Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization
Abstract
:1. Introduction
1.1. Concept (a): Preparation of Applicator Films
1.2. Concept (b): Optimization of the Dissolution Properties
1.3. Concept (c): Tandem Films for the Combination of Incompatible APIs
2. Materials and Methods
2.1. Materials for Film Casting
2.2. Solvent Casting
2.3. Construction and Evaluation of Prototype Models for Subdivision of the Coating Blade
2.4. Preparation of Tandem Films Made from Different Polymers
2.5. Solubility Parameter Calculations
2.6. Dynamic Viscosity
2.7. Basic Film Characterization and Wettability
2.8. Determination of the Films’ Mechanical Strength
2.9. Preparation of Applicator Films
2.10. Bisoprolol Content Determination
2.11. Preparation of Mucoadhesive Tandem Films for Rapid Release and Dissolution Testing
2.12. Dissolution Testing and Content Determination and of Mucoadhesive Films
3. Results and Discussion
3.1. Assessment of Model Constructions for the Preparation of Tandem Films
3.2. Preparation and Subsequent Evaluation of Tandem Films Based on Different Film Formers
3.3. Concept (a): Preparation of Applicator Films
3.4. Concept (b): Preparation of Mucoadhesive Tandem Films with Rapid Dissolution Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef]
- Giovinazzo, A.J.; Hedden, D.B.; Somer, M.d.; Bryson, N.J. Sublingual Apomorphine. Patent NZ-597237-A, 28 February 2014. [Google Scholar]
- Food and Drug Administration, Summary Review for Regulatory Action, Application Number 210875Orig1s000. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/210875Orig1s000SumR.pdf (accessed on 17 January 2022).
- Preis, M.; Woertz, C.; Schneider, K.; Kukawka, J.; Broscheit, J.; Roewer, N.; Breitkreutz, J. Design and evaluation of bilayered buccal film preparations for local administration of lidocaine hydrochloride. Eur. J. Pharm. Biopharm. 2014, 86, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [PubMed]
- Cook, M.T.; Smith, S.L.; Khutoryanskiy, V.V. Novel glycopolymer hydrogels as mucosa-mimetic materials to reduce animal testing. Chem. Commun. 2015, 51, 14447–14450. [Google Scholar] [CrossRef] [Green Version]
- Göbel, A.; Bassi da Silva, J.; Cook, M.; Breitkreutz, J. Development of buccal film formulations and their mucoadhesive performance in biomimetic models. Int. J. Pharm. 2021, 121233. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.-J. Development and Formulation of Carbomer 934P-Containing Mucoadhesive Pellets by Fluid-Bed Techniques Martin-Luther-Universität Halle-Wittenberg. Ph.D. Thesis, Universitäts- und Landesbibliothek Sachsen-Anhalt, Halle, Germany, 2007. [Google Scholar]
- Won, D.H.; Park, H.; Ha, E.-S.; Kim, H.-H.; Jang, S.W.; Kim, M.-S. Optimization of bilayer tablet manufacturing process for fixed dose combination of sustained release high-dose drug and immediate release low-dose drug based on quality by design (QbD). Int. J. Pharm. 2021, 605, 120838. [Google Scholar] [CrossRef]
- Nirmal, J.; Saisivam, S.; Peddanna, C.; Muralidharan, S.; Godwinkumar, S.; Nagarajan, M. Bilayer tablets of atorvastatin calcium and nicotinic acid: Formulation and evaluation. Chem. Pharm. Bull. 2008, 56, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Shiyani, B.; Gattani, S.; Surana, S. Formulation and evaluation of bi-layer tablet of metoclopramide hydrochloride and ibuprofen. AAPS PharmSciTech 2008, 9, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Rubio Badia, G.; Roig Carreras, M.; Salazar Macian, R.; Tubau Arino, P.; Rubio Rosell, N.; Sanchez Vega, O. Multi-Layered Controlled-Release Methylphenidate Pellet. United States Patent Application 20080069872, 2008. Available online: https://patentimages.storage.googleapis.com/09/a3/3d/4257f52cbf1357/CA2566497A1.pdf (accessed on 17 January 2022).
- Deshmane, S.; Channawar, M.; Chandewar, A.; Joshi, U.; Biyani, K. Chitosan based sustained release mucoadhesive buccal patches containing verapamil HCL. Int. J. Pharm. Pharm. Sci. 2009, 1, 216–229. [Google Scholar]
- Armenta Rojas, E.; Cornejo Bravo, J.M.; Serrano Medina, A.; López Maldonado, E.A.; Olivas Sarabia, A.; Castillo Martínez, N.A.; Vea Barragán, A.C. Chitosan mucoadhesive films as controlled release system of nystatin for buccal application. Rev. De Cienc. Tecnol. 2021, 4, 1–18. [Google Scholar] [CrossRef]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Parkinson’s Disease in Adults NICE Guideline; NICE: London, UK, 2017. [Google Scholar]
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf (accessed on 17 January 2022).
- Baumgartner, A.; Drame, K.; Geutjens, S.; Airaksinen, M. Does the Polypill Improve Patient Adherence Compared to Its Individual Formulations? A Systematic Review. Pharmaceutics 2020, 12, 190. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kwon, O.D.; Han, E.B.; Lee, C.M.; Oh, S.-W.; Joh, H.-K.; Oh, B.; Kwon, H.; Cho, B.; Choi, H.C. Impact of number of medications and age on adherence to antihypertensive medications: A nationwide population-based study. Medicine 2019, 98, e17825. [Google Scholar] [CrossRef]
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Nirmala, D.; Nandhini, S.; Sudhakar, M. Design and evaluation of fast dissolving oral films of Zolpidem by solvent casting method. Asian J. Pharm. Res. 2016, 6, 67. [Google Scholar] [CrossRef]
- Garsuch, V.I. Preparation and Chacacterization of Fast-Dissolving Oral Films for Pediatric Use. Ph.D. Thesis, Heinrich-Heine-University, Düsseldorf, Germany, 2009. [Google Scholar]
- Alves, F.V.d.C. Preparation and Characterization of Orodispersible Films. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2013. [Google Scholar]
- Naotaka Sakamoto, E.S. Differential Analysis of O-(2-hydroxypropyl cellulose by Using Two-Dimensional 1H-NMR Spectroscopy. Arch. Biomed. Sci. Eng. 2020, 6, 010–015. [Google Scholar] [CrossRef] [Green Version]
- Krull, S.M.; Ma, Z.; Li, M.; Davé, R.N.; Bilgili, E. Preparation and characterization of fast dissolving pullulan films containing BCS class II drug nanoparticles for bioavailability enhancement. Drug Dev. Ind. Pharm. 2016, 42, 1073–1085. [Google Scholar] [CrossRef]
- Vila, M.M.D.C.; Tardelli, E.R.; Chaud, M.V.; Tubino, M.; Balcão, V.M. Development of a buccal mucoadhesive film for fast dissolution: Mathematical rationale, production and physicochemical characterization. Drug Deliv. 2014, 21, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Just, S.; Sievert, F.; Thommes, M.; Breitkreutz, J. Improved group contribution parameter set for the application of solubility parameters to melt extrusion. Eur. J. Pharm. Biopharm. 2013, 85, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bagley, E.B.; Nelson, T.P.; Scigliano, J.M. Three-dimensional solubility parameters and their relationship to internal pressure measurements in polar and hydrogen bonding solvents. J. Paint Technol. 1971, 43, 35–42. [Google Scholar]
- Amores, S.; Domenech, J.; Colom, H.; Calpena, A.C.; Clares, B.; Gimeno, Á.; Lauroba, J. An improved cryopreservation method for porcine buccal mucosa in ex vivo drug permeation studies using Franz diffusion cells. Eur. J. Pharm. Sci. 2014, 60, 49–54. [Google Scholar] [CrossRef]
- Rowe, R.C. The molecular weight and molecular weight distribution of hydroxypropyl methylcellulose used in the film coating of tablets. J. Pharm. Pharmacol. 1980, 32, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-C.; Chen, T.-H.; Mandal, P.K. Enhancing the Mechanical and Tribological Properties of Cellulose Nanocomposites with Aluminum Nanoadditives. Polymers 2020, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Yoo, Y.J. Optimization of pH for high molecular weight pullulan. Biotechnol. Lett. 1993, 15, 1021–1024. [Google Scholar] [CrossRef]
- Ashland (Ed.) Product Grades Available. 2020. Available online: https://www.ashland.com/file_source/Ashland/Industries/Pharmaceutical/Articles/PC-11608.11_Pharma_Product_Grades.pdf (accessed on 17 January 2022).
- United States Pharmacopeial Convention. Polyvinylalcohol. In USP 41—NF 36; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018; pp. 3352–3353. [Google Scholar]
- DIN Deutsches Institut für Normung e.V. Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets; ISO: Geneva, Switzerland, 2018; ISO 5273:2018. [Google Scholar]
- Duramed Pharmaceuticals Zebeta (Bisoprolol Fumarate) Tablets Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019982s014lbl.pdf (accessed on 13 December 2021).
- Speer, I.; Steiner, D.; Thabet, Y.; Breitkreutz, J.; Kwade, A. Comparative study on disintegration methods for oral film preparations. Eur. J. Pharm. Biopharm. 2018, 132, 50–61. [Google Scholar] [CrossRef] [PubMed]
- EDQM. Bisoprolol fumarate. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2017; pp. 1986–1988. [Google Scholar]
- Krampe, R.; Sieber, D.; Pein-Hackelbusch, M.; Breitkreutz, J. A new biorelevant dissolution method for orodispersible films. Eur. J. Pharm. Biopharm. 2016, 98, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of human saliva as a platform for oral dissolution medium development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef]
- Sawinski, V.J.; Goldberg, A.F.; Loiselle, R.J. Osmolality of Normal Human Saliva at Body Temperature. Clin. Chem. 1966, 12, 513–514. [Google Scholar] [CrossRef]
- Erokhin, K.S.; Gordeev, E.G.; Ananikov, V.P. Revealing interactions of layered polymeric materials at solid-liquid interface for building solvent compatibility charts for 3D printing applications. Sci. Rep. 2019, 9, 20177. [Google Scholar] [CrossRef]
- Oosthuizen, G.A.; Hagedorn-Hansen, D.; Gerhold, T. Evaluation of rapid product development technologies for production of prosthesis in developing communities. In Proceedings of the SAIIE25 Proceedings, Stellenbosch, South Africa, 9 July 2013. [Google Scholar]
- Ujfalusi, Z.; Pentek, A.; Told, R.; Schiffer, A.; Nyitrai, M.; Maroti, P. Detailed Thermal Characterization of Acrylonitrile Butadiene Styrene and Polylactic Acid Based Carbon Composites Used in Additive Manufacturing. Polymers 2020, 12, 2960. [Google Scholar] [CrossRef]
- Velaga, S.; Nikjoo, D.; Vuddanda, P.R. Experimental Studies and Modeling of the Drying Kinetics of Multicomponent Polymer Films. AAPS PharmSciTech 2017, 19, 125–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pan, I.; Ziani, K.; Pedroza, R.; Maté, J. Effect of Drying Conditions on the Mechanical and Barrier Properties of Films Based on Chitosan. Dry. Technol. 2010, 28, 1350–1358. [Google Scholar] [CrossRef]
- Naseri, A.T.; Cetindag, E.; Bilgili, E.; Davé, R.N. A predictive transport model for convective drying of polymer strip films loaded with a BCS Class II drug. Eur. J. Pharm. Biopharm. 2019, 137, 164–174. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- ShinEtsu, Cloud Points of Aqueous Solution of Water-Soluble Cellulose Ethers. 2006. Available online: https://www.shinetsu.co.jp/cellulose/en/pharmaceutical/technical.html (accessed on 17 January 2022).
- Khuman, P.; Singh, W.; Devi, S.; Naorem, H. Viscosity-Temperature Behavior of Hydroxypropyl Cellulose Solution in Presence of an Electrolyte or a Surfactant: A Convenient Method to Determine the Cloud Point of Polymer Solutions. J. Macromol. Sci. 2014, 51, 924–930. [Google Scholar] [CrossRef]
- Amin, M.; Gangurde, A.B.; Alai, V. Oral Film Technology: Challenges and Future Scope for Pharmaceutical Industry. Int. J. Pharm. Pharm. Res. 2015, 3, 183–203. [Google Scholar]
- Sato, S.; Gondo, D.; Wada, T.; Kanehashi, S.; Nagai, K. Effects of various liquid organic solvents on solvent-induced crystallization of amorphous poly(lactic acid) film. J. Appl. Polym. Sci. 2013, 129, 1607–1617. [Google Scholar] [CrossRef]
- Rabek, C.L.; Van Stelle, R.; Dziubla, T.D.; Puleo, D.A. The effect of plasticizers on the erosion and mechanical properties of polymeric films. J. Biomater. Appl. 2014, 28, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Patel, H.V.; Li, M.; Bilgili, E.; Davé, R.N. Critical material attributes (CMAs) of strip films loaded with poorly water-soluble drug nanoparticles: I. Impact of plasticizer on film properties and dissolution. Eur. J. Pharm. Sci. 2016, 92, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Nyamweya, N.; Hoag, S.W. Assessment of polymer-polymer interactions in blends of HPMC and film forming polymers by modulated temperature differential scanning calorimetry. Pharm. Res. 2000, 17, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Fadnis, C.; Illiger, S.R.; Rao, K.P.; Demappa, T. Miscibility studies of HPMC/PVA blends in water by viscosity, density, refractive index and ultrasonic velocity method. Carbohydr. Polym. 2008, 74, 779–782. [Google Scholar] [CrossRef]
- Prasad, P.; Guru, G.S.; Shivakumar, H.R.; Rai, K.S. Miscibility, thermal, and mechanical studies of hydroxypropyl methylcellulose/pullulan blends. J. Appl. Polym. Sci. 2008, 110, 444–452. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry. Orally Disintegrating Tablets. In US Dep. Health Hum. Serv.; 2008. Available online: https://www.fda.gov/media/70877/download (accessed on 17 January 2022).
- Gijare, C.; Deshpande, A. Orodispersible Films: A Systematic Patent Review. Recent Pat. Drug Deliv. Formul. 2018, 12, 110–120. [Google Scholar] [CrossRef]
- EDQM. Uniformity of Dosage Units. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2017; pp. 398–400. [Google Scholar]
- Niese, S. Development of a Dosing System for Individualized Therapy with Oral Films. Ph.D. Thesis, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany, 2019. [Google Scholar]



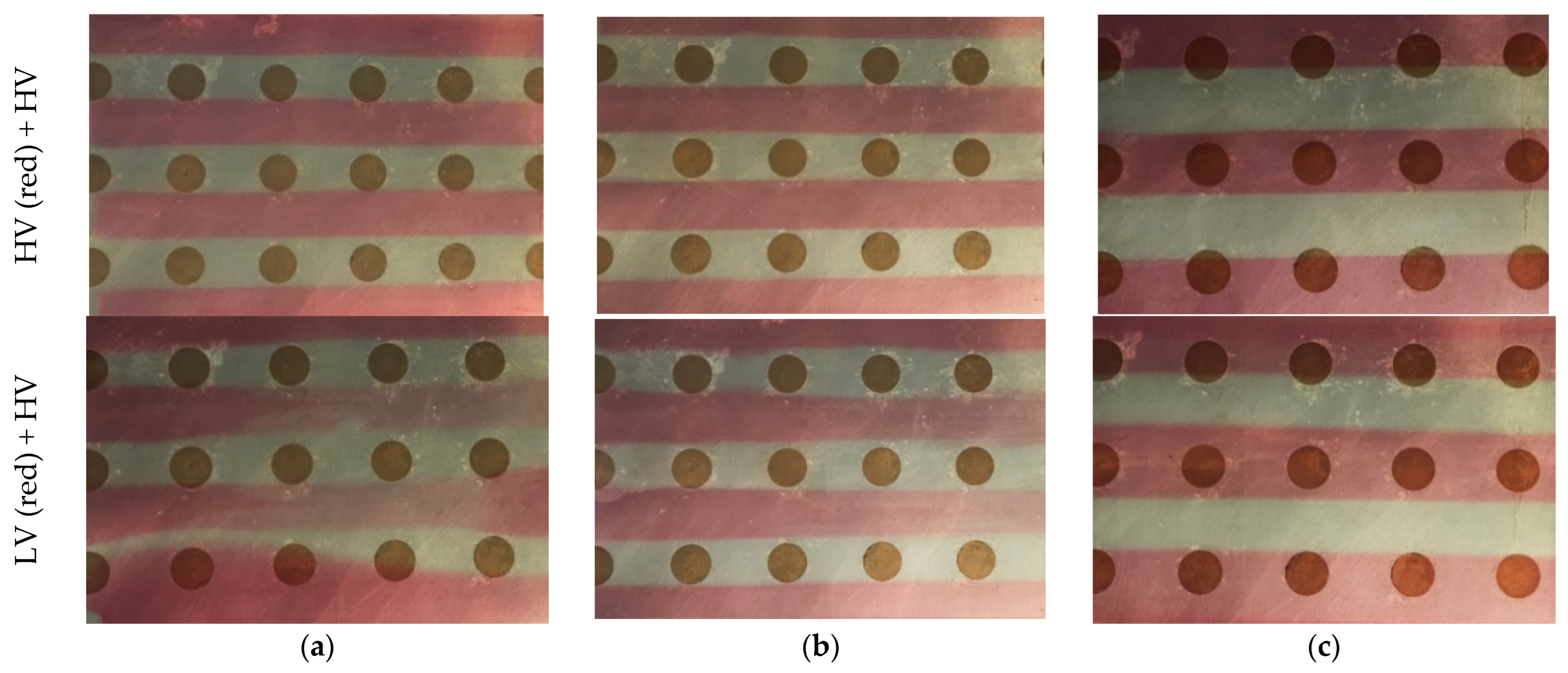






| Substance | HV (Red) | LV (Red) | HV |
|---|---|---|---|
| HPMC (P.606) | 15.0 | / | 15.0 |
| HPMC (P.603) | / | 15.0 | / |
| Glycerol | 3.0 | 3.0 | 3.0 |
| Amaranth solution | 0.15 | 0.15 | / |
| Deion. water | Ad 100.0 | Ad 100.0 | Ad 100.0 |
| Formulation Name | Film Former | Plasticizer | Thickening Agent | Solvent |
|---|---|---|---|---|
| HPMC (P.606) | HPMC (20.0) | Glycerol (4.0) | / | Water |
| HPC | HPC (20.0) | Glycerol (0.1) | / | Water |
| PVA | PVA (20.0) | Glycerol (3.0) | / | Water |
| Pullulan | Pullulan (20.0) | Glycerol (5.0) | Tragacanth (0.2) | Water |
| EC (N22) | EC (20.0) | Triethyl citrate (5.0) | / | Water (5.0) + ethanol |
| Ingredient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| HPMC (P.606) | 15.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Disintegrant | / | CMC 5.0 | PVP 5.0 | PVP Cl 5.0 | SSG 5.0 | MCC 5.0 | SSG 5.0 |
| Glycerol anh. | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| Bisoprolol | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 5.00 |
| Water | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 |
| Time (min) | Eluent A (% v/v) | Eluent B (% v/v) |
|---|---|---|
| 3.00 | 95.0 | 5.0 |
| 11.00 | 80.0 | 20.0 |
| 19.00 | 20.0 | 80.0 |
| 20.00 | 20.0 | 80.0 |
| 22.00 | 5.0 | 95.0 |
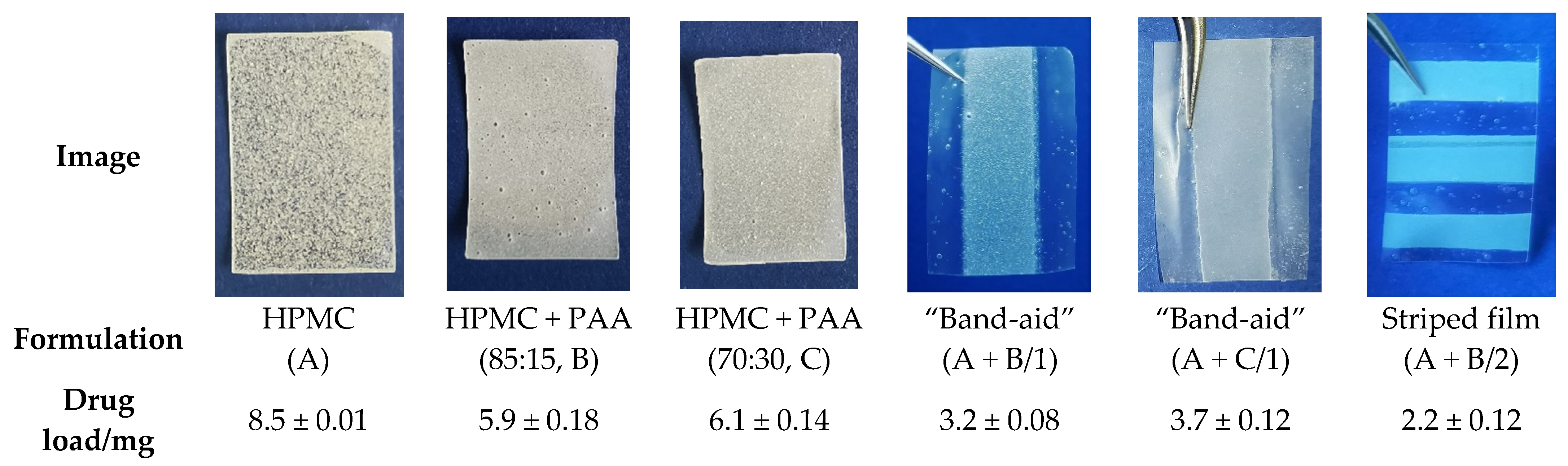
| Substance | A(v) | B(v) | C(v) | B(p) | C(p) |
|---|---|---|---|---|---|
| HPMC (P.606) | 10.0 | 8.5 | 7.0 | 8.5 | 7.0 |
| PAA | / | 1.5 | 3.0 | 1.5 | 3.0 |
| Glycerol | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Theophylline monohydrate | 2.0 | 2.0 | 2.0 | / | / |
| NaOH | / | q.s. ad pH 5.5 | q.s. ad pH 5.5 | q.s. ad pH 5.5 | q.s. ad pH 5.5 |
| Water | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 | Ad 100.0 |
| Parameter | Stainless Steel Constructions | 3D-Printed Inserts | ||
|---|---|---|---|---|
| Precursor | Optimized | PLA | ABS | |
| Robustness to thermal and mechanical stress [47,48] | ++ | ++ | - | - |
| Resistance to organic solvents/acids/bases [46,56] | ++ | ++ | + | - |
| Expense of manufacturing | - | - | ++ | ++ |
| Adjustability of compartment sizes | -- | -- | ++ | ++ |
| Quality of separation | - | + | ++ | ++ |
| Formulation | Viscosity of the Solution (Pa·s) (Mean ± sd) | Disintegration Time (s) | Dry Film Thickness (µm) (Mean ± sd) | Contact Angle (°) (Mean ± sd) | δd (MPa0.5) | δp (MPa0.5) | δh (MPa0.5) | δt (MPa0.5) | δv (MPa0.5) |
|---|---|---|---|---|---|---|---|---|---|
| HPMC | 4.2 ± 0.19 | 44 ± 3.0 | 70 ± 1.7 | 63 ± 8.1 | 16.1 | 13.5 | 4.2 | 21.5 | 21.1 |
| HPC | 1.3 ± 0.08 | 47 ± 2.4 | 65 ± 3.0 | 51 ± 5.1 | 15.1 | 15.5 | 8.5 | 23.3 | 21.6 |
| PVA | 0.8 ± 0.10 | 40 ± 5.5 | 156 ± 4.6 | 53 ± 2.1 | 16.8 | 10.9 | 12.4 | 23.5 | 20.0 |
| Pullulan | 2.2 ± 0.17 | 9 ± 1.2 | 143 ± 4.0 | 46 ± 1.5 | 20.0 | 19.3 | 12.9 | 30.6 | 27.8 |
| EC | 2.5 ± 0.31 | Insoluble | 73 ± 10.2 | 64 ± 1.9 | 16.1 | 11.4 | 7.2 | 21.0 | 19.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göbel, A.; Breitkreutz, J. Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization. Pharmaceutics 2022, 14, 264. https://doi.org/10.3390/pharmaceutics14020264
Göbel A, Breitkreutz J. Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization. Pharmaceutics. 2022; 14(2):264. https://doi.org/10.3390/pharmaceutics14020264
Chicago/Turabian StyleGöbel, Anja, and Jörg Breitkreutz. 2022. "Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization" Pharmaceutics 14, no. 2: 264. https://doi.org/10.3390/pharmaceutics14020264
APA StyleGöbel, A., & Breitkreutz, J. (2022). Concept of Orodispersible or Mucoadhesive “Tandem Films” and Their Pharmaceutical Realization. Pharmaceutics, 14(2), 264. https://doi.org/10.3390/pharmaceutics14020264







