Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review
Abstract
:1. Introduction
- (a)
- Limited solubility in aqueous solutions: most of the chemotherapy drugs are hydrophobic. Thus, solvents are used to solubilize these drugs, which increases their toxicity.
- (b)
- Poor specific targeting of the cancer cells, i.e., high toxic dosages, are delivered to healthy as well as cancer cells.
- (c)
- Cancer cells can develop resistance to chemotherapy drugs, a phenomenon known as multi-drug resistance (MDR). This results in minimal cell death and the expansion of drug-resistant tumors.
2. Nanoparticles as Drug Delivery Systems (DDS)
3. DOX Delivery Systems Based on Liposomes
4. DOX Delivery Systems Based on Micelles
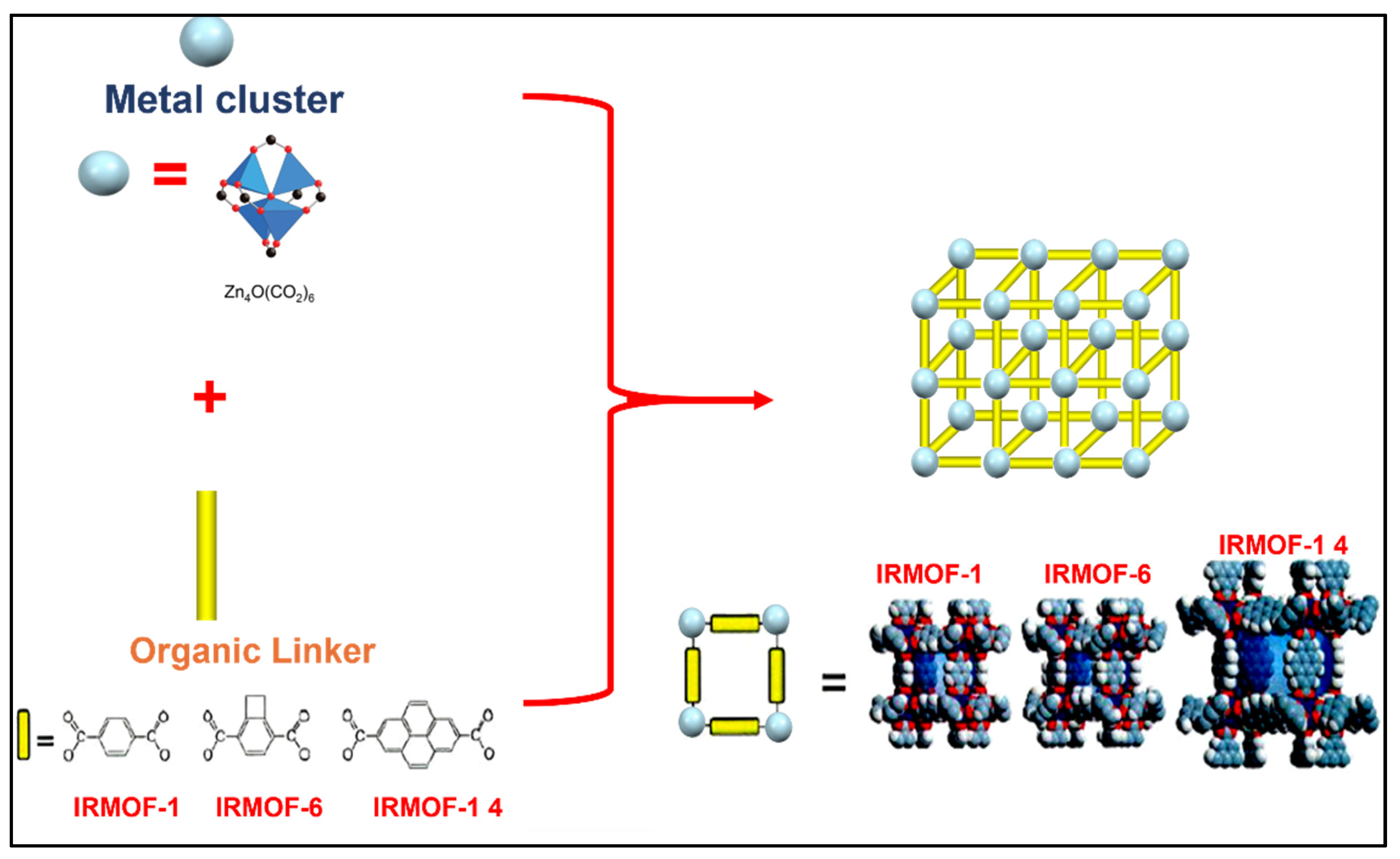
5. DOX Delivery Systems Based on Metal-Organic Frameworks (MOFs)
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husseini, G.A.; Jabbar, N.A.; Mjalli, F.S.; Pitt, W.G. Modeling and sensitivity analysis of acoustic release of Doxorubicin from unstabilized pluronic P105 using an artificial neural network model. Technol. Cancer Res. Treat. 2007, 6, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagos, E.G.; Ghaleb, A.M.; Dalton, W.B.; Bialkowska, A.B.; Yang, V.W. Mouse embryonic fibroblasts null for the Krüppel-like factor 4 gene are genetically unstable. Oncogene 2009, 28, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, E.; Zamora, P.; Feliu, J.; Barón, M.G. Classification of anticancer drugs—a new system based on therapeutic targets. Cancer Treat. Rev. 2003, 29, 515–523. [Google Scholar] [CrossRef]
- Espinosa, E.; Raposo, C.G. Classification of Anticancer Drugs Based on Therapeutic Targets. In Macromolecular Anticancer Therapeutics; Springer: New York, NY, USA, 2009; pp. 3–35. [Google Scholar] [CrossRef]
- Wu, X.-Z. A new classification system of anticancer drugs—Based on cell biological mechanisms. Med. Hypotheses 2006, 66, 883–887. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mukherjee, S. Cancer Therapy Using Antibiotics. J. Cancer Ther. 2015, 6, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Mier, W.; Hoffend, J.; Haberkorn, U.; Eisenhut, M. Current Strategies in Tumor-Targeting. In Apoptotic Pathways as Targets for Novel Therapies in Cancer and Other Diseases; Springer: Boston, MA, USA, 2005; pp. 343–355. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, I.; dos Santos Guimarães, I.; Daltoé, R.D.; Herlinger, A.L.; Madeira, K.P.; Ladislau, T.; Valadão, I.C.; Lyra, P.C.M., Jr.; Teixeira, S.F.; Amorim, G.M.; et al. Conventional cancer treatment. In Cancer Treatment—Conventional and Innovative Approaches; Rangel, L., Ed.; Intech: Rijeka, Croatia, 2013; pp. 3–35. [Google Scholar] [CrossRef] [Green Version]
- Kakde, D.; Kakde, R.; Patil, A.T.; Shrivastava, V.; Jain, D. Cancer Therapeutics-Opportunities, Challenges and Advances in Drug Delivery. J. Appl. Pharm. Sci. 2011, 1, 1–10. [Google Scholar]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Akhdar, H.; Legendre, C.; Aninat, C.; More, F. Anticancer Drug Metabolism: Chemotherapy Resistance and New Therapeutic Approaches. In Topics on Drug Metabolism; Paxton, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 138–170. [Google Scholar]
- Corrie, P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2011, 39, 717–722. [Google Scholar] [CrossRef]
- DeVita, V.T. The Evolution of Therapeutic Research in Cancer. N. Engl. J. Med. 1978, 298, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Osler, W. Principles Practice Medicine; D. Appleton and Company: New York, NY, USA, 1893; Volume 708. [Google Scholar]
- Fujiwara, A.; Hoshino, T.; Westley, J.W. Anthracycline Antibiotics. Crit. Rev. Biotechnol. 1985, 3, 133–157. [Google Scholar] [CrossRef]
- Ajaykumar, C. Overview on the Side Effects of Doxorubicin. In Advances in Precision Medicine Oncology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Tyleckova, J.; Hrabakova, R.; Mairychova, K.; Halada, P.; Radova, L.; Džubák, P.; Hajduch, M.; Gadher, S.J.; Kovarova, H. Cancer Cell Response to Anthracyclines Effects: Mysteries of the Hidden Proteins Associated with These Drugs. Int. J. Mol. Sci. 2012, 13, 15536–15564. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, A.; Dosio, F.; Mougin, J.; Ferrero, A.; Wack, S.; Reddy, L.H.; Weyn, A.A.; Lepeltier, E.; Bourgaux, C.; Stella, B.; et al. A Unique Squalenoylated and Nonpegylateddoxorubicin Nanomedicine with Systemiclong-Circulating Properties and Anticancer Activity. Proc. Natl. Acad. Sci. 2014, 111, E217–E226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkova, M. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Fernando, A.; Gatot, D.; Sitepu, Y.I.F. The Differences of Myelosuppression before and after Doxorubicin Chemotherapy in Breast Cancer Patients in Rsup. H. Adam Malik Medan. Int. J. Res. Rev. 2021, 8, 18–24. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk Factors for Doxorubicin-lnduced Congestive Heart Failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Galmarini, D.; Galmarini, C.M.; Galmarini, F.C. Cancer chemotherapy: A critical analysis of its 60 years of history. Crit. Rev. Oncol. 2012, 84, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. Semipermeable Microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. The in vivo Effects of Semipermeable Microcapsules containing L-Asparaginase on 6C3HED Lymphosarcoma. Nature 1971, 229, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.M.S. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif. Cells Nanomed. Biotechnol. 2019, 47, 997–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skipper, H.E.; Thomson, J.R.; Bell, M. Attempts at dual blocking of biochemical events in cancer chemotherapy. Cancer Res. 1954, 14, 503–507. [Google Scholar]
- Weber, G. Biochemical Strategy of Cancer Cells and the Design of Chemotherapy: G.H.A. Clowes Memorial Lecture. Cancer Res. 1983, 43, 3466–3492. [Google Scholar]
- Prabha, S.; Arya, G.; Chandra, R.; Ahmed, B.; Nimesh, S. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif. Cells Nanomed. Biotechnol. 2014, 44, 83–91. [Google Scholar] [CrossRef]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; ter Haar, G.; Allen, T.M.; Pitt, W.G.; Husseini, G.A. Ultrasound-Responsive Nanocarriers in Cancer Treatment: A Review. ACS Pharmacol. Transl. Sci. 2021, 4, 589–612. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Tomao, S.; Tomao, F.; Rossi, L.; Zaccarelli, E.; Caruso, D.; Zoratto, F.; Panici, P.B.; Papa, A. Angiogenesis and antiangiogenic agents in cervical cancer. OncoTargets Ther. 2014, 7, 2237–2248. [Google Scholar] [CrossRef] [Green Version]
- Dreher, M.; Liu, W.; Michelich, C.; Dewhirst, M.; Yuan, F.; Chilkoti, A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J. Natl. Cancer Inst. 2006, 5, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Tsukigawa, K.; Fang, J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy-Problems, Solutions, and Prospects. Microcirculation 2015, 23, 173–182. [Google Scholar] [CrossRef]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Al Basha, S.; Salkho, N.; Dalibalta, S.; Husseini, G.A.; Sameer, A.S.; Banday, M.Z.; Nissar, S.; Saeed, S.A. Liposomes in Active, Passive and Acoustically-Triggered Drug Delivery. Mini-Rev. Med. Chem. 2019, 19, 961–969. [Google Scholar] [CrossRef]
- Bangham, A.; Horne, R. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and Use of Liposomes as Models of Biological Membranes. In Methods in Membrane Biology; Korn, E., Ed.; Springer: Boston, MA, USA, 1974; pp. 1–68. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Buckland, R.A. Enzyme-containing Liposomes alleviate a Model for Storage Disease. Nature 1973, 244, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.; Mayer, L.; Bally, M.; Madden, T.; Hope, M. Generating and loading of liposomal systems for drug-delivery applications. Adv. Drug Deliv. Rev. 1989, 3, 267–282. [Google Scholar] [CrossRef]
- Chonn, A.; Cullis, P.R. Recent advances in liposomal drug-delivery systems. Curr. Opin. Biotechnol. 1995, 6, 698–708. [Google Scholar] [CrossRef]
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; Intech: Rijeka, Croatia, 2014. [Google Scholar]
- Crommelin, D.; van Bloois, L. Preparation and characterization of doxorubicin-containing liposomes. II. Loading capacity, long-term stability and doxorubicin-bilayer interaction mechanism. Int. J. Pharm. 1983, 17, 135–144. [Google Scholar] [CrossRef]
- Gubernator, J. Active methods of drug loading into liposomes: Recent strategies for stable drug entrapment and increasedin vivoactivity. Expert Opin. Drug Deliv. 2011, 8, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.; Bally, M.; Cullis, P. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim. Biophys. Acta (BBA) Biomembr. 1986, 857, 123–126. [Google Scholar] [CrossRef]
- Swenson, C.E.; Perkins, W.R.; Roberts, P.; Janoff, A.S. Liposome Technology and the Development of Myocet TM (Liposomal Doxorubicin Citrate). Breast 2001, 2, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Hirsh, D.J.; Cabral-Lilly, D.; Zirkel, A.; Gruner, S.M.; Janoff, A.S.; Perkins, W.R. Doxorubicin physical state in solution and inside liposomes loaded via a pH gradient. Biochim. Biophys. Acta (BBA) Biomembr. 1998, 1415, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Kanter, P.M.; A Bullard, G.; A Ginsberg, R.; Pilkiewicz, F.G.; Mayer, L.D.; Cullis, P.R.; Pavelic, Z.P. Comparison of the cardiotoxic effects of liposomal doxorubicin (TLC D-99) versus free doxorubicin in beagle dogs. Vivo 1993, 7, 17–26. [Google Scholar]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Alyane, M.; Barratt, G.; Lahouel, M. Remote loading of doxorubicin into liposomes by transmembrane pH gradient to reduce toxicity toward H9c2 cells. Saudi Pharm. J. 2015, 24, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Sakakibara, T.; Chen, F.-A.; Kida, H.; Kunieda, K.; Cuenca, R.E.; Martin, F.J.; Bankert3, R.B. Doxorubicin Encapsulated in Sterically Stabilized Liposomes Is Superior to Free Drug or Drug-Containing Conventional Liposomes at Suppressing Growth and Métastases of Human Lung Tumor Xenografts1. Cancer Res. 1996, 56, 3743–3746. [Google Scholar]
- Dosio, F.; Cattel, L. PEGylation of Proteins and Liposomes: A Powerful and Flexible Strategy to Improve the Drug Delivery. Curr. Drug Metab. 2012, 13, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Fritze, A.; Hens, F.; Kimpfler, A.; Schubert, R.; Peschka-Süss, R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
- Achim, M.; Precup, C.; Gonganău-Niţu, D.; Barbu-Tudoran, L.; Porfire, A.S.; Scurtu, R.; Ciuce, C. Thermosensitive Liposomes Containing Doxorubicin. Preparation and In Vitro Evaluation. Farmacia 2009, 57, 6. [Google Scholar]
- Pitt, W.G.; Husseini, G.A.; Roeder, B.L.; Dickinson, D.J.; Warden, D.R.; Hartley, J.M.; Jones, P.W. Preliminary Results of Combining Low Frequency Low Intensity Ultrasound and Liposomal Drug Delivery to Treat Tumors in Rats. J. Nanosci. Nanotechnol. 2011, 11, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Yuh, E.L.; Shulman, S.G.; Mehta, S.A.; Xie, J.; Chen, L.; Frenkel, V.; Bednarski, M.D.; Li, K. Delivery of Systemic Chemotherapeutic Agent to Tumors by Using Focused Ultrasound: Study in a Murine Model. Radiology 2005, 234, 431–437. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Li, Y.; Ye, F.F.; Yin, P.P.; Yu, X.; Hu, M.N.; Fu, Y.S.; Wang, C.; Shang, D.J. The Bifunctional Liposomes Constructed by Poly(2-ethyl-oxazoline)-cholesteryl Methyl Carbonate: An Effectual Approach to Enhance Liposomal Circulation Time, pH-Sensitivity and Endosomal Escape. Pharm. Res. 2014, 31, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef]
- Yang, B. Preclinical study of Doxorubicine-loaded liposomal drug delivery for the treatment of head and neck cancer: Optimization by Box-Behnken statistical design. Acta Biochim. Pol. 2020, 67, 149–155. [Google Scholar] [CrossRef]
- Elamir, A.; Ajith, S.; Al Sawaftah, N.; Abuwatfa, W.; Mukhopadhyay, D.; Paul, V.; Al-Sayah, M.H.; Awad, N.; Husseini, G.A. Ultrasound-triggered herceptin liposomes for breast cancer therapy. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS PharmSciTech 2020, 21, 202. [Google Scholar] [CrossRef]
- Yin, X.; Feng, S.; Chi, Y.; Liu, J.; Sun, K.; Guo, C.; Wu, Z. Estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides for the therapy of osteosarcoma. Drug Deliv. 2018, 25, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Kirchmeier, M.; Moase, E.; Zalipsky, S.; Allen, T. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1515, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Arabi, L.; Badiee, A.; Mosaffa, F.; Jaafari, M.R. Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin. J. Control. Release 2015, 220, 275–286. [Google Scholar] [CrossRef]
- Wang, W.; Shao, A.; Zhang, N.; Fang, J.; Ruan, J.J.; Ruan, B.H. Cationic Polymethacrylate-Modified Liposomes Significantly Enhanced Doxorubicin Delivery and Antitumor Activity. Sci. Rep. 2017, 7, 43036. [Google Scholar] [CrossRef] [Green Version]
- Ieranò, C.; Portella, L.; Lusa, S.; Salzano, G.; D’Alterio, C.; Napolitano, M.; Buoncervello, M.; Macchia, D.; Spada, M.; Barbieri, A.; et al. CXCR4-antagonist Peptide R-liposomes for combined therapy against lung metastasis. Nanoscale 2016, 8, 7562–7571. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Diao, W.; Xue, H.; Wu, F.; Wang, W.; Jiang, B.; Bai, J.; Lian, B.; Feng, W.; Sun, T.; et al. Improved efficacy of doxorubicin delivery by a novel dual-ligand-modified liposome in hepatocellular carcinoma. Cancer Lett. 2020, 489, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Safra, T.; Muggia, F.; Jeffers, S.; Tsao-Wei, D.D.; Groshen, S.; Lyass, O.; Henderson, R.; Berry, G.; Gabizon, A. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann. Oncol. 2000, 11, 1029–1034. [Google Scholar] [CrossRef]
- Kesterson, J.P.; Odunsi, K.; Lele, S. High cumulative doses of pegylated liposomal doxorubicin are not associated with cardiac toxicity in patients with gynecologic malignancies. Chemotherapy 2010, 56, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, A.; Tahara, K.; Hane, Y.; Matsui, T.; Sasaoka, S.; Hatahira, H.; Motooka, Y.; Hasegawa, S.; Naganuma, M.; Abe, J.; et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PloS ONE 2017, 12, e0185654. [Google Scholar] [CrossRef]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Tomkinson, B.; Bendele, R.; Giles, F.J.; Brown, E.; Gray, A.; Hart, K.; LeRay, J.D.; Meyer, D.; Pelanne, M.; Emerson, D.L. OSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALL. Leuk. Res. 2003, 27, 1039–1050. [Google Scholar] [CrossRef]
- Lyass, O.; Uziely, B.; Ben-Yosef, R.; Tzemach, D.; Heshing, N.I.; Lotem, M.; Brufman, G.; Gabizon, A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 2000, 89, 1037–1047. [Google Scholar] [CrossRef]
- Ranson, M.R.; Carmichael, J.; O’Byrne, K.; Stewart, S.; Smith, D.; Howell, A. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: Results of a multicenter phase II trial. J. Clin. Oncol. 1997, 15, 3185–3191. [Google Scholar] [CrossRef]
- FDA’s New Population Pharmacokinetics Guidance—What Drug/Biologic/Device Makers Need to Know | Guidehouse. Available online: https://guidehouse.com/insights/healthcare/2019/fdas-new-population-pharmacokinetics-guidance (accessed on 3 January 2022).
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Harris, L.; Batist, G.; Belt, R.; Rovira, D.; Navari, R.; Azarnia, N.; Welles, L.; Winer, E.; TLC D-99 Study Group. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002, 94, 25–36. [Google Scholar] [CrossRef]
- Lammers, T.; E Hennink, W.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef]
- Yoo, H.; Park, T.G. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA–PEG block copolymer. J. Control. Release 2001, 70, 63–70. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.S.; Oh, K.T.; Gao, Z.G.; Bae, Y.H. Doxorubicin-Loaded Polymeric Micelle Overcomes Multidrug Resistance of Cancer by Double-Targeting Folate Receptor and Early Endosomal pH. Small 2008, 4, 2043–2050. [Google Scholar] [CrossRef] [Green Version]
- Cuong, N.-V.; Jiang, J.-L.; Li, Y.-L.; Chen, J.-R.; Jwo, S.-C.; Hsieh, M.-F. Doxorubicin-Loaded PEG-PCL-PEG Micelle Using Xenograft Model of Nude Mice: Effect of Multiple Administration of Micelle on the Suppression of Human Breast Cancer. Cancers 2010, 3, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.-V.; Hsieh, M.-F.; Chen, Y.-T.; Liau, I. Synthesis and characterization of PEG-PCL-PEG triblock copolymers as carriers of doxorubicin for the treatment of breast cancer. J. Appl. Polym. Sci. 2010, 117, 3694–3703. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-HER2-targeted doxorubicin–core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, ume 14, 4105–4121. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Kulkarni, A.; Nagesha, D.; Sridhar, S. In Vitro Evaluation of Theranostic Polymeric Micelles for Imaging and Drug Delivery in Cancer. Theranostics 2012, 2, 714–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dube, N.; Shu, J.Y.; Dong, H.; Seo, J.W.; Ingham, E.; Kheirolomoom, A.; Chen, P.-Y.; Forsayeth, J.; Bankiewicz, K.; Ferrara, K.W.; et al. Evaluation of Doxorubicin-Loaded 3-Helix Micelles as Nanocarriers. Biomacromolecules 2013, 14, 3697–3705. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zou, Y.; Deng, C.; Cheng, R.; Meng, F.; Zhong, Z. Intracellular release of doxorubicin from core-crosslinked polypeptide micelles triggered by both pH and reduction conditions. Biomaterials 2013, 34, 5262–5272. [Google Scholar] [CrossRef]
- Sui, B.; Xu, H.; Jin, J.; Gou, J.; Liu, J.; Tang, X.; Zhang, Y.; Xu, J.; Zhang, H.; Jin, X. Self-Assembled Micelles Composed of Doxorubicin Conjugated Y-Shaped PEG-Poly(glutamic acid)2 Copolymers via Hydrazone Linkers. Molecules 2014, 19, 11915–11932. [Google Scholar] [CrossRef] [Green Version]
- Varela-Moreira, A.; Shi, Y.; Fens, M.H.A.M.; Lammers, T.; Hennink, W.E.; Schiffelers, R.M. Clinical application of polymeric micelles for the treatment of cancer. Mater. Chem. Front. 2017, 1, 1485–1501. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [Green Version]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef] [PubMed]
- Kadhom, M.; Deng, B. Metal-organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.-S.; Di Serio, M. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Liu, Y.; Yao, R.; Wang, X.; Cao, Y.; Ma, D.; Zou, M.; Cao, A.; Feng, X.; et al. Membrane adsorbers with ultrahigh metal-organic framework loading for high flux separations. Nat. Commun. 2019, 10, 4204. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.-G.; Hu, T.; Bu, X. Metal–Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2019, 32, e1806445. [Google Scholar] [CrossRef]
- Gautam, S.; Cole, D. CO2 Adsorption in Metal-Organic Framework Mg-MOF-74: Effects of Inter-Crystalline Space. Nanomaterials 2020, 10, 2274. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.M.; Madden, D.G.; Wheatley, A.E.H.; Fairen-Jimenez, D. Shaping the Future of Fuel: Monolithic Metal–Organic Frameworks for High-Density Gas Storage. J. Am. Chem. Soc. 2020, 142, 8541–8549. [Google Scholar] [CrossRef]
- Karam, L.; Reboul, J.; Casale, S.; Massiani, P.; El Hassan, N. Porous Nickel-Alumina Derived from Metal-Organic Framework (MIL-53): A New Approach to Achieve Active and Stable Catalysts in Methane Dry Reforming. ChemCatChem 2019, 12, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Karam, L.; Bacariza, M.C.; Lopes, J.M.; Henriques, C.; Reboul, J.; El Hassan, N.; Massiani, P. Mesoporous nickel-alumina catalysts derived from MIL-53(Al) metal-organic framework: A new promising path for synthesizing CO2 methanation catalysts. J. CO2 Util. 2021, 51, 101651. [Google Scholar] [CrossRef]
- El Taher, B.J.; Sabouni, R.; Ghommem, M. Luminescent metal organic framework for selective detection of mercury in aqueous media: Microwave-based synthesis and evaluation. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 607, 125477. [Google Scholar] [CrossRef]
- Strauss, I.; Chakarova, K.; Mundstock, A.; Mihaylov, M.; Hadjiivanov, K.; Guschanski, N.; Caro, J. UiO-66 and UiO-66-NH2 based sensors: Dielectric and FTIR investigations on the effect of CO2 adsorption. Microporous Mesoporous Mater. 2020, 302, 110227. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Yang, F.; Zhou, L.L.; Yin, B.; Wang, P.; Wang, L. Achieving high power density and excellent durability for high temperature proton exchange membrane fuel cells based on crosslinked branched polybenzimidazole and metal-organic frameworks. J. Membr. Sci. 2021, 630, 119288. [Google Scholar] [CrossRef]
- Ponnada, S.; Kiai, M.S.; Gorle, D.B.; Nowduri, A.; Sharma, R.K. Insight into the Role and Strategies of Metal–Organic Frameworks in Direct Methanol Fuel Cells: A Review. Energy Fuels 2021, 35, 15265–15284. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Lei, J.; Shen, H.; Ju, H. Multifunctional Metal–Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158. [Google Scholar] [CrossRef]
- Gallis, D.F.S.; Rohwer, L.E.S.; Rodriguez, M.A.; Barnhart-Dailey, M.C.; Butler, K.; Luk, T.S.; Timlin, J.A.; Chapman, K.W. Multifunctional, Tunable Metal–Organic Framework Materials Platform for Bioimaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 22268–22277. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Co-Ord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sabouni, R.; Husseini, G.A.; Karami, A.; Bai, R.G.; Mukhopadhyay, D. Facile Ultrasound-Triggered Release of Calcein and Doxorubicin from Iron-Based Metal-Organic Frameworks. J. Biomed. Nanotechnol. 2020, 16, 1359–1369. [Google Scholar] [CrossRef]
- Yang, X.-X.; Feng, P.; Cao, J.; Liu, W.; Tang, Y. Composition-Engineered Metal–Organic Framework-Based Microneedles for Glucose-Mediated Transdermal Insulin Delivery. ACS Appl. Mater. Interfaces 2020, 12, 13613–13621. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Rizvi, S.H.M.; Sushma; Mahdi, F.; Ahmad, I.; Singh, P.P.; Mahdi, A.A. Intranasal exposure to silica nanoparticles induces alterations in pro-inflammatory environment of rat brain. Toxicol. Ind. Health 2016, 33, 119–132. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Gao, H.; Chen, S.; Wang, J. Metal-organic framework-based nanomaterials for biomedical applications. Chin. Chem. Lett. 2019, 31, 1060–1070. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Kumari, A.; Sahu, S.K. IRMOF-3: A fluorescent nanoscale metal organic frameworks for selective sensing of glucose and Fe (III) ions without any modification. Mater. Sci. Eng. C 2018, 92, 913–921. [Google Scholar] [CrossRef]
- Ning, D.; Liu, Q.; Wang, Q.; Du, X.-M.; Ruan, W.-J.; Li, Y. Luminescent MOF nanosheets for enzyme assisted detection of H2O2 and glucose and activity assay of glucose oxidase. Sens. Actuators B: Chem. 2018, 282, 443–448. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellana-Tavra, C.; Köppen, M.; Li, A.; Stock, N.; Fairen-Jimenez, D. Biocompatible, Crystalline, and Amorphous Bismuth-Based Metal–Organic Frameworks for Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 5633–5641. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically porous metal–organic frameworks: Synthetic strategies and applications. Natl. Sci. Rev. 2019, 7, 1743–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabtamu, D.M.; Wu, Y.-N.; Li, F. Hierarchically porous metal–organic frameworks: Synthesis strategies, structure(s), and emerging applications in decontamination. J. Hazard. Mater. 2020, 397, 122765. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Vermeulen, N.A.; Malliakas, C.D.; Gómez-Gualdrón, D.A.; Howarth, A.J.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; O’Keeffe, M.; Farha, O.K. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 2017, 356, 624–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Cui, Y.; Yang, Y.; Hu, Q.; Qian, G. A biocompatible metal–organic framework as a pH and temperature dual-responsive drug carrier. Dalton Trans. 2018, 47, 15882–15887. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Wang, M.; Jiang, Z.; Qi, W.; Su, R.; He, Z. Constructing Redox-Responsive Metal–Organic Framework Nanocarriers for Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 16698–16706. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal-Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2018, 6, 1801526. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sébrié, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Borghi, F.; Manoli, F.; Manet, I.; Agostoni, V.; Reschiglian, P.; Gref, R.; Monti, S. Host–Guest Interactions in Fe(III)-Trimesate MOF Nanoparticles Loaded with Doxorubicin. J. Phys. Chem. B 2014, 118, 8532–8539. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.J.; Horcajada, P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2013, 2, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, C.; Das, A.; Chakraborty, A. Zeolitic Imidazole Framework (ZIF) Nanospheres for Easy Encapsulation and Controlled Release of an Anticancer Drug Doxorubicin under Different External Stimuli: A Way toward Smart Drug Delivery System. Mol. Pharm. 2015, 12, 3158–3166. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Kundu, T.; Mitra, S.; Patra, P.; Goswami, A.; Díaz, D.D.; Banerjee, R. Mechanical Downsizing of a Gadolinium(III)-based Metal-Organic Framework for Anticancer Drug Delivery. Chem. A Eur. J. 2014, 20, 10514–10518. [Google Scholar] [CrossRef]
- Tan, L.-L.; Li, H.; Qiu, Y.-C.; Chen, D.-X.; Wang, X.; Pan, R.-Y.; Wang, Y.; Zhang, S.X.-A.; Wang, B.; Yang, Y.-W. Stimuli-responsive metal–organic frameworks gated by pillar[5]arene supramolecular switches. Chem. Sci. 2014, 6, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.-X.; Zou, Q.; Sun, S.-K.; Yu, C.; Zhang, X.; Li, R.-J.; Fu, Y.-Y. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef] [Green Version]
- Yalamandala, B.N.; Shen, W.; Min, S.; Chiang, W.; Chang, S.; Hu, S. Advances in Functional Metal-Organic Frameworks Based On-Demand Drug Delivery Systems for Tumor Therapeutics. Adv. NanoBiomed. Res. 2021, 1, 2100014. [Google Scholar] [CrossRef]
- Fytory, M.; Arafa, K.K.; El Rouby, W.M.A.; Farghali, A.A.; Abdel-Hafiez, M.; El-Sherbiny, I.M. Dual-ligated metal organic framework as novel multifunctional nanovehicle for targeted drug delivery for hepatic cancer treatment. Sci. Rep. 2021, 11, 19808. [Google Scholar] [CrossRef]
- Li, X.; Porcino, M.; Qiu, J.; Constantin, D.; Martineau-Corcos, C.; Gref, R. Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials 2021, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yu, J.; Lu, F.; Jiang, H.; Wang, X. Enhancement of Cancer Chemotherapeutic Efficacy via Bone-Targeted Drug Delivery Carrier in Bone Metastases. Drug Des. Dev. Ther. 2021, ume 15, 4455–4468. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Sun, K.; Hu, J.; Chen, F.; Liu, W.; Chen, J.; Sun, B.; Hossain, A.M.S. A novel pH-responsive Fe-MOF system for enhanced cancer treatment mediated by the Fenton reaction. New J. Chem. 2021, 45, 3271–3279. [Google Scholar] [CrossRef]
- Kim, E.-M.; Jeong, H.-J. Liposomes: Biomedical Applications. Chonnam Med. J. 2021, 57, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly Enhanced Permeability and Retention Effects Induced by Photo-immunotherapy of Tumors. ACS Nano 2012, 7, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
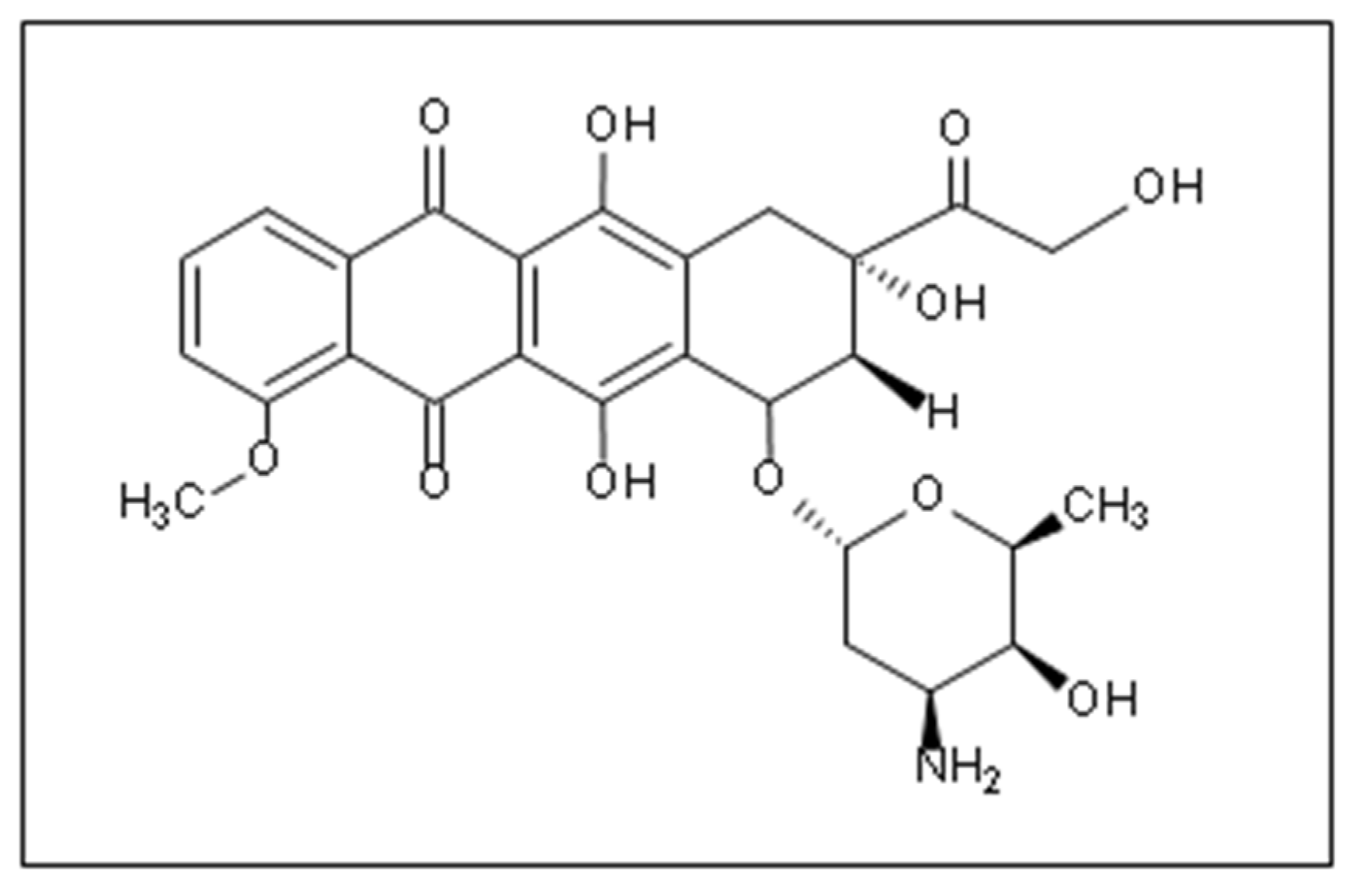

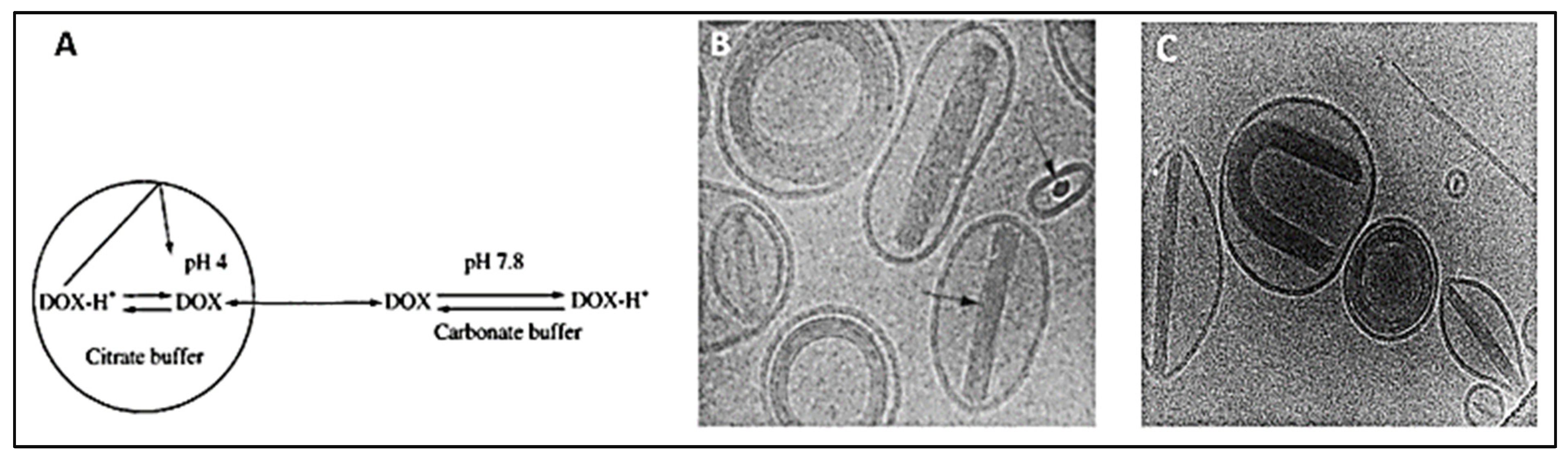


| Salt Gradient | Size ± SD (nm) | EE (%) |
|---|---|---|
| Ammonium Phosphate | 129.3 ± 3.7 | 98 |
| Ammonium Sulfate | 129.2 ± 2.9 | 95 |
| Ammonium Acetate | 115.9 ± 1.0 | 77 |
| Ammonium Citrate | 114.9 ± 1.2 | 100 |
| Sodium Phosphate | 113.4 ± 1.6 | 52 |
| Sodium Sulfate | 111.8 ± 1.9 | 44 |
| Sodium Acetate | 113.4 ± 1.6 | 16 |
| Sodium Citrate | 151.7 ± 3.8 | 54 |
| Preparation Method | Target Cancer | Functionalization | Study Model | Triggering Modality | Findings | Ref. |
|---|---|---|---|---|---|---|
| Ethanol injection | osteosarcoma | Estrogen | In vitro flow cytometry and MTT analysis on MG63 (estrogen overexpressing) cells and LO2 (negative liver cells). Ex vivo imaging of MG63 tumors extracted from Male BALB/c nude mice. | Redox-sensitivity and glutathione responsiveness | Loaded decorated liposomes size~110 nm. Exhibited high encapsulation efficiency. Ex vivo analysis of the functionalized liposomes showed more selective accumulation in tumor tissues compared to other vital organs, and in vitro results showed higher cytotoxicity towards overexpressing cells. | [67] |
| Thin-film hydration | Lymphoma | anti-CD19 moiety; PEG grafted by disulfide links (mPEG-S-S-DSPE) | In vitro MTT assay In vivo model: Female BALB/c Cr Alt B/M mice bearing Namalwa cells | pH sensitivity | Liposomes decorated with cleavable PEG chains rapidly dissociated in the plasma. The pH-sensitive liposomes, targeting the CD19 epitope excessively abundant on B-lymphoma cells, showed increased selective cytotoxicity towards these cells, and enhanced release kinetics at lower pH levels. | [68] |
| Post-insertion; mixing with preformed DOXIL | Cancer Stem Cells (CSCs) | anti-CD44 monoclonal antibody (mAb) | In vitro flow cytometry and MTT assay on C-26 and NIH-3T3 (non-tumor) cells. In vivo model: female BALB/c mice bearing C-26 colon carcinoma. | N/A | Functionalization of DOXIL liposomes significantly increased their size. The IC50 values were lower on the C-26 cell line overexpressing CD44, while higher values were reported for the negative cell line (NIH-3T3). | [69] |
| Solvent evaporation | Various cancers | Cationic Polymethacrylate Eudragit RL100 | In vitro flow cytometry and MTT assay on MCF7/adr and H22 cells. In vivo model: ICR mice bearing aggressive liver cancer H22 cells. | N/A | Functionalization of liposomes with Polymethacrylate derivatives increases their cellular internalization and antitumoral activity. The in vivo results showed that four injections of the functionalized formulation led to tumor size reduction by 60%. | [70] |
| Thin-film hydration | Metastatic lung cancer | CXCR4-antagonist cyclic peptide (peptide R) | In vitro cytotoxicity assay. In vivo model: C57BL/6 mice bearing B16 human melanoma cells | N/A | In vitro results showed that targeting significantly decreased the IC50 while reducing metastasis and regression in tumor size growth. | [71] |
| Film dispersion | hepatocellular carcinoma (HCC) | glycyrrhetinic acid (GA) and peanut agglutinin (PNA) | In vitro specific uptake of HepG2, MCF-7, and SMMC-7721 cells In vivo model: male BALB/C-nu mice bearing SMMC-7721 xenografts. | N/A | HepG2 cells showed the highest uptake towards the liposomes functionalized with GA alone, while MCF-7 showed the highest affinity towards the PNA functionalized liposomes. The dual-targeted liposomal formulation was most internalized by the SMMC-7721 | [72] |
| Formulation | Phase | Therapeutic Indication | Survival Rate (SR) | Progression-Free Survival | Incidence of AEs |
|---|---|---|---|---|---|
| All Presented Comparisons are Against Treatment with Free DOX | |||||
| Myocet® | III | Metastatic breast cancer | First-year SR: 69% vs. 64% | 4.3 vs. 3.6 months | Cardiac events: 13% vs. 29% Mucositis/stomatitis: 8.6% vs. 11.9% Nausea/vomiting: 12.3% vs. 20.3% |
| DOXIL® | Overall SR: 21 months vs. 22 months | 6.9 months vs. 7.8 months | Cardiotoxic implications: 3.9% vs. 18.8% Vomiting: 19% vs. 31% Alopecia: 20% vs. 66% Neutropenia: 4% vs. 10% PPE: 48% vs. 2% Stomatitis: 22% vs. 15% Mucositis: 23% vs. 13% | ||
| Formulation | Composition | Features | Preclinical Studies | Clinical Trials |
|---|---|---|---|---|
| SP1049C | Pluronic® L61 and L127 | Average size~30 nm Physical DOX loading EE~8.2% | In vitro Enhanced activity against multidrug-resistant (MDR) cells In vivo 2-fold higher AUC (14.6 vs. 7.1 μg hr/mL) Lewis lung tumor growth in mice got arrested in more than 50% of 9 tumor models | Phase I: patients with advanced solid tumors Administered doses ranged from 5 to 90 mg/m2, once every 3 weeks for six cycles The maximum tolerated dose (MTD) was 70 mg/m2 The micellar formulation showed a similar toxicity profile to free DOX 11.5% of the patients had a partial or complete response to the micellar treatment The median time for disease progression: 17.5 weeks in 30.8% of the patients Phase II: patients with advanced adenocarcinoma of the esophagus or gastroesophageal junction Administered dose was 75 mg/m2, once every 3 weeks for six cycles Grade 3 or 4 neutropenia was observed in 62% of the patient Median overall survival: 9.96 months Median progression-free survival: 6.6 months Phase III: approved |
| NK911 | poly(ethylene glycol)-b-poly(α,βaspartic acid) | Average size~40 nm DOX was covalently conjugated to 50% of the micelles’ carboxylic groups as well as physically loaded into the cores | In vivo 29-fold higher AUC (120 vs. 4 μg hr/mL) in mice bearing colon-26 carcinoma 3.4-folds higher accumulation at the tumor site (1605 vs. 474 μg hr/mL) effectively arrested the growth of sarcoma, lung, colon and breast cancer in different mouse models | Phase I: patients with metastatic/recurrent solid tumors refractory to conventional DOX chemotherapy Administered doses ranged from 6 to 67 mg/m2, once every 3 weeks MTD was 67 mg/m2 Grade 3 or 4 neutropenia was observed at doses of 50 mg/m2 with AUC of 3.2 vs. 1.6 μg hr/mL The maximum tolerated dose was 70 mg/m2/and the recommended dose for phase II trials was 50 mg/m2 to be administered once every 3 weeks Phase II: currently undergoing |
| NC-6300 | PEG-p(Asp-Hyd) | Average size~65 nm Modifications to the NK911 formulation by using pH hydrolyzable linkers (hydrazone bonds) for the chemical conjugation of DOX to the micelles | In vivo 15-fold higher AUC (859 vs. 59 μg hr/mL) in mice bearing colon-26 carcinoma 4-folds higher accumulation at the tumor site MTD (40 mg/kg vs. 10 mg/kg) | Phase I: -pending results |
| Composition | Target Cancer | Functionalization | DOX Loading | Study Model | Triggering Modality | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| nanoscale Zr (IV)-based nanoMOFs (NH2-UiO-66) | hepatocellular carcinoma (HCC) | folic acid (FA), lactobionic acid (LA), glycyrrhetinic acid (GA) | Physical loading at dark conditions for 72 h where 100 mg of each MOFs formulation was added to 35 mg of DOX solution, followed by pelleting and vacuum drying at 40 °C. | Biocompatibility testing by SRB assay on human fibroblast skin cells. In vitro flow cytometry and MTT assay on HepG2 cells. | pH-responsiveness | MOF nanocarriers are biocompatible and safe (cell viability of h 77 ± 0.71% was observed at the highest MOFs concentration of 1000 μg/mL). Drug release from dual-ligand LA-GA formulation was sensitive to pH, releasing 60% and 100% of the encapsulated DOX at pH 7.4 and 4.0, respectively. Dual-targeting was the most efficient approach as these MOFs exhibited the best anti-tumor activity, approaching that of free DOX. | [138] |
| MIL-100(Al) nanoMOFs | hepatocellular carcinoma (HCC) | γ-cyclodextrincitrate oligomers (CD-CO) coatings | DOX loading was carried out by pelleting the MOFs and dispersing them in water before mixing 1 mL of aqueous MOFs (2 mg/mL) with 1 mL of DOX solution). The mixture was mixed for 1 to 6 days. The loaded MOFs were centrifuged and collected. | Solid-state NMR (ssNMR) spectroscopy. DOX release in phosphate buffer saline (PBS). | N/A | DOX encapsulation efficiency was a function of the weight ratio of DOX to MOFs during the loading process and the time of impregnation. A higher DOX payload was observed with the increase in the weight ratio and the impregnation time. DOX encapsulation had no significant effects on the MOFs’ morphologies or colloidal stability. | [139] |
| Alendronate (Aln) modified ZIF-8 based MOFs | Bone metastasis | N/A | 2 mL of DOX solution (6.8 mg in 50 mL methanol) was mixed with 100 mg of MOFs or Aln-MOFs powder. The mixture was gently mixed for two days, followed by centrifugation, washing, and freeze-drying. | In vitro Cck-8 assay and flow cytometry analysis of mouse breast cancer 4T1 cells. In vivo model: Balb/c mice inoculated with 4T1 cells to establish a bone metastasis model. | pH-responsiveness | DOX entrapment into both types of MOFs resulted in a loaded capacity of 0.65 μg/mg. Release from both types was sustained for 12 h period, while enhanced kinetics were observed at a lower pH (~5.5) than neutral conditions. The modified MOFs (Aln-MOF-DOX) showed superior anti-tumor activity compared to the unmodified MOFs. However, the tumor growth was arrested for 12 days only after which it regrows again. | [140] |
| Fe-MOFs | Different cancers | cationic polymer MV-PAH multilayers (PEM) | DOX was loaded into Fe-MOFs by mixing 10 mg of DOX with 20 mg of Fe-MOFs overnight, followed by centrifugation. Loaded Fe-MOFs were then coated with PEM using the LBL technique. | The in vitro dialysis bag diffusion technique to study pH-dependent release kinetics, MTT assay to evaluate toxicity to A549 and MCF-7 cells. In vitro Annexin V-FITC apoptosis detection assay. | pH-responsiveness | Both functionalized and unfunctionalized MOFs showed stability and long circulation capabilities. The release at pH 5.0 after 12-h incubation reached 72% in the functionalized MOFs, while unfunctionalized MOFs at pH 7.4 released <4% after the same incubation period. Coating with PEM increased the sensitivity of the DDS towards pH changes. | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. https://doi.org/10.3390/pharmaceutics14020254
Ibrahim M, Abuwatfa WH, Awad NS, Sabouni R, Husseini GA. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics. 2022; 14(2):254. https://doi.org/10.3390/pharmaceutics14020254
Chicago/Turabian StyleIbrahim, Mihad, Waad H. Abuwatfa, Nahid S. Awad, Rana Sabouni, and Ghaleb A. Husseini. 2022. "Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review" Pharmaceutics 14, no. 2: 254. https://doi.org/10.3390/pharmaceutics14020254
APA StyleIbrahim, M., Abuwatfa, W. H., Awad, N. S., Sabouni, R., & Husseini, G. A. (2022). Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics, 14(2), 254. https://doi.org/10.3390/pharmaceutics14020254







