantoic membrane as a model platform for imaging-navigated biomedical research. Cells 2021, 463, 463. [Google Scholar] [CrossRef]
- Wong, E.A.; Uni, Z. Centennial review: The chicken yolk sac is a multifunctional organ. Poult. Sci. 1974, 100, 100821. [Google Scholar] [CrossRef]
- Valls, R.; Vega, E.; Garcia, M.L.; Egea, M.A.; Valls, J.O. Transcorneal permeation in a corneal device of non-steroidal anti-inflammatory drugs in drug delivery systems. Open Med. Chem. J. 2008, 2, 66–71. [Google Scholar] [CrossRef]






| Effect | Score | ||
|---|---|---|---|
| 0.5 min | 2 min | 5 min | |
| Hemorrhage | 7 | 5 | 3 |
| Vascular lysis | 5 | 3 | 1 |
| Coagulation | 9 | 7 | 5 |
| Batch | Particle Size (nm) | PDI |
|---|---|---|
| Without pH adjustment of the chitosan solution | 559 ± 52 | 0.58 ± 0.02 |
| With pH adjustment to 4.6 using 0.1 M sodium hydroxide | 221 ± 16 | 0.47 ± 0.03 |
| Batch | With pH Adjustment to 4.6 | |
|---|---|---|
| Stirring Time | Particle Size ± SD (nm) | PDI ± SD |
| 1 h | 221 ± 16 | 0.47 ± 0.03 |
| 4 h | 206 ± 16 | 0.45 ± 0.03 |
| 7 h | 182 ± 02 | 0.41 ± 0.06 |
| 20 h | 155 ± 02 | 0.22 ± 0.01 |
| Batch | With pH Adjustment to 4.6 with 20 h Stirring | |
|---|---|---|
| Sonication Time | Mean Particle Size ± SD (nm) | Mean PDI ± SD |
| 1 min | 118 ± 1 | 0.27 ± 0.004 |
| 2 min | 102 ± 1.7 | 0.20 ± 0.005 |
| 3 min | 113 ± 0.8 | 0.28 ± 0.02 |
| 4 min | 143 ± 4.1 | 0.35 ± 0.02 |
| 5 min | 99 ± 1.4 | 0.19 ± 0.002 |
| S. No. | Mass Ratio Alginate (10 mg/mL): VCZ-MA | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| 1. | 1:1 | 1583 ± 16 | 0.67 ± 0.15 | −42 ± 2.1 |
| 2. | 1:2 | 862 ± 17 | 0.68 ± 0.09 | −40 ± 4.4 |
| 3. | 1:3 | 758 ± 24 | 0.79 ± 0.21 | −38 ± 5.0 |
| 4. | 1:4 | 699 ± 22 | 0.95 ± 0.08 | −33 ± 3.2 |
| 5. | 1:5 | 652 ± 11 | 0.55 ± 0.09 | −34 ± 4.8 |
| Alginate Concentration | Mass Ratio Alginate: VCZ-MA-NPs | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| 10 mg/mL | 1:5 | 652 ± 11.2 | 0.55 ± 0.09 | −34 ± 4.8 |
| 5 mg/mL | 1:5 | 341 ± 13.1 | 0.43 ± 0.01 | −29 ± 0.9 |
| 2 mg/mL | 1:5 | 185 ± 1 | 0.20 ± 0.01 | −24 ± 0.9 |
| S. No. | Batch Specifications | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| 1. | Chitosan (5–20 Mpa) in 1 % acetic acid at 4.6 pH adjusted with 0.1 N NaOH and STPP (2 mg/mL), 5 min sonication | 99 ± 1 | 0.19 ± 0.002 | +15 ± 2.9 |
| 2. | Chitosan (5–20 Mpa) in 1 % Acetic acid at 4.6 pH adjusted with 0.1 N NaOH, VCZ (20 mg) and STPP (2 mg/mL), 5 min sonication | 116 ± 2 | 0.23 ± 0.004 | +16 ± 0.9 |
| 3. | Optimized VCZ-MA-NPs and sodium alginate solution (2 mg/mL) in 5:1 | 185 ± 1 | 0.20 ± 0.01 | −24 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhosale, V.A.; Srivastava, V.; Valamla, B.; Yadav, R.; Singh, S.B.; Mehra, N.K. Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics 2022, 14, 2802. https://doi.org/10.3390/pharmaceutics14122802
Bhosale VA, Srivastava V, Valamla B, Yadav R, Singh SB, Mehra NK. Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics. 2022; 14(12):2802. https://doi.org/10.3390/pharmaceutics14122802
Chicago/Turabian StyleBhosale, Vaishnavi A., Vaibhavi Srivastava, Bhavana Valamla, Rati Yadav, Shashi Bala Singh, and Neelesh Kumar Mehra. 2022. "Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease" Pharmaceutics 14, no. 12: 2802. https://doi.org/10.3390/pharmaceutics14122802
APA StyleBhosale, V. A., Srivastava, V., Valamla, B., Yadav, R., Singh, S. B., & Mehra, N. K. (2022). Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics, 14(12), 2802. https://doi.org/10.3390/pharmaceutics14122802





