Accelerated Wound Healing in Minipigs by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
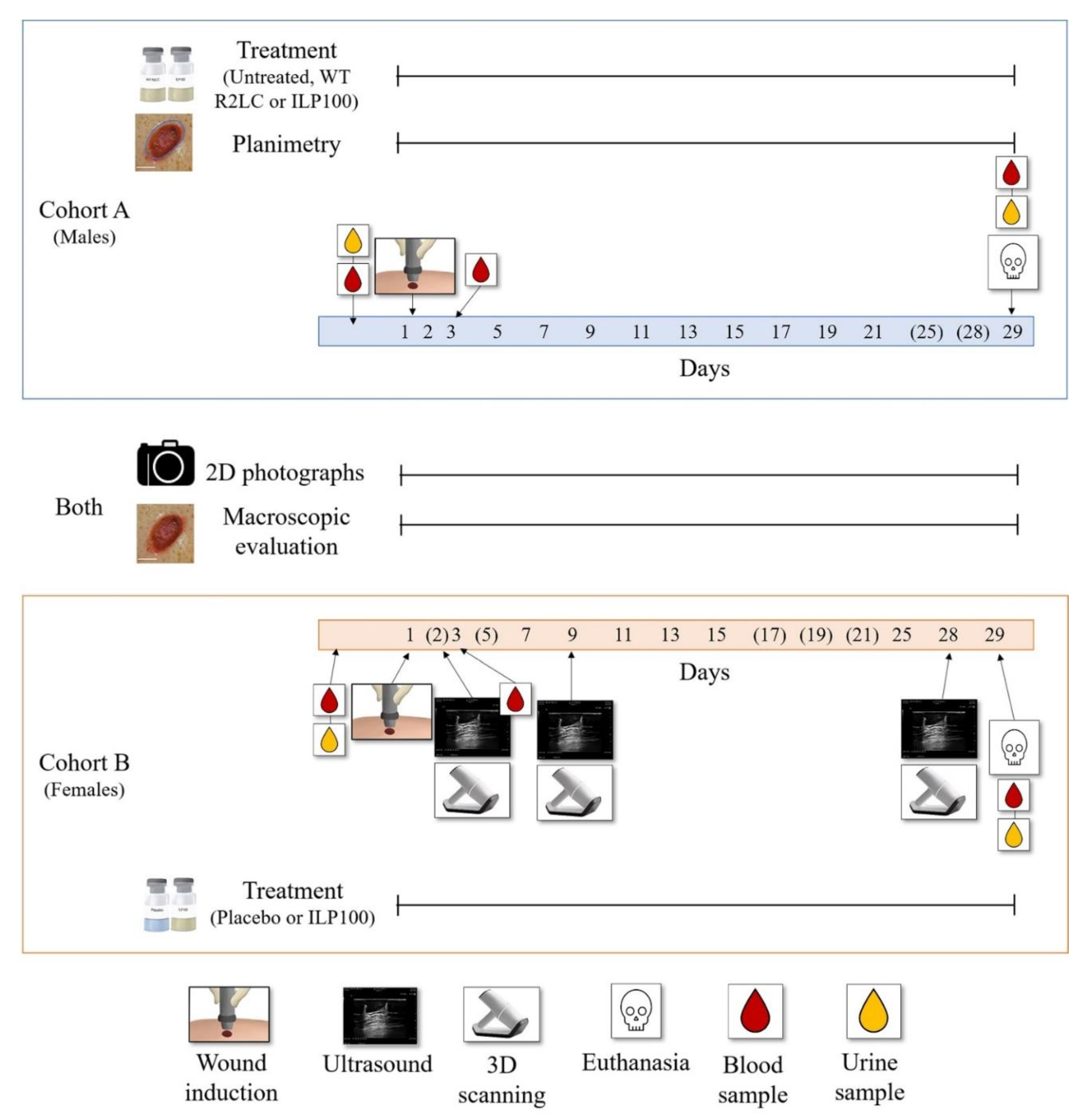
2.1. Study Design
2.2. Animals
2.3. Limosilactobacillus reuteri R2LC Encoding Human CXCL12
2.4. Wound Induction
2.5. Blood Sampling
2.6. Urinalysis
2.7. Histopathology
2.8. Wound Treatment
2.9. Wound Evaluation
2.9.1. Two-Dimensional Photographs of Wounds
2.9.2. Planimetric Assessments of Wounds
2.9.3. Three-Dimensional Scanning of Wounds
2.9.4. Ultrasound Imaging of Wounds
2.10. Data Management and Statistics
3. Results
3.1. Evaluation of Methods Assessing Wound Granulation, Re-Epithelialization and Area
3.2. Treatment with CXCL12-Producing L. reuteri R2LC Accelerates Wound Healing
3.3. Safety Assessments
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Organs and Tissue for Histopathology | |
|---|---|
| Abnormalities (gross lesions) | Muscle, skeletal * |
| Artery, aorta (thoracic and aortic arch) | Nerve, optic ** |
| Bone marrow, sternum | Nerve, sciatic * |
| Bone, femur (medial condyl) * | Pancreas |
| Brain | Skin, untreated |
| Esophagus | Small intestine, duodenum |
| Eye (with lens) ** | Small intestine, jejunum |
| Gallbladder | Small intestine, ileum |
| Gland, adrenal ** | Spinal cord, thoracic |
| Gland, mammary ** (Cohort A only *) | Spinal cord, lumbar |
| Gland, parathyroid (when possible) ** | Spleen |
| Gland, pituitary | Stomach |
| Gland, salivary, parotid * | Thymus |
| Gland, salivary, sublingual * | Tongue |
| Gland, salivary, submandibular * | Ureter ** |
| Gland, thyroid | Urinary bladder |
| Heart | Wounds |
| Joint, femorotibial * | |
| Kidney ** | Cohort A |
| Large intestine, cecum | Gland, prostate |
| Large intestine, colon | Testis |
| Large intestine, rectum | |
| Larynx | Cohort B |
| Liver (all main lobes) | Lymph node, axillary |
| Lung (cranial and caudal lobes, both sides) | Ovary ** |
| Lymph node, mandibular * | Uterus (horn **, cervix and oviduct **) |
| Lymph node, mesenteric | Vagina |


| Wound Edge Inflammation (Mean ± SEM) | |||||
|---|---|---|---|---|---|
| Cohort A | Day 3 | Day 5 | Day 7 | Day 9 | n;N |
| Untreated | 0.17 (± 0.09) | 0.00 (± 0.00) | 0.17 (± 0.09) | 0.00 (± 0.00) | 3;18 |
| Wild type R2LC | 0.33 (± 0.14) | 0.00 (± 0.00) | 0.00 (± 0.00) | 0.00 (± 0.00) | 3;12 |
| ILP100 | 0.40 (± 0.09) | 0.17 (± 0.69) | 0.13 (± 0.8) | 0.14 (± 0.07) | 6;30 |
| Cohort B | Day 3 | - | Day 7 | Day 9 | n;N |
| Placebo | 0.11 (± 0.08) | - | 0.00 (± 0.00) | 0.00 (± 0.00) | 3;18 |
| ILP100 | 0.19 (± 0.07) | - | 0.06 (± 0.04) | 0.00 (± 0.00) | 6;36 |
| Surrounding Skin Inflammation (Mean ± SEM) | |||||
| Cohort A | Day 3 | Day 15 | Day 21 | Day 29 | n;N |
| Untreated | 0.11 (± 0.08) | 0.00 (± 0.00) | 0.00 (± 0.00) | 0.00 (± 0.00) | 3;18 |
| Wild type R2LC | 0.25 (± 0.45) | 0.00 (± 0.00) | 0.25 (± 0.45) | 0.58 (± 0.29) | 3;12 |
| ILP100 | 0.03 (± 0.03) | 0.00 (± 0.00) | 0.13 (± 0.06) | 0.57 (± 0.18) * | 6;30 |
| Cohort B | Day 3 | Day 15 | Day 21 | Day 28 | n;N |
| Placebo | 0.00 (± 0.00) | 0.22 (± 0.10) | 0.17 (± 0.09) | 0.11 (± 0.08) | 3;18 |
| ILP100 | 0.00 (± 0.00) | 0.00 (± 0.00) * | 0.00 (± 0.00) * | 0.00 (± 0.00) | 6;36 |
| Hemorrhage (Mean ± SEM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cohort A | Day 2 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | n;N |
| Untreated | 0.50 (± 0.62) | 0.22 (± 0.10) | 0.33 (± 0.11) | 0.28 (± 0.14) | 0.89 (± 0.08) | 0.11 (± 0.08) | 0.03 (± 0.03) | 3;18 |
| Wild type R2LC | 0.75 (± 0.22) | 0.08 (± 0.08) | 0.33 (± 0.14) | 1.42 (± 0.15) * | 0.83 (± 0.17) | 0.00 (± 0.00) | 0.00 (± 0.00) | 3;12 |
| ILP100 | 0.17 (± 0.07) | 0.10 (± 0.06) | 0.23 (± 0.08) | 1.17 (± 0.11) * | 0.30 (± 0.09) * | 0.00 (± 0.00) | 0.00 (± 0.00) | 6;30 |
| Cohort B | - | Day 3 | - | Day 7 | Day 9 | Day 11 | Day 13 | n;N |
| Placebo | - | 1.17 (± 0.12) | - | 0.39 (± 0.12) | 0.56 (± 0.12) | 0.61 (± 0.12) | 0.11 (± 0.08) | 3;18 |
| ILP100 | - | 0.47 (± 0.13) * | - | 0.94 (± 0.09) * | 0.47 (± 0.08) | 0.22 (± 0.07) * | 0.06 (± 0.04) | 6;36 |
| Exudate (Mean ± SEM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort A | Day 2 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 | Day 13 | Day 19 | n;N |
| Untreated | 1.28 (± 0.11) | 1.39 (± 0.16) | 1.72 (± 0.11) | 0.61 (± 0.12) | 0.39 (± 0.14) | 0.17 (± 0.09) | 0.00 (± 0.00) | 0.00 (± 0.00) | 3;18 |
| Wild type R2LC | 0.83 (± 0.11) * | 0.67 (± 0.14) * | 0.92 (± 0.15) * | 0.17 (± 0.11) | 0.25 (± 0.13) | 0.08 (± 0.08) | 0.00 (± 0.00) | 0.08 (± 0.08) | 3;12 |
| ILP100 | 0.63 (± 0.09) * | 0.67 (± 0.10) * | 0.80 (± 0.12) * | 0.57 (± 0.11) | 0.50 (± 0.10) | 0.03 (± 0.03) | 0.07 (± 0.05) | 0.00 (± 0.00) | 6;30 |
| Cohort B | - | Day 3 | - | Day 7 | Day 9 | Day 11 | Day 13 | - | n;N |
| Placebo | - | 1.56 (± 0.12) | - | 1.17 (± 0.17) | 0.56 (± 0.12) | 0.00 (± 0.00) | 0.00 (± 0.00) | - | 3;18 |
| ILP100 | - | 1.50 (± 0.08) | - | 0.75 (± 0.11) | 0.47 (± 0.08) | 0.17 (± 0.06) | 0.06 (± 0.04) | - | 6;36 |
| Hypergranulation (Mean ± SEM) | ||||
|---|---|---|---|---|
| Cohort A | Day 7 | Day 9 | Day 11 | n;N |
| Untreated | 0.00 (± 0.00) | 0.17 (± 0.09) | 0.06 (± 0.06) | 3;18 |
| Wild type R2LC | 0.25 (± 0.13) | 0.17 (± 0.11) * | 0.00 (± 0.00) | 3;12 |
| ILP100 | 0.00 (± 0.00) | 0.00 (± 0.00) | 0.00 (± 0.00) | 6;30 |
| Cohort B | Day 7 | Day 9 | Day 11 | n;N |
| Placebo | 0.00 (±0.00) | 0.00 (±0.00) | 0.33 (±0.11) | 3;18 |
| ILP100 | 0.03 (±0.03) | 0.08 (±0.05) | 0.22 (±0.07) | 6;36 |
References
- Ashcroft, G.S.; Horan, M.A.; Ferguson, M.W. The Effects of Ageing on Cutaneous Wound Healing in Mammals. J. Anat. 1995, 187 Pt 1, 1–26. [Google Scholar] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 1 Normal and Chronic Wounds Biology, Causes, and Approaches to Care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound Assessment. BMJ 2006, 332, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-Illness Studies in Chronic Ulcers: A Systematic Review. J. Wound Care 2017, 26, S4–S14. [Google Scholar] [CrossRef] [Green Version]
- Statens beredning för medicinsk utvärdering. Svårläkta sår hos Äldre: Prevention och Behandling: En Systematisk Litteraturöversikt; Statens Beredning för Medicinsk utvärdering (SBU): Stockholm, Sweden, 2014; ISBN 978-91-85413-67-6. [Google Scholar]
- Öhnstedt, E.; Lofton Tomenius, H.; Vågesjö, E.; Phillipson, M. The Discovery and Development of Topical Medicines for Wound Healing. Expert Opin. Drug Discov. 2019, 14, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Yager, D.R.; Nwomeh, B.C. The Proteolytic Environment of Chronic Wounds. Wound Repair Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Vågesjö, E.; Öhnstedt, E.; Mortier, A.; Lofton, H.; Huss, F.; Proost, P.; Roos, S.; Phillipson, M. Accelerated Wound Healing in Mice by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 1895–1900. [Google Scholar] [CrossRef] [Green Version]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Zomer, H.D.; Trentin, A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A Review of Animal Models from 2015 to 2020 for Preclinical Chronic Wounds Relevant to Human Health. J. Tissue Viability 2021, 30, 291–300. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds—Developing Products for Treatment; U.S. Department of Health and Human Services Food and Drug Administration: Silver Spring, MA, USA, 2006.
- Dotson, P. Who Said Persistence Doesn’t Pay Off? FDA Closer to New Primary Wound Care Endpoints. Today’s Wound Clin. 2020, 14, 18–19. [Google Scholar]
- Driver, V.R.; Gould, L.J.; Dotson, P.; Allen, L.L.; Carter, M.J.; Bolton, L.L. Evidence Supporting Wound Care End Points Relevant to Clinical Practice and Patients’ Lives. Part 2. Literature Survey: Validation of Wound Care End Points. Wound Repair Regen. 2019, 27, 80–89. [Google Scholar] [CrossRef]
- Driver, V.R.; Gould, L.J.; Dotson, P.; Gibbons, G.W.; Li, W.W.; Ennis, W.J.; Kirsner, R.S.; Eaglstein, W.H.; Bolton, L.L.; Carter, M.J. Identification and Content Validation of Wound Therapy Clinical Endpoints Relevant to Clinical Practice and Patient Values for FDA Approval. Part 1. Survey of the Wound Care Community: Identification and Content Validation of Endpoints. Wound Repair Regen. 2017, 25, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Salameh, F.; Koren, A.; Sprecher, E.; Artzi, O. Novel Stereoscopic Optical System for Objectively Measuring Above-Surface Scar Volume—First-Time Quantification of Responses to Various Treatment Modalities. Dermatol. Surg. 2018, 44, 848–854. [Google Scholar] [CrossRef]
- Mangelsdorf, S.; Vergou, T.; Sterry, W.; Lademann, J.; Patzelt, A. Comparative Study of Hair Follicle Morphology in Eight Mammalian Species and Humans. Ski. Res. Technol. 2014, 20, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Richter, H.; Schaefer, H.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Variations of Hair Follicle Size and Distribution in Different Body Sites. J. Investig. Dermatol. 2004, 122, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Darwin, E.; Tomic-Canic, M. Healing Chronic Wounds: Current Challenges and Potential Solutions. Curr. Dermatol. Rep. 2018, 7, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gulf Diabetic Foot Working Group Identification and Management of Infection in Diabetic Foot Ulcers: International Consensus; Wounds international: London, UK, 2017.
- Sussman, C.; Bates-Jensen, B.M. Wound Care: A Collaborative Practice Manual for Health Professionals; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 978-1-60831-715-8. [Google Scholar]
- Jeong, E.-G.; Cho, S.S.; Lee, S.-H.; Lee, K.-M.; Woo, S.-K.; Kang, Y.; Yun, J.-S.; Cha, S.-A.; Kim, Y.-J.; Ahn, Y.-B.; et al. Depth and Combined Infection Is Important Predictor of Lower Extremity Amputations in Hospitalized Diabetic Foot Ulcer Patients. Korean J. Intern. Med. 2018, 33, 952–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.P.H.; Chin, W.Y.; Wan, E.Y.F.; Lam, C.L.K. Evaluation of the Internal and External Responsiveness of the Pressure Ulcer Scale for Healing ( PUSH ) Tool for Assessing Acute and Chronic Wounds. J. Adv. Nurs. 2016, 72, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, S.E.; Frantz, R.A.; Bergquist, S.; Shin, C.D. A Prospective Study of the Pressure Ulcer Scale for Healing (PUSH). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 93–97. [Google Scholar] [CrossRef]
- Stotts, N.A.; Rodeheaver, G.T.; Thomas, D.R.; Frantz, R.A.; Bartolucci, A.A.; Sussman, C.; Ferrell, B.A.; Cuddigan, J.; Maklebust, J. An Instrument to Measure Healing in Pressure Ulcers: Development and Validation of the Pressure Ulcer Scale for Healing (PUSH). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M795–M799. [Google Scholar] [CrossRef]
- Sussman, C.; Swanson, G. Utility of the Sussman Wound Healing Tool in Predicting Wound Healing Outcomes in Physical Therapy. Adv. Wound Care 1997, 10, 74–77. [Google Scholar]
- Hasatsri, S.; Aramwit, P. Nontraditional Methods to Evaluate Wound Healing. Dermatol. Surg. 2017, 43, 342–350. [Google Scholar] [CrossRef]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME Concept: What Have We Learned in the Past 10 Years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- St-Supery, V.; Tahiri, Y.; Sampalis, J.; Brutus, J.-P.; Harris, P.G.; Nikolis, A. Wound Healing Assessment: Does the Ideal Methodology for a Research Setting Exist? Ann. Plast. Surg. 2011, 67, 193–200. [Google Scholar] [CrossRef]
- Gould, L.J.; Liu, J.; Wan, R.; Carter, M.J.; Dotson, M.(P.); Driver, V.R. Evidence Supporting Wound Care End Points Relevant to Clinical Practice and Patients’ Lives. Part 3: The Patient Survey. Wound Repair Regen. 2021, 29, 60–69. [Google Scholar] [CrossRef]
- Rogers, L.C.; Bevilacqua, N.J.; Armstrong, D.G.; Andros, G. Digital Planimetry Results in More Accurate Wound Measurements: A Comparison to Standard Ruler Measurements. J. Diabetes Sci. Technol. 2010, 4, 799–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thawer, H.A.; Houghton, P.E.; Woodbury, M.G.; Keast, D.; Campbell, K. A Comparison of Computer-Assisted and Manual Wound Size Measurement. Ostomy Wound Manag. 2002, 48, 46–53. [Google Scholar]
- Jørgensen, L.B.; Sørensen, J.A.; Jemec, G.B.; Yderstraede, K.B. Methods to Assess Area and Volume of Wounds—A Systematic Review: Review of Methods to Assess Wound Size. Int. Wound J. 2016, 13, 540–553. [Google Scholar] [CrossRef]
- Davis, K.E.; Constantine, F.C.; MacAslan, E.C.; Bills, J.D.; Noble, D.L.; Lavery, L.A. Validation of a Laser-Assisted Wound Measurement Device for Measuring Wound Volume. J. Diabetes Sci. Technol. 2013, 7, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Jun, D.; Choi, H.; Kim, J.; Lee, M.; Kim, S.; Jo, D.; Kim, C.; Shin, D. Efficacy of the Mobile Three-Dimensional Wound Measurement System in Pressure Ulcer Assessment. J. Wound Manag. Res. 2019, 15, 78–84. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; Skov-Jeppesen, S.M.; Halekoh, U.; Rasmussen, B.S.; Sørensen, J.A.; Jemec, G.B.E.; Yderstræde, K.B. Validation of Three-dimensional Wound Measurements Using a Novel 3D-WAM Camera. Wound Repair Regen. 2018, 26, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Hendel, K.; Ortner, V.K.; Fuchs, C.S.K.; Eckhouse, V.; Haedersdal, M. Dermatologic Scar Assessment With Stereoscopic Imaging and Digital Three-Dimensional Models: A Validation Study. Lasers Surg. Med. 2021, 53, 1043–1049. [Google Scholar] [CrossRef]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential Roles of Macrophages in Diverse Phases of Skin Repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Zapata, M.J.; Martí-Carvajal, A.J.; Solà, I.; Expósito, J.A.; Bolíbar, I.; Rodríguez, L.; Garcia, J.; Zaror, C. Autologous Platelet-Rich Plasma for Treating Chronic Wounds. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Lakmal, K.; Basnayake, O.; Hettiarachchi, D. Systematic Review on the Rational Use of Amniotic Membrane Allografts in Diabetic Foot Ulcer Treatment. BMC Surg. 2021, 21, 87. [Google Scholar] [CrossRef]
- Cazzell, S.; Vayser, D.; Pham, H.; Walters, J.; Reyzelman, A.; Samsell, B.; Dorsch, K.; Moore, M. A Randomized Clinical Trial of a Human Acellular Dermal Matrix Demonstrated Superior Healing Rates for Chronic Diabetic Foot Ulcers over Conventional Care and an Active Acellular Dermal Matrix Comparator: Clinical Trial of D-ADM Demonstrated Superior Healing Rates for Chronic Diabetic Foot Ulcers. Wound Repair Regen. 2017, 25, 483–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbany, S.Y.; Pastore, J.; Yamamoto, M.; Miller, T.; Rafii, S.; Aras, R.; Penn, M. Continuous Delivery of Stromal Cell-Derived Factor-1 from Alginate Scaffolds Accelerates Wound Healing. Cell Transplant. 2010, 19, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [Green Version]
- Schultz, G.S.; Wysocki, A. Interactions between Extracellular Matrix and Growth Factors in Wound Healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öhnstedt, E.; Lofton Tomenius, H.; Frank, P.; Roos, S.; Vågesjö, E.; Phillipson, M. Accelerated Wound Healing in Minipigs by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria. Pharmaceutics 2022, 14, 229. https://doi.org/10.3390/pharmaceutics14020229
Öhnstedt E, Lofton Tomenius H, Frank P, Roos S, Vågesjö E, Phillipson M. Accelerated Wound Healing in Minipigs by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria. Pharmaceutics. 2022; 14(2):229. https://doi.org/10.3390/pharmaceutics14020229
Chicago/Turabian StyleÖhnstedt, Emelie, Hava Lofton Tomenius, Peter Frank, Stefan Roos, Evelina Vågesjö, and Mia Phillipson. 2022. "Accelerated Wound Healing in Minipigs by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria" Pharmaceutics 14, no. 2: 229. https://doi.org/10.3390/pharmaceutics14020229
APA StyleÖhnstedt, E., Lofton Tomenius, H., Frank, P., Roos, S., Vågesjö, E., & Phillipson, M. (2022). Accelerated Wound Healing in Minipigs by On-Site Production and Delivery of CXCL12 by Transformed Lactic Acid Bacteria. Pharmaceutics, 14(2), 229. https://doi.org/10.3390/pharmaceutics14020229






