Thymoquinone: Hydroxypropyl-β-cyclodextrin Loaded Bacterial Cellulose for the Management of Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media and Reagents
2.2. Microorganisms
2.3. Active Pharmaceutical Ingredient (API) Extraction
2.4. Production and Purification of Bacterial Cellulose
2.5. Preparation of THQ:HPβCD-Inclusion Complex
2.6. Preparation of Formulations
2.7. Thermal Degradation
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Permeation Studies
2.10. Adhesion Testing
2.11. Crystal Growth
2.12. Statistical Analysis
3. Results and Discussion
3.1. Solubility of API
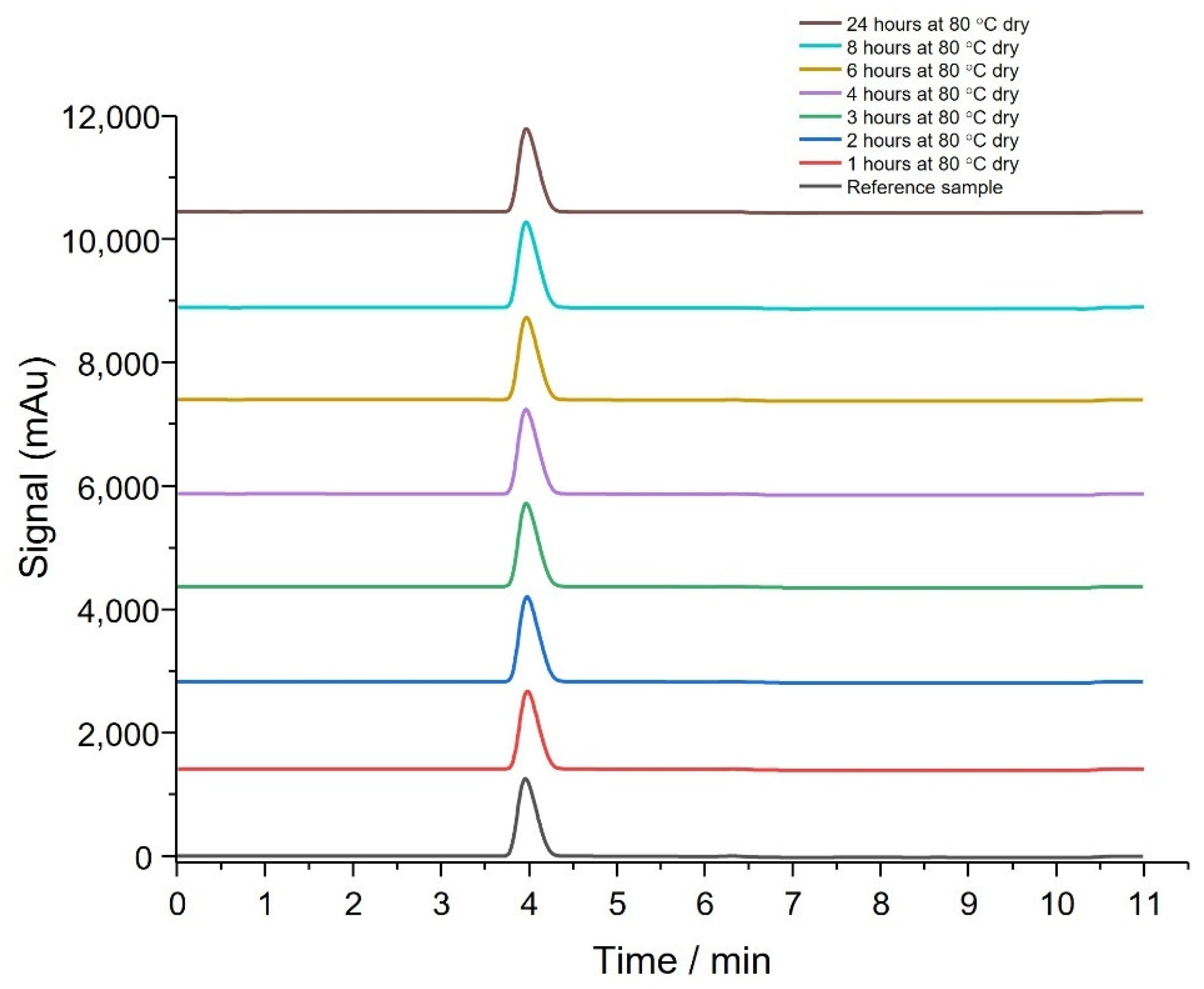
3.2. Thermal Stability of API
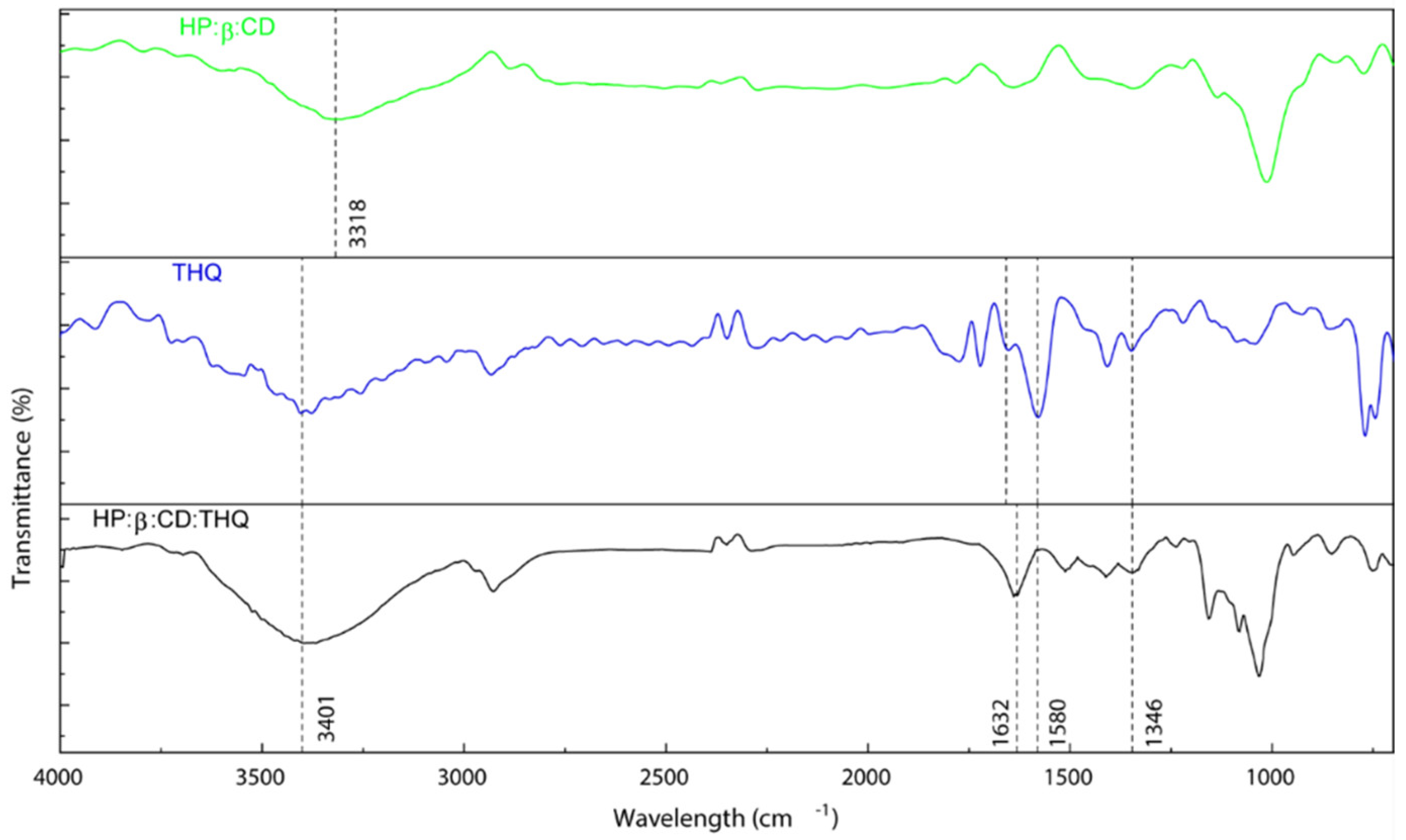
3.3. FTIR Confirmation
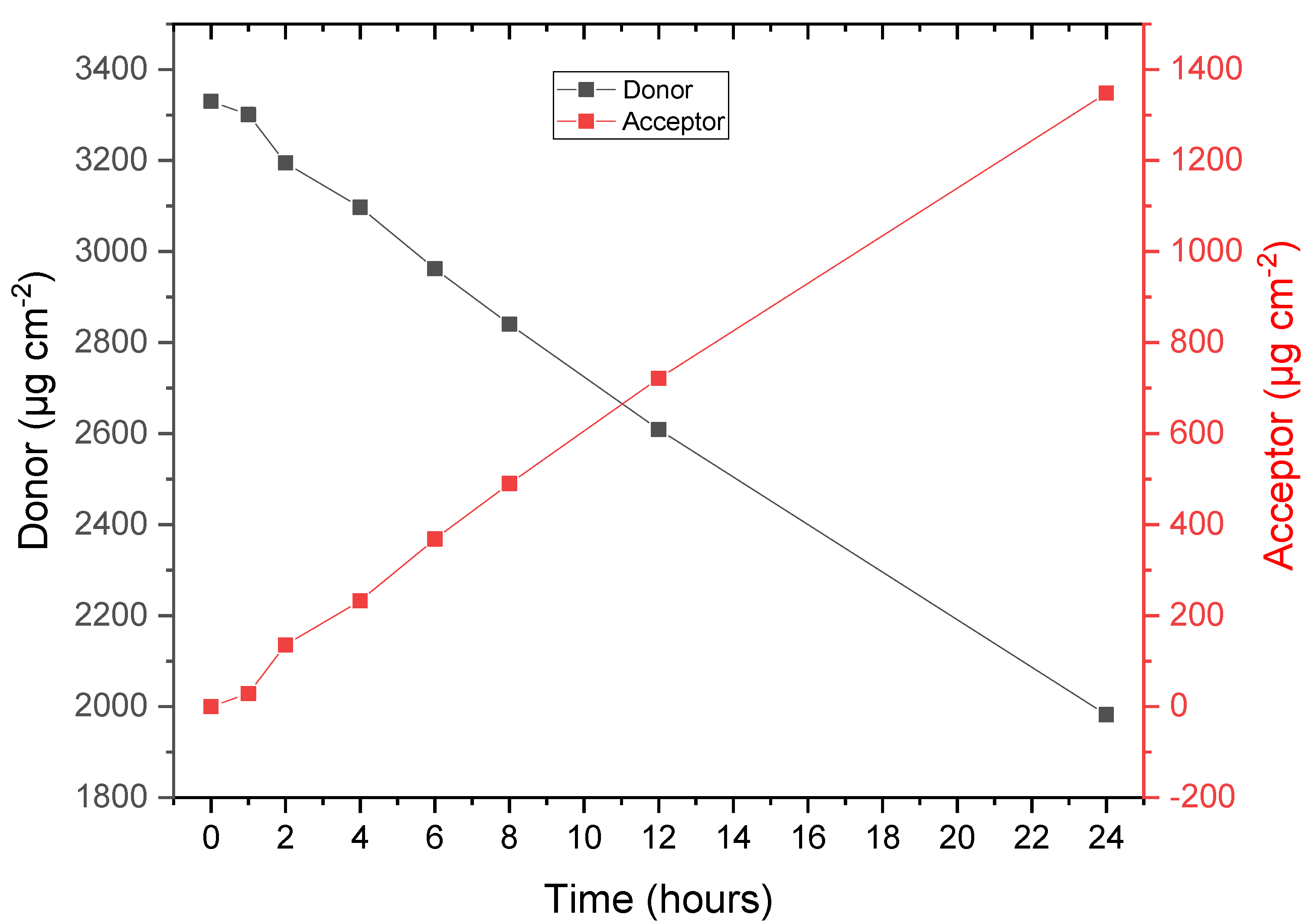
3.4. API Permeation and Extraction
3.5. Adhesion
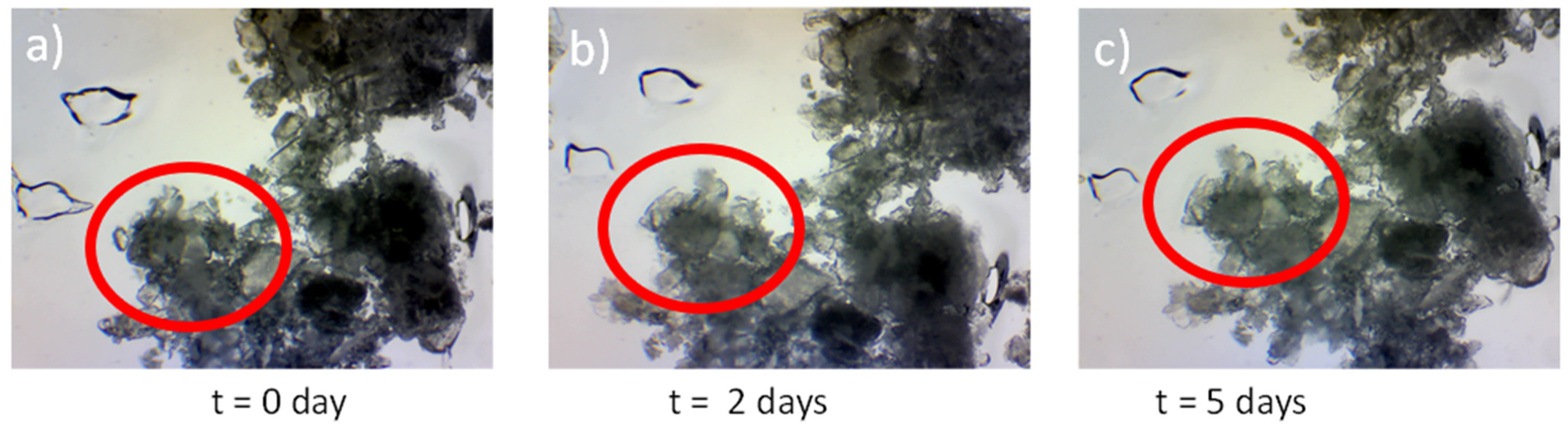
3.6. Crystal Growth
4. Further Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Heaselgrave, W.; Kowalczuk, M.; Adamus, G.; Radecka, I. The Mould War: Developing an Armamentarium against Fungal Pathogens Utilising Thymoquinone, Ocimene, and Miramistin within Bacterial Cellulose Matrices. Materials 2021, 14, 2654. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.D. Bacteria and Antibiotics in Wound Healing. Surg. Clin. N. Am. 2020, 100, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Riccardi, N.; Vena, A.; Bassetti, M. Mould Infections of Traumatic Wounds: A Brief Narrative Review. Infect. Dis. Ther. 2020, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Caref. 2014, 3, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Volova, T.G.; Shumilova, A.A.; Nikolaeva, E.D.; Kirichenko, A.K.; Shishatskaya, E.I. Biotechnological Wound Dressings Based on Bacterial Cellulose and Degradable Copolymer P(3HB/4HB). Int. J. Biol Macromol. 2019, 131, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Weir, D. Wound Dressings in Local Wound Care for Dermatologists; Alavi, A., Maibach, H.I., Eds.; Springer Nature: Geneva, Switzerland, 2020; pp. 25–34. [Google Scholar] [CrossRef]
- Ajiteru, O.; Lee, O.J.; Kim, J.-H.; Lee, Y.J.; Lee, J.S.; Lee, H.; Sultan, M.T.; Park, C.H. Fabrication and Characterization of a Myrrh Hydrocolloid Dressing for Dermal Wound Healing. Colloid Interface Sci. Commun. 2022, 48, 100617. [Google Scholar] [CrossRef]
- Anton-Sales, I.; Beekmann, U.; Laromaine, A.; Roig, A.; Kralisch, D. Opportunities of Bacterial Cellulose to Treat Epithelial Tissues. Curr. Drug Targets 2019, 20, 808–822. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic Polymeric Biomaterials for Wound Healing: A Review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Yang, H.; Zeng, L.; Hu, H.; Hu, C. Production and Characterization of Bacterial Cellulose Obtained by Gluconacetobacter Xylinus Utilizing the By-Products from Baijiu Production. Bioprocess Biosyst. Eng. 2020, 43, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and Metabolic Analysis of Komagataeibacter Xylinus DSM 2325 Producing Bacterial Cellulose Nanofiber. Biotechnol. Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin Tissue Regeneration for Burn Injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional Cellulose-Based Hydrogels as Extracellular Matrices for Tissue Engineering. J. Biol. Eng. 2019, 13, 55. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Ahmed, J.; Gunduz, O.; Edirisinghe, M. Fiber Forming Capability of Binary and Ternary Compositions in the Polymer System: Bacterial Cellulose–Polycaprolactone–Polylactic Acid. Polymers 2019, 11, 1148. [Google Scholar] [CrossRef] [Green Version]
- Takahama, R.; Kato, H.; Takayama, G.; Tajima, K.; Kondo, T. Physical Characteristics and Cell-Adhesive Properties of in Vivo Fabricated Bacterial Cellulose/Hyaluronan Nanocomposites. Cellulose 2022, 29, 3239–3251. [Google Scholar] [CrossRef]
- Khodamoradi, N.; Babaeipour, V.; Sirousazar, M. Bacterial Cellulose/Montmorillonite Bionanocomposites Prepared by Immersion and In-Situ Methods: Structural, Mechanical, Thermal, Swelling and Dehydration Properties. Cellulose 2019, 26, 7847–7861. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and Characterisation of Bacterial Cellulose Hydrogels Loaded with Curcumin Encapsulated in Cyclodextrins as Wound Dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Gobinath, P.; Packialakshmi, P.; Hatamleh, A.A.; Al-Dosary, M.A.; Al-Wasel, Y.A.; Balasubramani, R.; Surendrakumar, R.; Idhayadhulla, A. Calotropis Gigantea Assisted Synthesis of Zinc Oxide Nanoparticle Catalysis: Synthesis of Novel 3-Amino Thymoquinone Connected 1,4-Dihyropyridine Derivatives and Their Cytotoxic Activity. J. Nanomater. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals 2022, 15, 408. [Google Scholar] [CrossRef]
- Alam, M.; Hasan, G.M.; Ansari, M.M.; Sharma, R.; Yadav, D.K.; Hassan, M.I. Therapeutic Implications and Clinical Manifestations of Thymoquinone. Phytochemistry 2022, 200, 113213. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A Small Molecule from Nature with High Therapeutic Potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef]
- Rayene, K.; Imane, D.; Abdelaziz, B.; Leila, N.; Fatiha, M.; Abdelkrim, G.; Bouzid, G.; Ismahan, L.; Brahim, H.; Rabah, O. Molecular Modeling Study of Structures, Hirschfield Surface, NBO, AIM, RDG, IGM and 1HNMR of Thymoquinone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex from QM Calculations. J. Mol. Struct. 2022, 1249, 131565. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Kumar, R.; Nehul, S.; Singh, A.; Tomar, S. Identification and Evaluation of Antiviral Potential of Thymoquinone, a Natural Compound Targeting Chikungunya Virus Capsid Protein. Virology 2021, 561, 36–46. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for 90 Degree Peel Resistance of Adhesives. Available online: https://www.astm.org/d6862-11r21.html (accessed on 15 October 2022).
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Khan, A.A.; Rahmani, A.H. Thymoquinone, an Active Compound of Nigella Sativa: Role in Prevention and Treatment of Cancer. Curr. Pharm. Biotechnol. 2020, 21, 1028–1041. [Google Scholar] [CrossRef]
- Negi, P.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O.P. Thymoquinone a Potential Therapeutic Molecule from the Plant Nigella Sativa: Role of Colloidal Carriers in Its Effective Delivery. Recent Pat. Drug Deliv. Formul. 2018, 12, 3–22. [Google Scholar] [CrossRef]
- Alamoudi, R.A.; Alamoudi, S.A.; Alamoudi, R.A. Biological Potential of the Main Component, Thymoquinone, of Nigella Sativa in Pulp Therapy—in Vitro Study. Life 2022, 12, 1434. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of Thymoquinone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex: Application to Anti-Allergy Properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, F.; Zhang, H.; Huang, Y.; Yang, J.; Sun, D. Recent Approaches and Future Prospects of Bacterial Cellulose-Based Electroconductive Materials. J. Mater. Sci. 2016, 51, 5573–5588. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The Growing Merits and Dwindling Limitations of Bacterial Cellulose-Based Tissue Engineering Scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Cardoso, T.; Galhano, C.I.C.; Ferreira Marques, M.F.; Moreira da Silva, A. Thymoquinoneβ-Cyclodextrin Nanoparticles System: A Preliminary Study. Spectrosc-Int. J. 2012, 27, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Dinh, L.; Lee, S.; Abuzar, S.M.; Park, H.; Hwang, S.-J. Formulation, Preparation, Characterization, and Evaluation of Dicarboxylic Ionic Liquid Donepezil Transdermal Patches. Pharmaceutics 2022, 14, 205. [Google Scholar] [CrossRef]
- European Medicine Agency. Committee for Medicinal Products for Human Use (CHMP) Guideline on Quality of Transdermal Patches; European Medicine Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hasrawati, A.; Rizaldi, I.; Febrianti, D.; Mursyid, A.M.; Bakri, N.A. Preparation and characterization of thymoquinone nanoparticles pegylated as drug delivery system. Univers. J. Pharm. Res. 2021, 5, 13–17. [Google Scholar] [CrossRef]
- Pagola, S.; Benavente, A.; Raschi, A.; Romano, E.; Molina, M.A.A.; Stephens, P.W. Crystal Structure Determination of Thymoquinone by High-Resolution X-Ray Powder Diffraction. AAPS PharmSciTech 2004, 5, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Shown, I.; Banerjee, S.; Ramchandran, A.V.; Geckeler, K.E.; Murthy, C.N. Synthesis of Cyclodextrin and Sugar-Based Oligomers for the Efavirenz Drug Delivery. Macromol. Symp. 2010, 287, 51–59. [Google Scholar] [CrossRef]
- Alrawashdeh, L.; Assaf, K.I.; Alshaer, W.; Odeh, F.; Bani-Atta, S.A. Preparation, Characterization, and Biological Activity Study of Thymoquinone-Cucurbit[7]Uril Inclusion Complex. RSC Adv. 2022, 12, 1982–1988. [Google Scholar] [CrossRef]
- European Medicines Agency; International Council for Harmonisation. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology Step 5 Note for Guidance on Validation of Analytical Procedures: Text And Methodology; European Medicine Agency: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Peimanfard, S.; Zarrabi, A.; Trotta, F.; Matencio, A.; Cecone, C.; Caldera, F. Developing Novel Hydroxypropyl-β-Cyclodextrin-Based Nanosponges as Carriers for Anticancer Hydrophobic Agents: Overcoming Limitations of Host–Guest Complexes in a Comparative Evaluation. Pharmaceutics 2022, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of Phytochemicals with Cyclodextrins and Their Derivatives- an Update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef] [PubMed]
- Medicines & Healthcare Products Agency. A Guide to What Is a Medicinal Product MHRA Guidance Note 8; Medicines & Healthcare Products Agency: Brussels, Belgium, 2020.
- Rathore, C.; Hemrajani, C.; Sharma, A.K.; Gupta, P.K.; Jha, N.K.; Aljabali, A.A.A.; Gupta, G.; Singh, S.K.; Yang, J.-C.; Dwivedi, R.P.; et al. Self-Nanoemulsifying Drug Delivery System (SNEDDS) Mediated Improved Oral Bioavailability of Thymoquinone: Optimization, Characterization, Pharmacokinetic, and Hepatotoxicity Studies. Drug Deliv. Transl. Res. 2022, 13, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Shariare, M.H.; Khan, M.A.; Al-Masum, A.; Khan, J.H.; Uddin, J.; Kazi, M. Development of Stable Liposomal Drug Delivery System of Thymoquinone and Its in Vitro Anticancer Studies Using Breast Cancer and Cervical Cancer Cell Lines. Molecules 2022, 27, 6744. [Google Scholar] [CrossRef]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral Bioavailability Enhancement and Hepatoprotective Effects of Thymoquinone by Self-Nanoemulsifying Drug Delivery System. Mater. Sci. Eng. C. 2017, 76, 319–329. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound Healing Effects of Nanoemulsion Containing Clove Essential Oil. Artif. Cells Nanomed. Biotechnol. 2016, 45, 591–597. [Google Scholar] [CrossRef]




| Item No | Ingredient | Mass Fraction, % | Scale, mg | |
|---|---|---|---|---|
| 1 | Adhesive | Dry BC | 83.0 | 36.10 |
| 2 | IC (aq) | THQ:HPβCD | 7.0 | 3.04 |
| 3 | Excipient | DMSO | 10.0 | 4.34 |
| Formulation | Dry BC, wt% | THQ:HPβCD, wt% | DMSO, wt% | Loading, Min |
|---|---|---|---|---|
| THQ.009 | 83 | 7 | 10 | 55 |
| THQ.010 | 83 | 7 | 10 | 45 |
| THQ.011 | 83 | 7 | 10 | 50 |
| THQ.012 | 83 | 7 | 10 | 60 |
| THQ.013 | 83 | 7 | 10 | 50 |
| Parameter/API | THQ:HPβCD |
|---|---|
| Release lag time, h | 0 |
| Total released amount (0–24 h), µg cm−2 | 1347.9 |
| Average flux after lag, µg h−1 cm−2 | 56.2 |
| Permeation lag time, h | 2 |
| Total permeated amount (0–24 h), µg cm−2 | 538.8 |
| Average flux after lag, µg h−1 cm−2 | 22.4 |
| Area, mAu | Extracted THQ, mg | THQ Dose, mg cm−2 | Calculation | Dose, mg cm−2 | ±, mg cm−2 | Variation, % |
|---|---|---|---|---|---|---|
| 1956.65 | 1.67 | 3.32 | Mean | 3.31 | ±0.06 | 1.79 |
| 1982.42 | 1.69 | 3.37 | ||||
| 1963.84 | 1.67 | 3.33 | ||||
| 1969.25 | 1.68 | 3.34 | Median | 3.33 | ±0.03 | 0.80 |
| 1891.00 | 1.61 | 3.21 | ||||
| 1918.86 | 1.64 | 3.26 |
| Formulation | Replicates | Max peak | Area, N/mm | Average, N/mm |
|---|---|---|---|---|
| THQ.010 | 1 | 3.0 | 220.9 | 2.0 |
| 2 | 3.1 | 217.4 | 1.9 | |
| 3 | 3.4 | 250.3 | 2.2 | |
| Average | 3.2 | 229.6 | 2.1 | |
| Std Dev | 0.2 | 18.1 | 0.2 | |
| Relative Std Dev, % | 6.2 | 7.9, | 7.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Adamus, G.; Heaselgrave, W.; Radecka, I. Thymoquinone: Hydroxypropyl-β-cyclodextrin Loaded Bacterial Cellulose for the Management of Wounds. Pharmaceutics 2022, 14, 2816. https://doi.org/10.3390/pharmaceutics14122816
Swingler S, Gupta A, Gibson H, Kowalczuk M, Adamus G, Heaselgrave W, Radecka I. Thymoquinone: Hydroxypropyl-β-cyclodextrin Loaded Bacterial Cellulose for the Management of Wounds. Pharmaceutics. 2022; 14(12):2816. https://doi.org/10.3390/pharmaceutics14122816
Chicago/Turabian StyleSwingler, Sam, Abhishek Gupta, Hazel Gibson, Marek Kowalczuk, Grazyna Adamus, Wayne Heaselgrave, and Iza Radecka. 2022. "Thymoquinone: Hydroxypropyl-β-cyclodextrin Loaded Bacterial Cellulose for the Management of Wounds" Pharmaceutics 14, no. 12: 2816. https://doi.org/10.3390/pharmaceutics14122816