Genetic Factors of Renin–Angiotensin System Associated with Major Bleeding for Patients Treated with Direct Oral Anticoagulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Genotyping Methods
2.3. Statistical Analysis
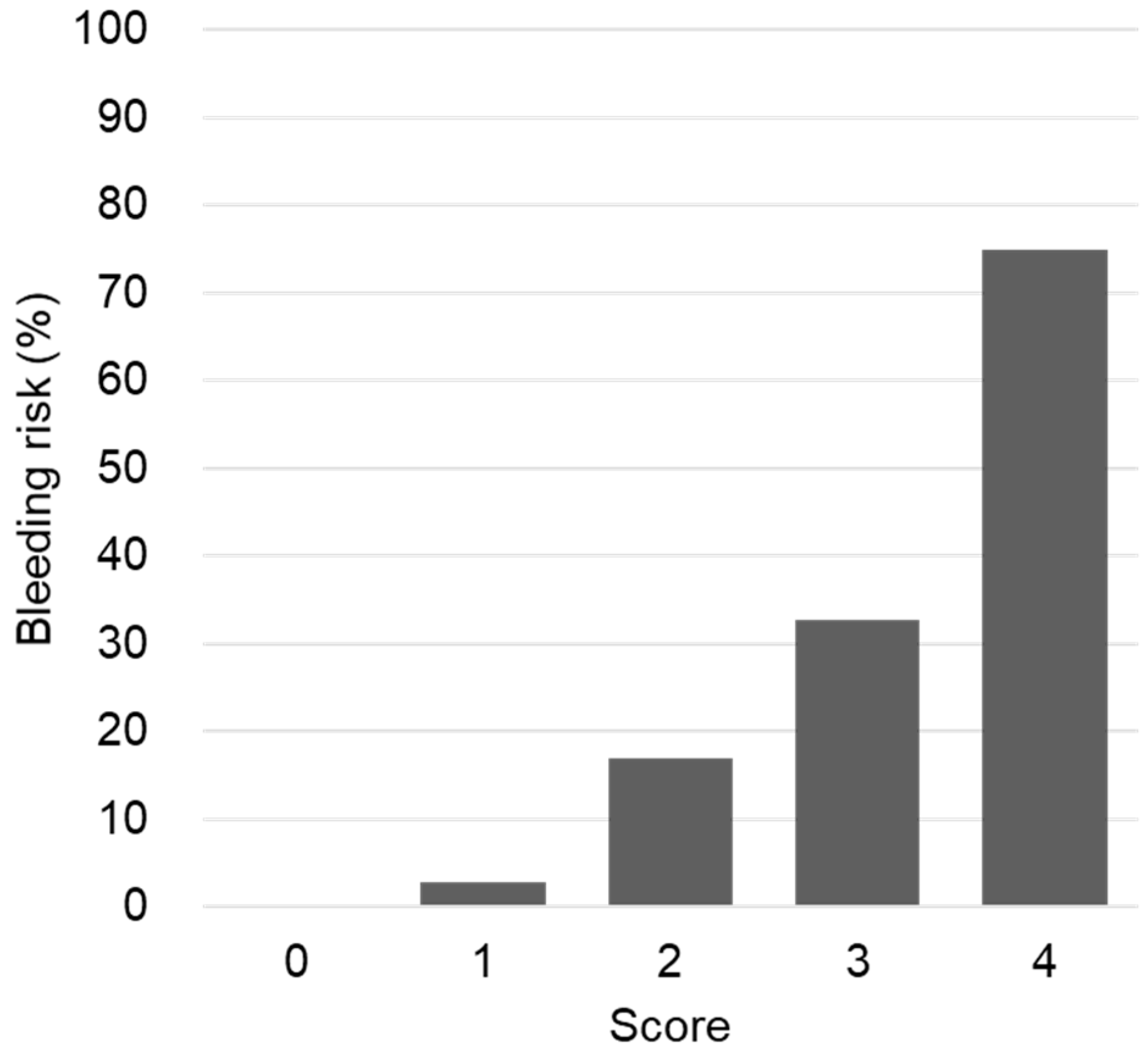
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Mekaj, A.; Mekaj, Y.; Duci, S.; Miftari, E. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Werth, S.; Breslin, T.; NiAinle, F.; Beyer-Westendorf, J. Bleeding Risk, Management and Outcome in Patients Receiving Non-VKA Oral Anticoagulants (NOACs). Am. J. Cardiovasc. Drugs 2015, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Keshishian, A.; Li, X.; Hamilton, M.; Masseria, C.; Gupta, K.; Luo, X.; Mardekian, J.; Friend, K.; Nadkarni, A.; et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients. Stroke 2018, 49, 2933–2944. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Papavasileiou, V.; Makaritsis, K.; Vemmos, K.; Michel, P.; Lip, G.Y. Real-World Setting Comparison of Nonvitamin-K Antagonist Oral Anticoagulants Versus Vitamin-K Antagonists for Stroke Prevention in Atrial Fibrillation: A Systematic Review and Meta-Analysis. Stroke 2017, 48, 2494–2503. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Farmer, C.; Luo, X.; Li, X.; Vo, L.; Mardekian, J.; Fahrbach, K.; Ashaye, A. Comparison of major bleeding risk in patients with non-valvular atrial fibrillation receiving direct oral anticoagulants in the real-world setting: A network meta-analysis. Curr. Med. Res. Opin. 2017, 34, 487–498. [Google Scholar] [CrossRef]
- Gelosa, P.; Castiglioni, L.; Tenconi, M.; Baldessin, L.; Racagni, G.; Corsini, A.; Bellosta, S. Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs). Pharmacol. Res. 2018, 135, 60–79. [Google Scholar] [CrossRef]
- Gong, I.Y.; Kim, R.B. Importance of Pharmacokinetic Profile and Variability as Determinants of Dose and Response to Dabigatran, Rivaroxaban, and Apixaban. Can. J. Cardiol. 2013, 29, S24–S33. [Google Scholar] [CrossRef]
- Chan, N.; Sager, P.T.; Lawrence, J.; Ortel, T.; Reilly, P.; Berkowitz, S.; Kubitza, D.; Eikelboom, J.; Florian, J.; Stockbridge, N.; et al. Is there a role for pharmacokinetic/pharmacodynamic-guided dosing for novel oral anticoagulants? Am. Heart J. 2018, 199, 59–67. [Google Scholar] [CrossRef]
- Tseng, A.S.; Patel, R.D.; Quist, H.E.; Kekic, A.; Maddux, J.T.; Grilli, C.B.; Shamoun, F.E. Clinical Review of the Pharmacogenomics of Direct Oral Anticoagulants. Cardiovasc. Drugs Ther. 2018, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, S.H.; Kreutz, R.P. Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. J. Pers. Med. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atlas, S.A. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, D.E. The renin-angiotensin system and fibrinolysis. Am. J. Cardiol. 1997, 79, 12–16. [Google Scholar] [CrossRef]
- Felmeden, D.C.; Lip, G.Y. The renin-angiotensin-aldosterone system and fibrinolysis. J. Renin-Angiotensin-Aldosterone Syst. 2000, 1, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Soccio, M.; De Caterina, R. Aspects of gene polymorphisms in cardiovascular disease: The renin-angiotensin system. Eur. J. Clin. Investig. 2001, 31, 476–488. [Google Scholar] [CrossRef]
- Wang, J.-G.; Staessen, J.A. Genetic polymorphisms in the renin–angiotensin system: Relevance for susceptibility to cardiovascular disease. Eur. J. Pharmacol. 2000, 410, 289–302. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A Novel User-Friendly Score (HAS-BLED) To Assess 1-Year Risk of Major Bleeding in Patients with Atrial Fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourgholi, L.; Goodarzynejad, H.; Mandegary, A.; Ziaee, S.; Talasaz, A.H.; Jalali, A.; Boroumand, M. Gene polymorphisms and the risk of warfarin-induced bleeding complications at therapeutic international normalized ratio (INR). Toxicol. Appl. Pharmacol. 2016, 309, 37–43. [Google Scholar] [CrossRef] [PubMed]
- ReidI, A.; Morris, B.J.; Ganong, W.F. The Renin-Angiotensin System. Annu. Rev. Physiol. 1978, 40, 377–410. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Hilgers, K.F.; Schlaich, M.P.; Schmidt, B.M. Renin-angiotensin system and cardiovascular risk. Lancet 2007, 369, 1208–1219. [Google Scholar] [CrossRef]
- Jeunemaitre, X.; Soubrier, F.; Kotelevtsev, Y.V.; Lifton, R.P.; Williams, C.S.; Charru, A.; Hunt, S.C.; Hopkins, P.N.; Williams, R.R.; Lalouel, J.-M.; et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell 1992, 71, 169–180. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, N.; Posadas-Romero, C.; Molina, T.V.; Vallejo, M.; Del-Valle-Mondragón, L.; Rámirez-Bello, J.; Valladares, A.; Cruz-López, M.; Vargas-Alarcón, G. Single Nucleotide Polymorphisms of the Angiotensin-Converting Enzyme (ACE) Gene Are Associated with Essential Hypertension and Increased ACE Enzyme Levels in Mexican Individuals. PLoS ONE 2013, 8, e65700. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fu, L.; Pi, L.; Che, D.; Xu, Y.; Zheng, H.; Long, H.; Zeng, L.; Huang, P.; Zhang, L.; et al. An Angiotensinogen Gene Polymorphism (rs5050) Is Associated with the Risk of Coronary Artery Aneurysm in Southern Chinese Children with Kawasaki Disease. Dis. Markers 2019, 2019, 2849695. [Google Scholar] [CrossRef] [Green Version]
- Lynch, A.I.; Tang, W.; Shi, G.; Devereux, R.B.; Eckfeldt, J.H.; Arnett, D.K. Epistatic effects of ACE I/D and AGT gene variants on left ventricular mass in hypertensive patients: The HyperGEN study. J. Hum. Hypertens. 2012, 26, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Negovan, A.; Voidăzan, S.; Pantea, M.; Moldovan, V.; Bățagă, S.; Cozlea, L.; Mocan, S.; Banescu, C. AGT A-20C (rs5050) gene polymorphism and ulcer occurrence in patients treated with low-dose aspirin: A case-control study. Rev. Romana Med. Lab. 2015, 23, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yee, J.; Chang, B.C.; Gwak, H.S. Gene Polymorphisms of the Renin-Angiotensin System and Bleeding Complications of Warfarin: Genetic-Based Machine Learning Models. Pharmaceuticals 2021, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Inoue, I.; Nakajima, T.; Williams, C.S.; Quackenbush, J.; Puryear, R.; Powers, M.; Cheng, T.; Ludwig, E.H.; Sharma, A.M.; Hata, A.; et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J. Clin. Investig. 1997, 99, 1786–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Perdomo-Pantoja, A.; Mejía-Pérez, S.I.; Reynoso-Noverón, N.; Gómez-Flores-Ramos, L.; Soto-Reyes, E.; Sánchez-Correa, T.E.; Guerra-Calderas, L.; Castro-Hernandez, C.; Vidal-Millán, S.; Sánchez-Corona, J.; et al. Angiotensinogen rs5050 germline genetic variant as potential biomarker of poor prognosis in astrocytoma. PLoS ONE 2018, 13, e0206590. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, F.; Martin, S.; Alonso, A.; Visvikis, S.; Tiret, L.; Matsuda, F.; Lathrop, G.M.; Farrall, M. High-resolution genetic mapping of the ACE-linked QTL influencing circulating ACE activity. Eur. J. Hum. Genet. 2002, 10, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Pagani, F.; Baralle, F.E. Genomic variants in exons and introns: Identifying the splicing spoilers. Nat. Rev. Genet. 2004, 5, 389–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Dong, J.; Li, L.; Dong, Z.; Deng, X. Application of a multiplex SNP genotyping system in predicting genetic susceptibility to CAD in Chinese people of Han ethnicity. Med. Sci. Monit. 2010, 16, BR384–BR395. [Google Scholar]
- Shoeb, M.; Fang, M.C. Assessing bleeding risk in patients taking anticoagulants. J. Thromb. Thrombolysis 2013, 35, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Gersh, B.J.; Sangaralingham, L.R.; Kent, D.M.; Shah, N.D.; Abraham, N.S.; Noseworthy, P.A. Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA Risk Scores in Predicting Non-Vitamin K Antagonist Oral Anticoagu-lants-Associated Bleeding in Patients with Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Menke, J.; Sollinger, D.; Schinzel, H.; Thürmel, K. Haemostasis in chronic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolakis, S.; Guo, Y.; Lane, D.A.; Buller, H.; Lip, G.Y. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: The AMADEUS trial. Eur. Heart J. 2013, 34, 3572–3579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gage, B.F.; Yan, Y.; Milligan, P.E.; Waterman, A.D.; Culverhouse, R.; Rich, M.W.; Radford, M. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am. Heart J. 2006, 151, 713–719. [Google Scholar] [CrossRef]
| Characteristics | Cases (n = 33) | Controls (n = 139) | p |
|---|---|---|---|
| Sex | 0.073 | ||
| Male | 20 (60.6) | 88 (63.3) | |
| Female | 13 (39.4) | 51 (36.7) | |
| Age (y) | 0.021 | ||
| <65 | 6 (18.2) | 55 (39.6) | |
| ≥65 | 27 (81.8) | 84 (60.4) | |
| Body weight (kg) | 0.416 | ||
| <60 | 11 (33.3) | 36 (26.3) | |
| ≥60 | 22 (66.7) | 101 (73.7) | |
| Creatinine clearance (mL/min) | 0.013 | ||
| <50 | 8 (25.0) | 11 (8.1) | |
| ≥50 | 24 (75.0) | 124 (91.9) | |
| Systolic blood pressure (mmHg) | 127.6 ± 17.9 | 131.4 ± 20.0 | 0.334 |
| Diastolic blood pressure (mmHg) | 73.0 ± 11.2 | 77.3 ± 14.4 | 0.118 |
| Modified HAS-BLED | |||
| <2 | 6 (18.2) | 52 (37.4) | 0.036 |
| ≥2 | 27 (81.8) | 87 (62.6) | |
| Type of DOAC | 1.000 | ||
| Direct thrombin inhibitors | 4 (12.1) | 16 (11.5) | |
| Factor Xa inhibitors | 29 (87.9) | 123 (88.5) | |
| Dose of DOAC a | 0.572 | ||
| Underdose | 13 (39.4) | 44 (31.7) | |
| Standard dose | 20 (60.6) | 93 (66.9) | |
| Overdose | 0 (0.0) | 2 (1.4) | |
| Alcohol | 7 (21.2) | 45 (32.8) | 0.193 |
| Smoking | 5 (15.6) | 24 (17.5) | 0.798 |
| Comorbidity | |||
| Chronic heart failure | 7 (21.2) | 34 (24.5) | 0.694 |
| Hypertension | 24 (72.7) | 96 (69.1) | 0.680 |
| Diabetes mellitus | 9 (27.3) | 33 (23.7) | 0.671 |
| Dyslipidemia | 6 (18.2) | 26 (18.7) | 0.474 |
| Hepatic abnormality | 1 (3.0) | 2 (1.4) | 0.474 |
| Renal abnormality | 2 (6.1) | 1 (0.7) | 0.095 |
| Previous stroke/TIA/thromboembolism | 17 (51.5) | 62 (44.6) | 0.474 |
| Previous bleeding events | 4 (12.1) | 13 (9.4) | 0.745 |
| Comedication | |||
| Antiplatelet drugs | 3 (9.1) | 7 (5.0) | 0.407 |
| ACEI/ARB | 16 (48.5) | 53 (38.1) | 0.275 |
| Beta-blocker | 23 (69.7) | 111 (79.9) | 0.206 |
| Calcium channel blocker | 8 (30.8) | 30 (22.1) | 0.337 |
| Diuretics | 14 (42.4) | 36 (25.9) | 0.060 |
| Statins | 20 (60.6) | 62 (44.6) | 0.098 |
| Gene Polymorphism | Minor Allele Frequency | Grouped Genotypes | Cases (n = 33) | Controls (n = 139) | p |
|---|---|---|---|---|---|
| AGT | 0.12 | GG | 27 (81.8) | 111 (81.0) | 0.916 |
| rs7079 | GT, TT | 6 (18.2) | 26 (19.0) | ||
| AGT | 0.18 | AA, AG | 9 (27.3) | 42 (30.4) | 0.721 |
| rs699 | GG | 24 (72.7) | 96 (69.6) | ||
| AGT | 0.36 | TT, TC | 25 (75.8) | 123 (89.1) | 0.083 |
| rs11122576 | CC | 8 (24.2) | 15 (10.9) | ||
| AGT | 0.20 | TT | 26 (78.8) | 76 (55.1) | 0.013 |
| rs5050 | TG, GG | 7 (21.2) | 62 (44.9) | ||
| REN | 0.19 | CC | 21 (63.6) | 92 (67.2) | 0.701 |
| rs2368564 | CT, TT | 12 (36.4) | 45 (32.8) | ||
| REN | 0.37 | GG | 12 (36.4) | 54 (39.1) | 0.769 |
| rs12750834 | GA, AA | 21 (63.6) | 84 (60.9) | ||
| ACE | 0.45 | CC, CT | 27 (81.8) | 90 (65.2) | 0.065 |
| rs1800764 | TT | 6 (18.2) | 48 (34.8) | ||
| ACE | 0.42 | GG, GC | 26 (78.8) | 89 (64.5) | 0.116 |
| rs4341 | CC | 7 (21.2) | 49 (35.5) | ||
| ACE | 0.45 | AA, AG | 28 (84.8) | 91 (66.4) | 0.038 |
| rs4353 | GG | 5 (15.2) | 46 (33.6) | ||
| AGTR1 | 0.13 | TT | 26 (78.8) | 105 (77.2) | 0.845 |
| rs275651 | AT, AA | 7 (21.2) | 31 (22.8) | ||
| AGTR1 | 0.15 | AA, AG | 8 (24.2) | 36 (26.1) | 0.828 |
| rs2640543 | GG | 25 (75.8) | 102 (73.9) | ||
| AGTR1 | 0.25 | CC, CT | 16 (48.5) | 55 (39.9) | 0.366 |
| rs5182 | TT | 17 (51.5) | 83 (60.1) | ||
| AGTR1 | 0.04 | AA | 32 (97.0) | 125 (91.2) | 0.467 |
| rs5186 | AC, CC | 1 (3.0) | 12 (8.8) | ||
| AGTR2 | 0.34 | GG, GA | 17 (51.5) | 49 (35.8) | 0.096 |
| rs1403543 | AA | 16 (48.5) | 88 (64.2) |
| Variables | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
| Female | 1.12 (0.51–2.44) | |
| Age (≥65 years) | 2.95 (1.14–7.60) | 3.08 (1.12–8.46) * |
| CrCl (<50 mL/min) | 3.76 (1.37–10.32) | 4.16 (1.41–12.31) * |
| AGT rs5050 TT | 3.03 (1.23–7.45) | 3.56 (1.35–9.43) * |
| ACE rs4353 AA, AG | 2.83 (1.03–7.81) | 3.14 (1.06–9.31) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, J.; Song, T.-J.; Yoon, H.-Y.; Park, J.; Gwak, H.-S. Genetic Factors of Renin–Angiotensin System Associated with Major Bleeding for Patients Treated with Direct Oral Anticoagulants. Pharmaceutics 2022, 14, 231. https://doi.org/10.3390/pharmaceutics14020231
Yee J, Song T-J, Yoon H-Y, Park J, Gwak H-S. Genetic Factors of Renin–Angiotensin System Associated with Major Bleeding for Patients Treated with Direct Oral Anticoagulants. Pharmaceutics. 2022; 14(2):231. https://doi.org/10.3390/pharmaceutics14020231
Chicago/Turabian StyleYee, Jeong, Tae-Jin Song, Ha-Young Yoon, Junbeom Park, and Hye-Sun Gwak. 2022. "Genetic Factors of Renin–Angiotensin System Associated with Major Bleeding for Patients Treated with Direct Oral Anticoagulants" Pharmaceutics 14, no. 2: 231. https://doi.org/10.3390/pharmaceutics14020231
APA StyleYee, J., Song, T.-J., Yoon, H.-Y., Park, J., & Gwak, H.-S. (2022). Genetic Factors of Renin–Angiotensin System Associated with Major Bleeding for Patients Treated with Direct Oral Anticoagulants. Pharmaceutics, 14(2), 231. https://doi.org/10.3390/pharmaceutics14020231








