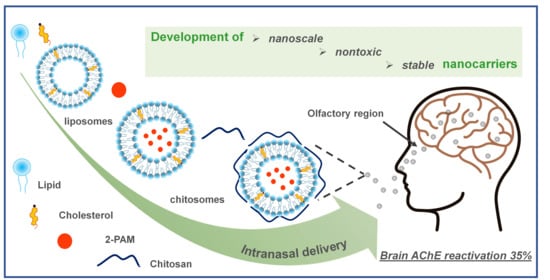
Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Arginine-Chitosan
2.3. Preparation of Chitosomes
2.4. Vesicle Size, Zeta Potential and PdI
2.5. Encapsulation Efficiency
2.6. In Vitro Release of Rhodamine B/2-PAM from Chitosomes
2.7. Hemolytic Activity
2.8. Cytotoxicity Assay and Cellular Uptake Studies
2.9. Animals
2.10. AChE Reactivation in Rat Brain
3. Results
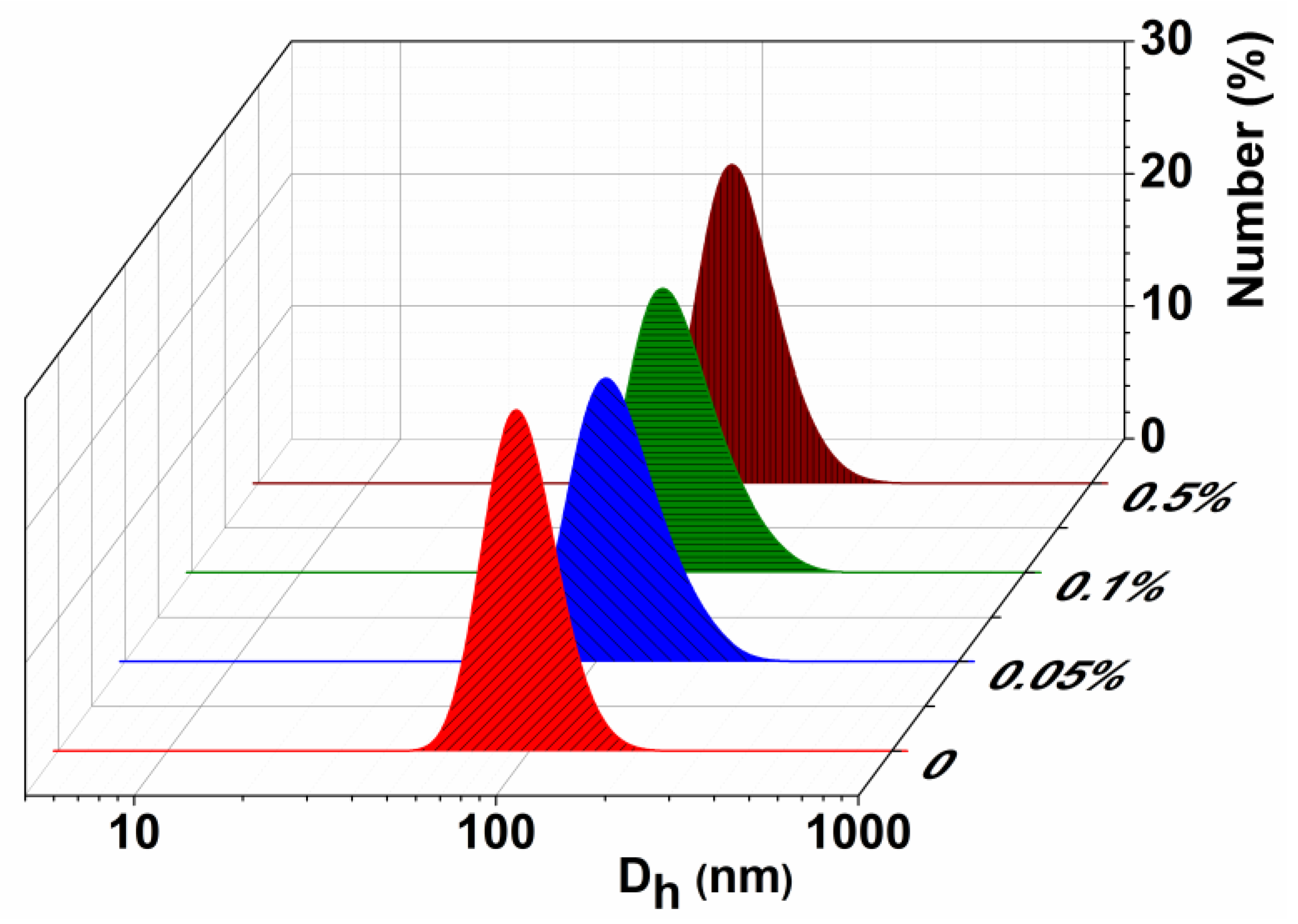
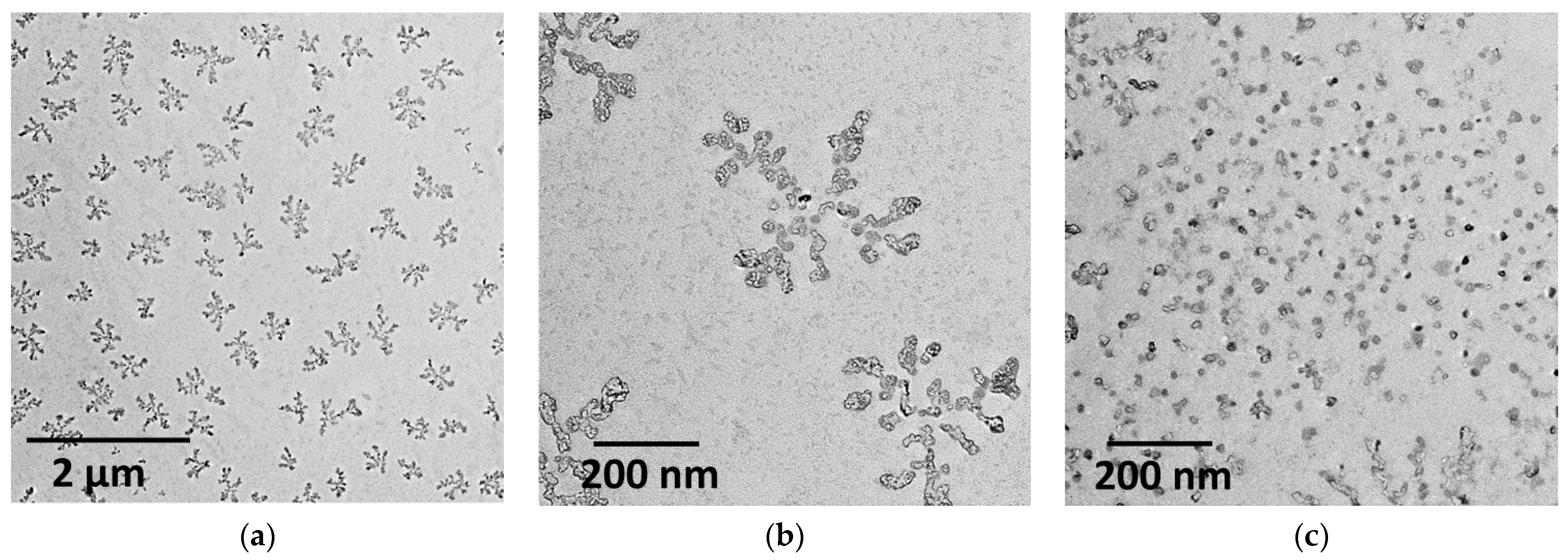
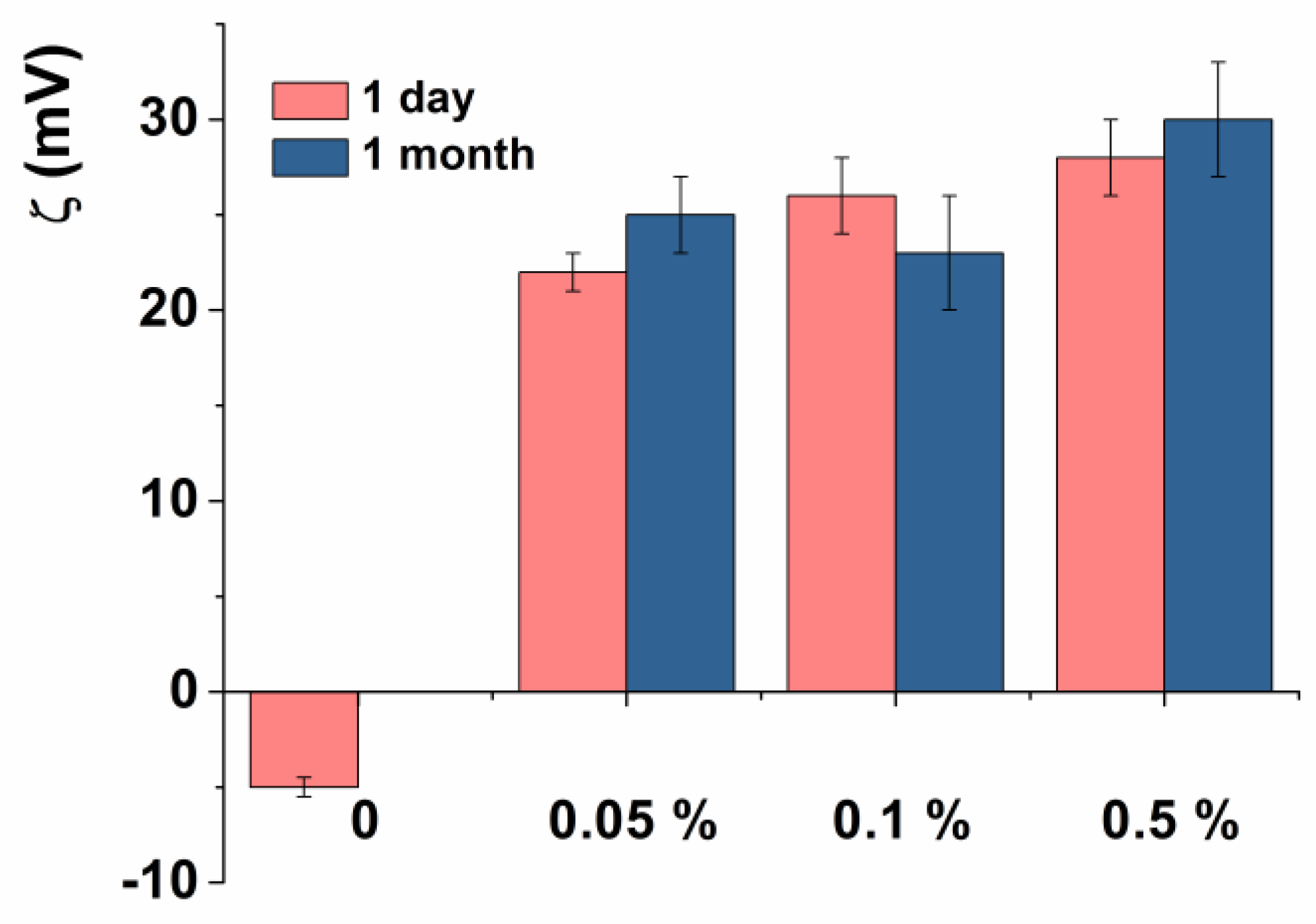
3.1. Characterization of the Empty Chitosan-Coated Liposomes
3.2. Characterization of the Substrate-Loaded Chitosomes
3.3. Encapsulation Efficiency (EE)
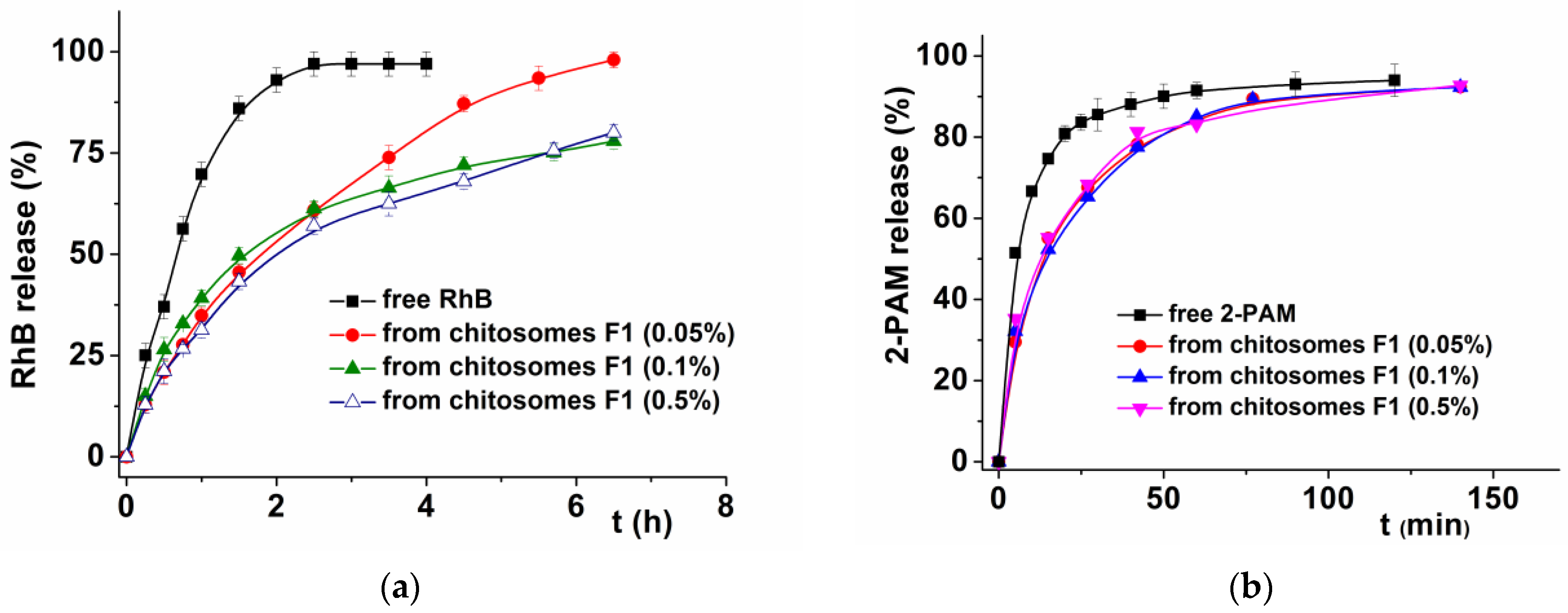
3.4. In Vitro Release Study and Kinetics
3.5. Analysis of Hemolytic Activity and Cytotoxicity
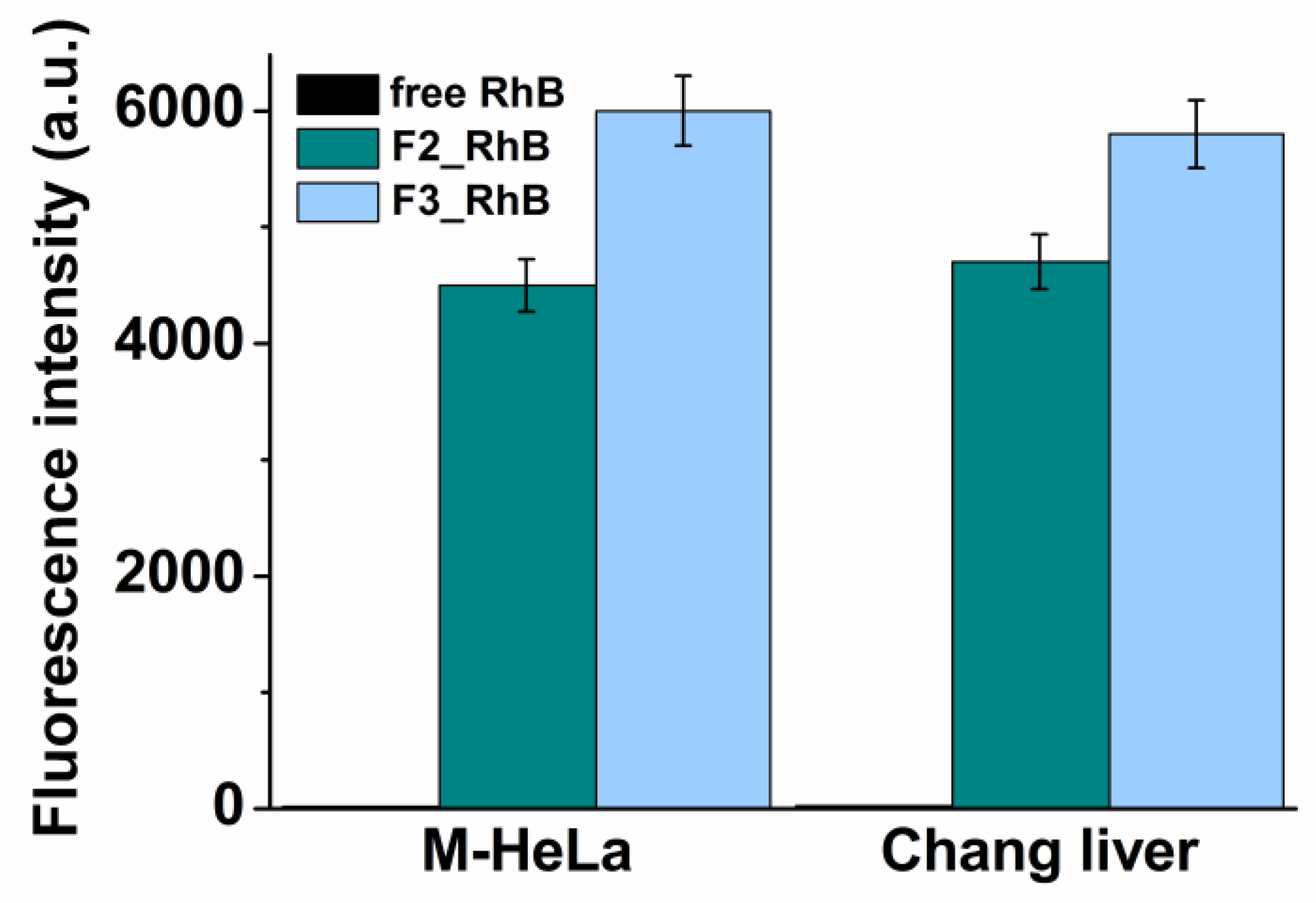
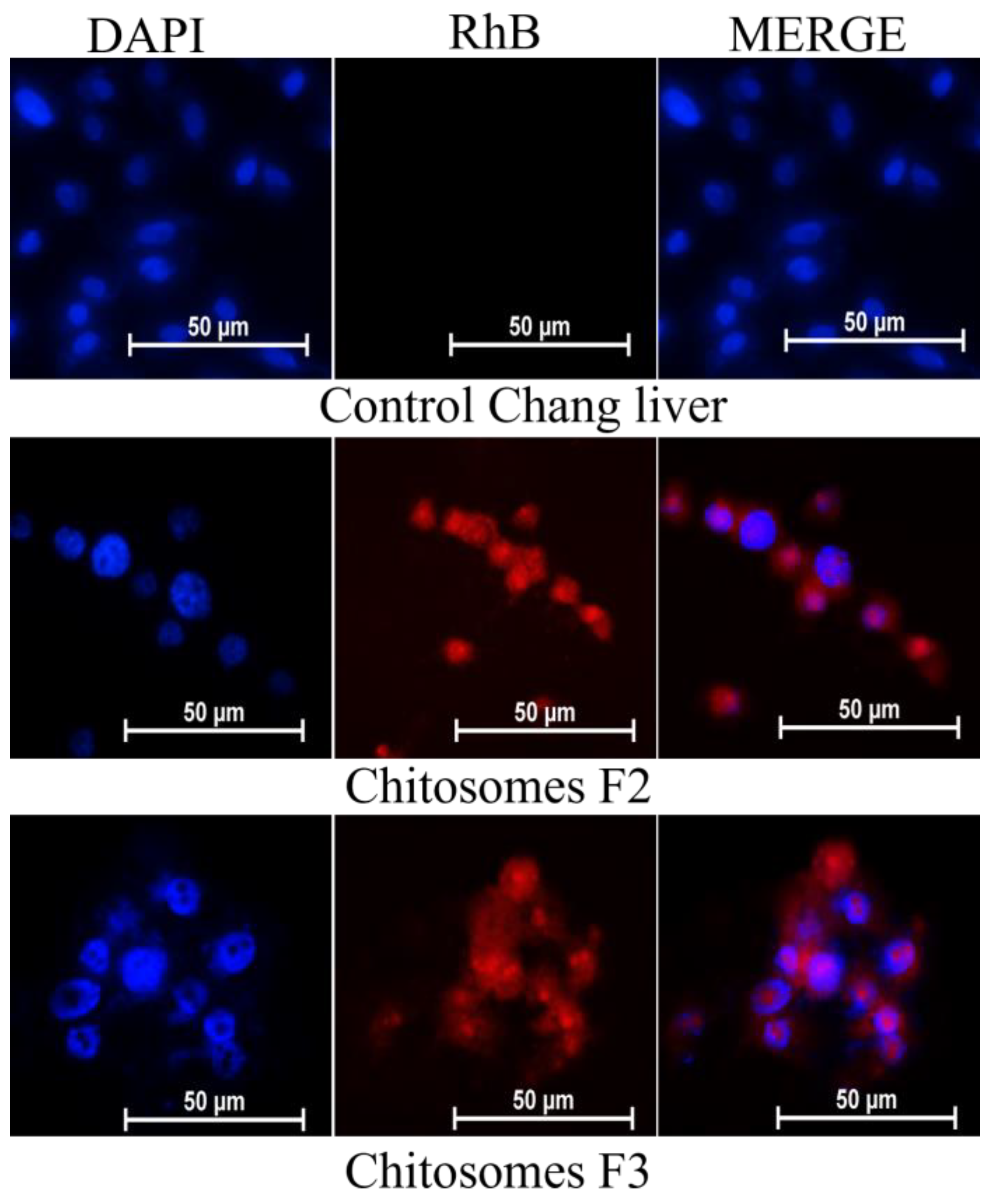
3.6. The Cellular Uptake of RhB Loaded Chitosomes
3.7. Brain AChE Reactivation In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sette, K.N.; Alugubelly, N.; Glenn, L.B.; Guo-Ross, S.X.; Parkes, M.K.; Wilson, J.R.; Seay, C.N.; Carr, R.L. The Mechanistic Basis for the Toxicity Difference between Juvenile Rats and Mice Following Exposure to the Agricultural Insecticide Chlorpyrifos. Toxicology 2022, 480, 153317. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, J.R.; Nguyen, A.; Klas, J.; Gahagan, S.; Checkoway, H.; Lopez-Paredes, D.; Jacobs, D.R.; Noble, M. Associations of Acetylcholinesterase Inhibition Between Pesticide Spray Seasons with Depression and Anxiety Symptoms in Adolescents, and the Role of Sex and Adrenal Hormones on Gender Moderation. Expo. Health 2021, 13, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, M.; Babacan, Ş.N.; Demir, Y.; Sert, Y.; Koca, İ.; Gülçin, İ. Discovery of Sulfadrug–Pyrrole Conjugates as Carbonic Anhydrase and Acetylcholinesterase Inhibitors. Arch. Pharm. 2022, 355, 2100242. [Google Scholar] [CrossRef]
- Vinotha Alex, A.; Mukherjee, A. Review of Recent Developments (2018–2020) on Acetylcholinesterase Inhibition Based Biosensors for Organophosphorus Pesticides Detection. Microchem. J. 2021, 161, 105779. [Google Scholar] [CrossRef]
- Masson, P.; Nachon, F. Cholinesterase Reactivators and Bioscavengers for Pre- and Post-Exposure Treatments of Organophosphorus Poisoning. J. Neurochem. 2017, 142, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuča, K.; Petroianu, G.A. Oximes as Pretreatment before Acute Exposure to Paraoxon. J. Appl. Toxicol. 2019, 39, 1506–1515. [Google Scholar] [CrossRef]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. Acute Organophosphorus Poisoning. Clin. Chim. Acta 2014, 431, 66–76. [Google Scholar] [CrossRef]
- Antonijevic, B.; Stojiljkovic, M.P. Unequal Efficacy of Pyridinium Oximes in Acute Organophosphate Poisoning. Clin. Med. Res. 2007, 5, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Acc. Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef]
- Dhuguru, J.; Zviagin, E.; Skouta, R. FDA-Approved Oximes and Their Significance in Medicinal Chemistry. Pharmaceuticals 2022, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Hrabinova, M.; Soukup, O.; Tobin, G.; Karasova, J.; Pohanka, M. Pralidoxime—The Gold Standard of Acetylcholinesterase Reactivators—Reactivation in Vitro Efficacy. Bratisl. Lek. Listy 2010, 111, 502–504. [Google Scholar]
- Da Silva, O.; Probst, N.; Landry, C.; Hanak, A.-S.; Warnault, P.; Coisne, C.; Calas, A.-G.; Gosselet, F.; Courageux, C.; Gastellier, A.-J.; et al. A New Class of Bi- and Trifunctional Sugar Oximes as Antidotes against Organophosphorus Poisoning. J. Med. Chem. 2022, 65, 4649–4666. [Google Scholar] [CrossRef] [PubMed]
- Čadež, T.; Kolić, D.; Šinko, G.; Kovarik, Z. Assessment of Four Organophosphorus Pesticides as Inhibitors of Human Acetylcholinesterase and Butyrylcholinesterase. Sci. Rep. 2021, 11, 21486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, B.; Sahu, A.K.; Acharya, J.; Satnami, M.L.; Ghosh, K.K. Synthesis and In-Vitro Reactivation Screening of Imidazolium Aldoximes as Reactivators of Sarin and VX-Inhibited Human Acetylcholinesterase (HAChE). Chem. Biol. Interact. 2016, 259, 85–92. [Google Scholar] [CrossRef]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Petukhova, E.O.; Gubaidullina, L.M.; Krylova, E.S.; Saifina, L.F.; Lenina, O.A.; Petrov, K.A. Novel Acetylcholinesterase Inhibitors Based on Uracil Moiety for Possible Treatment of Alzheimer Disease. Molecules 2020, 25, 4191. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gaynanova, G.A.; Vasilieva, E.A.; Pavlov, R.V.; Zueva, I.V.; Babaev, V.M.; Kuznetsov, D.M.; Voloshina, A.D.; Petrov, K.A.; Zakharova, L.Y.; et al. Oxime Therapy for Brain AChE Reactivation and Neuroprotection after Organophosphate Poisoning. Pharmaceutics 2022, 14, 1950. [Google Scholar] [CrossRef]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Babaev, V.M.; Lukashenko, S.S.; Rizvanov, I.K.; Souto, E.B.; Nikolsky, E.E.; Zakharova, L.Y.; Masson, P.; et al. Nanoparticle-Delivered 2-PAM for Rat Brain Protection against Paraoxon Central Toxicity. ACS Appl. Mater. Interfaces 2017, 9, 16922–16932. [Google Scholar] [CrossRef]
- Parvaz, S.; Taheri-Ledari, R.; Esmaeili, M.S.; Rabbani, M.; Maleki, A. A Brief Survey on the Advanced Brain Drug Administration by Nanoscale Carriers: With a Particular Focus on AChE Reactivators. Life Sci. 2020, 240, 117099. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Hong, S.-S.; Oh, K.T.; Choi, H.-G.; Lim, S.-J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Martin, V.; Hoekman, J.; Aurora, S.K.; Shrewsbury, S.B. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J. Clin. Med. 2021, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.-R.; Chiu, G.N.C. Liposomes as Sterile Preparations and Limitations of Sterilisation Techniques in Liposomal Manufacturing. Asian J. Pharm. Sci. 2013, 8, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Vhora, I.; Khatri, N.; Desai, J.; Thakkar, H.P. Caprylate-Conjugated Cisplatin for the Development of Novel Liposomal Formulation. AAPS PharmSciTech 2014, 15, 845–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Saraswat, A.; Vartak, R.; Patki, M.; Patel, K. Liposomal Formulation. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 79–102. ISBN 978-0-323-85041-4. [Google Scholar]
- Kuznetsova, D.A.; Vasilieva, E.A.; Kuznetsov, D.M.; Lenina, O.A.; Filippov, S.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Enhancement of the Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs Using Liposomes Containing Cationic Surfactants. ACS Omega 2022, 7, 25741–25750. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Ahtamyanova, L.R.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel Hybrid Liposomal Formulations Based on Imidazolium-Containing Amphiphiles for Drug Encapsulation. Colloids Surf. B Biointerfaces 2019, 178, 352–357. [Google Scholar] [CrossRef]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsova, D.A.; Vasileva, L.A.; Zueva, I.V.; Sapunova, A.S.; Buzyurova, D.N.; Babaev, V.M.; Voloshina, A.D.; Lukashenko, S.S.; et al. Biomedical Potentialities of Cationic Geminis as Modulating Agents of Liposome in Drug Delivery across Biological Barriers and Cellular Uptake. Int. J. Pharm. 2020, 587, 119640. [Google Scholar] [CrossRef]
- Khalili, L.; Dehghan, G.; Sheibani, N.; Khataee, A. Smart Active-Targeting of Lipid-Polymer Hybrid Nanoparticles for Therapeutic Applications: Recent Advances and Challenges. Int. J. Biol. Macromol. 2022, 213, 166–194. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Gaynanova, G.; Vasileva, L.; Kashapov, R.; Kuznetsova, D.; Kushnazarova, R.; Tyryshkina, A.; Vasilieva, E.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembling Drug Formulations with Tunable Permeability and Biodegradability. Molecules 2021, 26, 6786. [Google Scholar] [CrossRef] [PubMed]
- Colino, C.I.; Velez Gomez, D.; Alonso Horcajo, E.; Gutierrez-Millan, C. A Comparative Study of Liposomes and Chitosomes for Topical Quercetin Antioxidant Therapy. J. Drug Deliv. Sci. Technol. 2022, 68, 103094. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M. Effect of Chitosan Coating on the Characteristics of DPPC Liposomes. J. Adv. Res. 2010, 1, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Sebaaly, C.; Haydar, S.; Greige-Gerges, H. Eugenol Encapsulation into Conventional Liposomes and Chitosan-Coated Liposomes: A Comparative Study. J. Drug Deliv. Sci. Technol. 2022, 67, 102942. [Google Scholar] [CrossRef]
- Noorulla, K.M.; Yasir, M.; Muzaffar, F.; Roshan, S.; Ghoneim, M.M.; Almurshedi, A.S.; Tura, A.J.; Alshehri, S.; Gebissa, T.; Mekit, S.; et al. Intranasal Delivery of Chitosan Decorated Nanostructured Lipid Carriers of Buspirone for Brain Targeting: Formulation Development, Optimization and In-Vivo Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102939. [Google Scholar] [CrossRef]
- Abdelbary, G. Ocular Ciprofloxacin Hydrochloride Mucoadhesive Chitosan-Coated Liposomes. Pharm. Dev. Technol. 2011, 16, 44–56. [Google Scholar] [CrossRef]
- Sahatsapan, N.; Pamornpathomkul, B.; Rojanarata, T.; Ngawhirunpat, T.; Poonkhum, R.; Opanasopit, P.; Patrojanasophon, P. Feasibility of Mucoadhesive Chitosan Maleimide-Coated Liposomes for Improved Buccal Delivery of a Protein Drug. J. Drug Deliv. Sci. Technol. 2022, 69, 103173. [Google Scholar] [CrossRef]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved Oral Bioavailability of the Anticancer Drug Catechin Using Chitosomes: Design, in-Vitro Appraisal and in-Vivo Studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Abbas, H.; Sayed, N.S.E.; Youssef, N.A.H.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in Nasal Delivery Systems for Therapeutic Drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Dornish, M.; Wood, E.J. Involvement of Protein Kinase C in Chitosan Glutamate-Mediated Tight Junction Disruption. Biomaterials 2005, 26, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral Drug Absorption Enhancement by Chitosan and Its Derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Aspden, T.J.; Mason, J.D.T.; Jones, N.S.; Lowe, J.; Skaugrud, Ø.; Illum, L. Chitosan as a Nasal Delivery System: The Effect of Chitosan Solutions on in Vitro and in Vivo Mucociliary Transport Rates in Human Turbinates and Volunteers. J. Pharm. Sci. 1997, 86, 509–513. [Google Scholar] [CrossRef]
- Balde, A.; Kim, S.-K.; Benjakul, S.; Nazeer, R.A. Pulmonary Drug Delivery Applications of Natural Polysaccharide Polymer Derived Nano/Micro-Carrier Systems: A Review. Int. J. Biol. Macromol. 2022, 220, 1464–1479. [Google Scholar] [CrossRef]
- Garcia, B.B.M.; Mertins, O.; da Silva, E.R.; Mathews, P.D.; Han, S.W. Arginine-Modified Chitosan Complexed with Liposome Systems for Plasmid DNA Delivery. Colloids Surf. B Biointerfaces 2020, 193, 111131. [Google Scholar] [CrossRef]
- Fernandes Patta, A.C.M.; Mathews, P.D.; Madrid, R.R.M.; Rigoni, V.L.S.; Silva, E.R.; Mertins, O. Polyionic Complexes of Chitosan-N-Arginine with Alginate as PH Responsive and Mucoadhesive Particles for Oral Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 148, 550–564. [Google Scholar] [CrossRef]
- Bahnsen, J.S.; Franzyk, H.; Sandberg-Schaal, A.; Nielsen, H.M. Antimicrobial and Cell-Penetrating Properties of Penetratin Analogs: Effect of Sequence and Secondary Structure. Biochim. Biophys. Acta BBA-Biomembr. 2013, 1828, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Bechara, C.; Sagan, S. Cell-penetrating Peptides: 20 Years Later, Where Do We Stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Mertins, O.; Dimova, R. Insights on the Interactions of Chitosan with Phospholipid Vesicles. Part II: Membrane Stiffening and Pore Formation. Langmuir 2013, 29, 14552–14559. [Google Scholar] [CrossRef]
- Mathews, P.D.; Mertins, O. Chitosan and Lipid Composites as Versatile Biomedical Material. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–291. ISBN 978-0-12-816913-1. [Google Scholar]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed Cationic Liposomes for Brain Delivery of Drugs by the Intranasal Route: The Acetylcholinesterase Reactivator 2-PAM as Encapsulated Drug Model. Colloids Surf. B Biointerfaces 2018, 171, 358–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.-X.; Zhang, Z.-H.; Wang, X.-P.; Cheng, Q.-Q.; Wang, W.; Huang, X.-H.; Zhou, J.-P.; Zhang, Q.; Hou, L.-L.; Huo, W. A Biomimetic Chitosan Derivates: Preparation, Characterization and Transdermal Enhancement Studies of N-Arginine Chitosan. Molecules 2011, 16, 6778–6790. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, D.A.; Vasileva, L.A.; Gaynanova, G.A.; Vasilieva, E.A.; Lenina, O.A.; Nizameev, I.R.; Kadirov, M.K.; Petrov, K.A.; Zakharova, L.Y.; Sinyashin, O.G. Cationic Liposomes Mediated Transdermal Delivery of Meloxicam and Ketoprofen: Optimization of the Composition, in Vitro and in Vivo Assessment of Efficiency. Int. J. Pharm. 2021, 605, 120803. [Google Scholar] [CrossRef]
- Vasilieva, E.A.; Lukashenko, S.S.; Voloshina, A.D.; Strobykina, A.S.; Vasileva, L.A.; Zakharova, L.Y. The Synthesis and Properties of Homologous Series of Surfactants Containing the Pyrrolidinium Head Group with Hydroxyethyl Moiety. Russ. Chem. Bull. 2018, 67, 1280–1286. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Kuznetsov, D.M.; Vasileva, L.A.; Amerhanova, S.K.; Valeeva, D.N.; Salakhieva, D.V.; Nikolaeva, V.A.; Nizameev, I.R.; Islamov, D.R.; Usachev, K.S.; et al. Complexation of Oligo- and Polynucleotides with Methoxyphenyl-Functionalized Imidazolium Surfactants. Pharmaceutics 2022, 14, 2685. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zaru, M.; Manca, M.-L.; Fadda, A.M.; Antimisiaris, S.G. Chitosan-Coated Liposomes for Delivery to Lungs by Nebulisation. Colloids Surf. B Biointerfaces 2009, 71, 88–95. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S. Experimental Design Based Optimization and Ex Vivo Permeation of Desmopressin Acetate Loaded Elastic Liposomes Using Rat Skin. Pharmaceutics 2021, 13, 1047. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Park, H.-J. Factors Effect on the Loading Efficiency of Vitamin C Loaded Chitosan-Coated Nanoliposomes. Colloids Surf. B Biointerfaces 2010, 76, 16–19. [Google Scholar] [CrossRef]
- Efimova, A.A.; Popov, A.S.; Krivtsov, G.G. Anionic Liposomes in Contact with Cationic Chitosan Particles. Russ. J. Gen. Chem. 2020, 90, 2156–2162. [Google Scholar] [CrossRef]
- Laye, C.; McClements, D.J.; Weiss, J. Formation of Biopolymer-Coated Liposomes by Electrostatic Deposition of Chitosan. J. Food Sci. 2008, 73, N7–N15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, C.; Salama, M.; Xia, M.; Huang, X.; Sheng, L.; Cai, Z. Encapsulation Efficiency and Oral Delivery Stability of Chitosan–Liposome-encapsulated Immunoglobulin Y. J. Food Sci. 2022, 87, 1708–1720. [Google Scholar] [CrossRef]
- Esposto, B.S.; Pinho, S.G.B.; Thomazini, M.; Ramos, A.P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. TPP-Chitosomes as Potential Encapsulation System to Protect Carotenoid-Rich Extract Obtained from Carrot by-Product: A Comparison with Liposomes and Chitosomes. Food Chem. 2022, 397, 133857. [Google Scholar] [CrossRef]
- Sokolov, S.V.; Tschulik, K.; Batchelor-McAuley, C.; Jurkschat, K.; Compton, R.G. Reversible or Not? Distinguishing Agglomeration and Aggregation at the Nanoscale. Anal. Chem. 2015, 87, 10033–10039. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-Loaded Chitosomes for Treatment of Malignant Pleural Mesothelioma—A Rare Thoracic Cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The Stabilization and Release Performances of Curcumin-Loaded Liposomes Coated by High and Low Molecular Weight Chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Li, R.; Deng, L.; Cai, Z.; Zhang, S.; Wang, K.; Li, L.; Ding, S.; Zhou, C. Liposomes Coated with Thiolated Chitosan as Drug Carriers of Curcumin. Mater. Sci. Eng. C 2017, 80, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Esposto, B.S.; Jauregi, P.; Tapia-Blácido, D.R.; Martelli-Tosi, M. Liposomes vs. Chitosomes: Encapsulating Food Bioactives. Trends Food Sci. Technol. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and Characterization of Chitosan-Coated Nanoliposomes for Encapsulation of Caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Megahed, M.A.; El-Sawy, H.S.; Reda, A.M.; Abd-Allah, F.I.; Abu Elyazid, S.K.; Lila, A.E.; Ismael, H.R.; El-Say, K.M. Effect of Nanovesicular Surface-Functionalization via Chitosan and/or PEGylation on Cytotoxicity of Tamoxifen in Induced-Breast Cancer Model. Life Sci. 2022, 307, 120908. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Shi, Y.; Liang, Y.; Liu, L.; Sun, K.; Li, Y. Relationship and Improvement Strategies between Drug Nanocarrier Characteristics and Hemocompatibility: What Can We Learn from the Literature. Asian J. Pharm. Sci. 2021, 16, 551–576. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.L.; Littlejohn, L. Review of New Topical Hemostatic Dressings for Combat Casualty Care. Mil. Med. 2014, 179, 497–514. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.B.; Sharma, C.P. Use of Chitosan as a Biomaterial: Studies on Its Safety and Hemostatic Potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Hirano, S.; Noishiki, Y. The Blood Compatibility of Chitosan AndN-Acylchitosans. J. Biomed. Mater. Res. 1985, 19, 413–417. [Google Scholar] [CrossRef]
- Chan, L.W.; Kim, C.H.; Wang, X.; Pun, S.H.; White, N.J.; Kim, T.H. PolySTAT-Modified Chitosan Gauzes for Improved Hemostasis in External Hemorrhage. Acta Biomater. 2016, 31, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, J.M.; Sarmento, R.R.; de Souza, J.R.; Brayner, F.A.; Feitosa, A.P.S.; Padilha, R.; Alves, L.C.; Porto, I.J.; Batista, R.F.B.D.; de Oliveira, J.E.; et al. Evaluation of Hemagglutination Activity of Chitosan Nanoparticles Using Human Erythrocytes. BioMed Res. Int. 2015, 2015, 247965. [Google Scholar] [CrossRef] [PubMed]
- Niazvand, F.; Rajendra Wagh, P.; Khazraei, E.; Borzouyan Dastjerdi, M.; Patil, C.; Ahmad Najar, I. Application of Carbon Allotropes Composites for Targeted Cancer Therapy Drugs: A Review. J. Compos. Compd. 2021, 3, 140–151. [Google Scholar] [CrossRef]
- Krishnan, J.K.S.; Arun, P.; Appu, A.P.; Vijayakumar, N.; Figueiredo, T.H.; Braga, M.F.M.; Baskota, S.; Olsen, C.H.; Farkas, N.; Dagata, J.; et al. Intranasal Delivery of Obidoxime to the Brain Prevents Mortality and CNS Damage from Organophosphate Poisoning. NeuroToxicology 2016, 53, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurada, K.; Matsubara, K.; Shimizu, K.; Shiono, H.; Seto, Y.; Tsuge, K.; Yoshino, M.; Sakai, I.; Mukoyama, H.; Takatori, T. Pralidoxime Iodide (2-PAM) Penetrates across the Blood Brain Barrier. Neurochem. Res. 2003, 28, 1401–1407. [Google Scholar] [CrossRef]
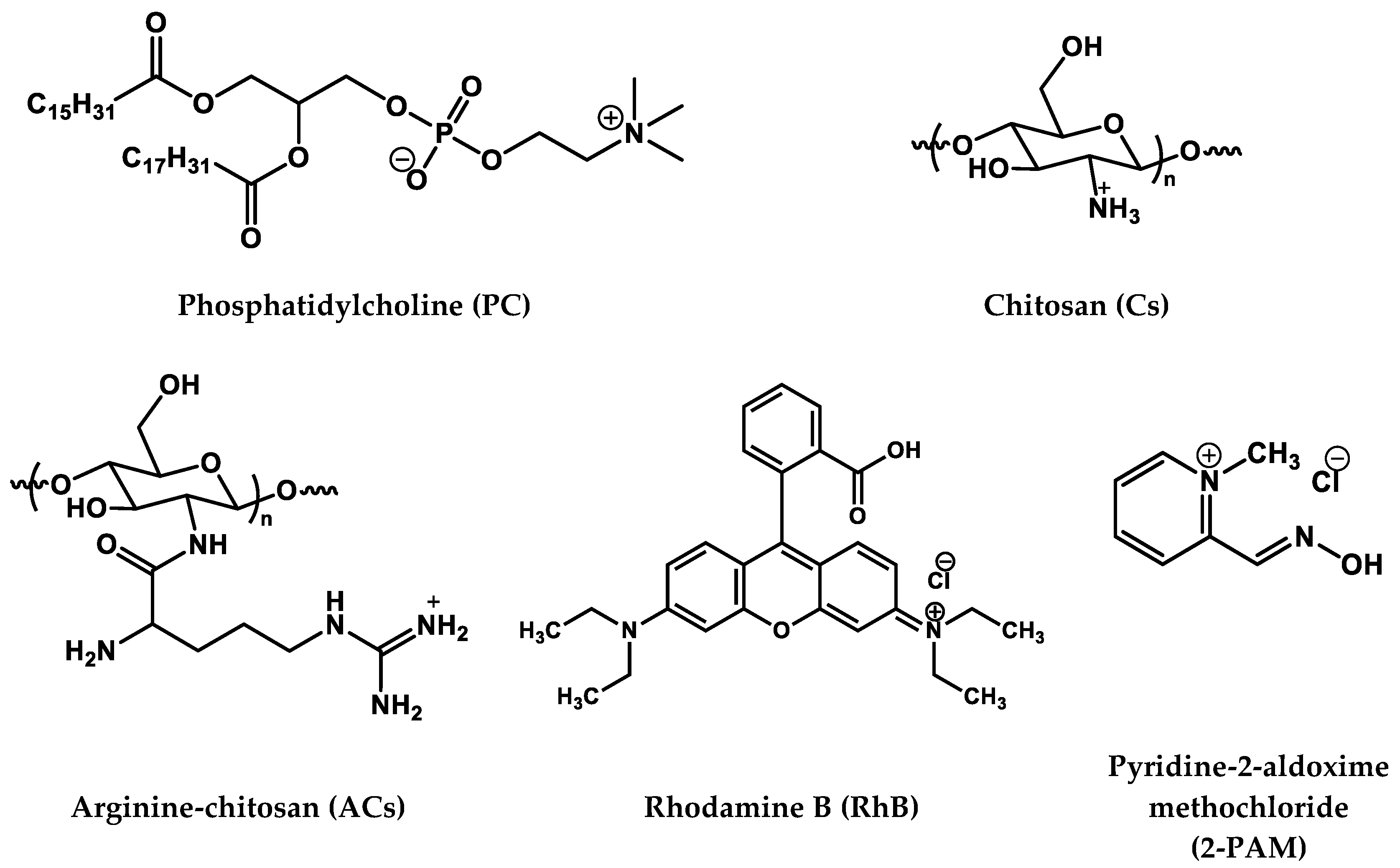



| Polymer | H (%) | C (%) | N (%) | C/N (%) | DS (%) |
|---|---|---|---|---|---|
| Cs | 6.70 | 34.53 | 6.86 | 4.96 | - |
| ACs | 5.65 | 36.35 | 5.46 | 6.65 | 28 |
| System | CCs, % | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| 1 day | 2 months | ||||||
| PC (10 mM) | - | 106 ± 1 | 0.07 ± 0.01 | −15 ± 1 | Not stable | ||
| F1 | 0.05 | 131 ± 1 | 0.17 ± 0.01 | 18 ± 1 | 134 ± 1 | 0.17 ± 0.01 | 37 ± 1 |
| F1 | 0.1 | 135 ± 1 | 0.19 ± 0.01 | 17 ± 1 | 131 ± 1 | 0.14 ± 0.01 | 35 ± 1 |
| F1 | 0.5 | 128 ± 1 | 0.16 ± 0.01 | 20 ± 1 | 135 ± 1 | 0.28 ± 0.02 | 46 ± 1 |
| F2 | 0.05 | 110 ± 1 | 0.05 ± 0.01 | 5 ± 1 | 109 ± 1 | 0.07 ± 0.02 | 8 ± 1 |
| F2 | 0.1 | 111 ± 2 | 0.06 ± 0.04 | 5 ± 1 | 111 ± 1 | 0.07 ± 0.02 | 11 ± 1 |
| F3 | 0.05 | 111 ± 2 | 0.06 ± 0.01 | 4 ± 1 | 111 ± 1 | 0.07 ± 0.01 | 22 ± 1 |
| F3 | 0.1 | 115 ± 1 | 0.08 ± 0.01 | 5 ± 1 | 115 ± 1 | 0.08 ± 0.01 | 19 ± 1 |
| System | CCs, % | Dh, nm | PdI | ζ, mV | Dh, nm | PdI | ζ, mV |
|---|---|---|---|---|---|---|---|
| RhB | |||||||
| 1 day | 1 month | ||||||
| PC (10 mM) | - | 105 ± 2 | 0.10 ± 0.02 | −5 ± 1 | Not stable | ||
| F1 | 0.05 | 131 ± 1 | 0.10 ± 0.03 | 22 ± 1 | 133 ± 1 | 0.12 ± 0.01 | 29 ± 1 |
| F2 | 0.05 | 119 ± 1 | 0.19 ± 0.01 | 32 ± 1 | 121 ± 1 | 0.21 ± 0.01 | 25 ± 1 |
| F3 | 0.05 | 100 ± 1 | 0.12 ± 0.01 | 34 ± 1 | 115 ± 1 | 0.13 ± 0.09 | 30 ± 1 |
| 2-PAM | |||||||
| PC (10 mM) | - | 118 ± 1 | 0.06 ± 0.01 | −7 ± 1 | Not stable | ||
| F1 | 0.05 | 134 ± 1 | 0.06 ± 0.01 | 9 ± 1 | 134 ± 1 | 0.13 ± 0.01 | 16 ± 1 |
| F2 | 0.05 | 113 ± 1 | 0.07 ± 0.01 | 6 ± 1 | 116 ± 1 | 0.07 ± 0.02 | 11 ± 1 |
| F3 | 0.05 | 116 ± 1 | 0.07 ± 0.01 | 6 ± 1 | 115 ± 1 | 0.08 ± 0.01 | 7 ± 1 |
| Kinetic Model | Parameters | Chitosomes | ||
|---|---|---|---|---|
| 0.05% | 0.1% | 0.5% | ||
| Zero order | k0 | 1.755 | 1.745 | 1.774 |
| R2 | 0.588 | 0.606 | 0.501 | |
| Higuchi Model | kn | 12.008 | 11.916 | 12.209 |
| R2 | 0.964 | 0.973 | 0.943 | |
| Korsmeyer–Peppas Model | kKP | 18.737 | 18.535 | 22.033 |
| n | 0.376 | 0.377 | 0.336 | |
| R2 | 0.987 | 0.997 | 0.989 | |
| System | CPC, mM | Hemolysis, % | HC50, mM * | IC50, mM * Chang Liver/M-HeLa |
|---|---|---|---|---|
| F1_2-PAM | 5 | 29.6 | >5 | |
| 2.5 | 20.7 | |||
| 1.25 | 7.1 | |||
| 0.625 | 4.6 | |||
| F2_2-PAM | 5 | 23.6 | >5 | >5/>5 |
| 2.5 | 16.6 | |||
| 1.25 | 7.8 | |||
| 0.625 | 4.5 | |||
| F3_2-PAM | 5 | 22.3 | >5 | 3.5/3 |
| 2.5 | 16.6 | |||
| 1.25 | 8.2 | |||
| 0.625 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, E.A.; Kuznetsova, D.A.; Valeeva, F.G.; Kuznetsov, D.M.; Zakharov, A.V.; Amerhanova, S.K.; Voloshina, A.D.; Zueva, I.V.; Petrov, K.A.; Zakharova, L.Y. Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes. Pharmaceutics 2022, 14, 2846. https://doi.org/10.3390/pharmaceutics14122846
Vasilieva EA, Kuznetsova DA, Valeeva FG, Kuznetsov DM, Zakharov AV, Amerhanova SK, Voloshina AD, Zueva IV, Petrov KA, Zakharova LY. Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes. Pharmaceutics. 2022; 14(12):2846. https://doi.org/10.3390/pharmaceutics14122846
Chicago/Turabian StyleVasilieva, Elmira A., Darya A. Kuznetsova, Farida G. Valeeva, Denis M. Kuznetsov, Andrey V. Zakharov, Syumbelya K. Amerhanova, Alexandra D. Voloshina, Irina V. Zueva, Konstantin A. Petrov, and Lucia Ya. Zakharova. 2022. "Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes" Pharmaceutics 14, no. 12: 2846. https://doi.org/10.3390/pharmaceutics14122846
APA StyleVasilieva, E. A., Kuznetsova, D. A., Valeeva, F. G., Kuznetsov, D. M., Zakharov, A. V., Amerhanova, S. K., Voloshina, A. D., Zueva, I. V., Petrov, K. A., & Zakharova, L. Y. (2022). Therapy of Organophosphate Poisoning via Intranasal Administration of 2-PAM-Loaded Chitosomes. Pharmaceutics, 14(12), 2846. https://doi.org/10.3390/pharmaceutics14122846