A Novel Multilayer Natural Coating for Fed-State Gastric Protection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Tablet Preparation
2.3. Preparation of Coating System
2.3.1. Casted Film
2.3.2. Coated Tablets
2.4. Model Tablet Preparation
2.5. Morphology of the Coating
2.6. Disintegration Test
2.7. Water Uptake
2.8. In Vitro Drug Testing
2.8.1. pH Change Release Studies Method
2.8.2. Krebs’s Bicarbonate Buffer Test
2.8.3. Release at Elevated Gastric pH
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Characteristics of Coated Tablets
3.2. Acid Uptake and Disintegration
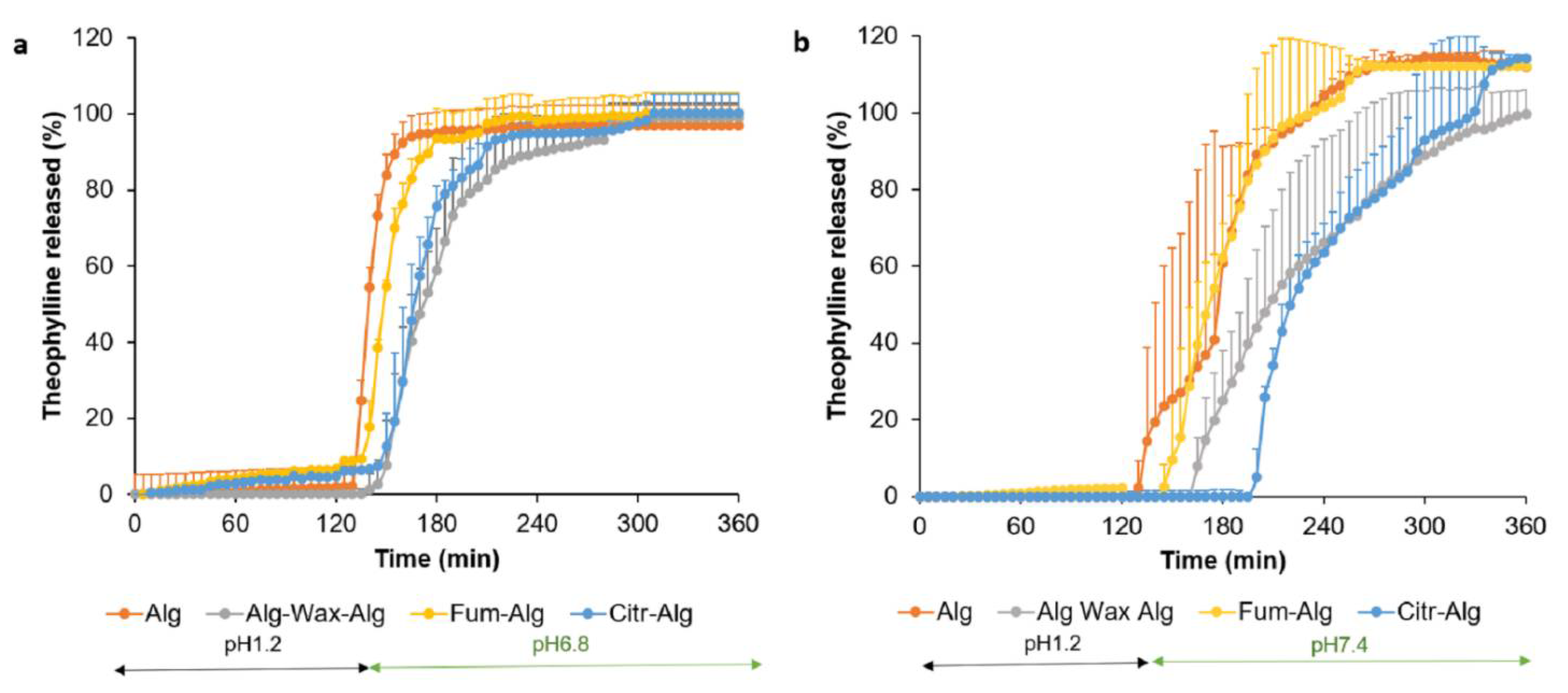
3.3. In Vitro Drug Release Study
- Gastric pH 1.2
- b.
- Intestinal release
- c.
- Fed state gastric pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murthy, A. Nutraceuticals Industry Market Report. 2017. Available online: http://blog.pipecandy.com/nutraceuticals-industry-market-report-2017/ (accessed on 9 September 2018).
- Czarnocka, J.K.; Alhnan, M.A. Gastro-resistant characteristics of GRAS-grade enteric coatings for pharmaceutical and nutraceutical products. Int. J. Pharm. 2015, 486, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Farag, F.; Leopold, C.S. Physicochemical properties of various shellac types. Dissolut. Technol. 2008, 16, 33–39. [Google Scholar] [CrossRef]
- Habashy, R.; Khoder, M.; Zhang, S.; Pereira, B.; Bohus, M.; Tzu-Wen Wang, J.; Isreb, A.; Alhnan, M.A. An innovative wax-based enteric coating for pharmaceutical and nutraceutical oral products. Int. J. Pharm. 2020, 591, 119935. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, G.; Fullarton, G.M.; Crean, G.P.; McColl, K.E. Comparison of gastric body and antral pH: A 24 hour ambulatory study in healthy volunteers. Gut 1989, 30, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendtsen, F.; Rosenkilde-Gram, B.; Tage-Jensen, U.; Ovesen, L.; Rune, S.J. Duodenal bulb acidity in patients with duodenal ulcer. Gastroenterology 1987, 93, 1263–1269. [Google Scholar] [CrossRef]
- Al-Gousous, J.; Tsume, Y.; Fu, M.; Salem, I.I.; Langguth, P. Unpredictable Performance of pH-Dependent Coatings Accentuates the Need for Improved Predictive in Vitro Test Systems. Mol. Pharm. 2017, 14, 4209–4219. [Google Scholar] [CrossRef]
- Matsui, K.; Tsume, Y.; Amidon, G.E.; Amidon, G.L. In Vitro Dissolution of Fluconazole and Dipyridamole in Gastrointestinal Simulator (GIS), Predicting in Vivo Dissolution and Drug–Drug Interaction Caused by Acid-Reducing Agents. Mol. Pharm. 2015, 12, 2418–2428. [Google Scholar] [CrossRef]
- Barbosa, J.A.C.; Abdelsadig, M.S.E.; Conway, B.R.; Merchant, H.A. Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their pKa. Int. J. Pharm. 2019, 1, 100024. [Google Scholar] [CrossRef]
- Khoder, M.; Schropp, V.; Zeitler, S.; Pereira, B.; Habashy, R.; Royall, P.G.; Wang, J.T.; Alhnan, M.A. A novel natural GRAS-grade enteric coating for pharmaceutical and nutraceutical products. Int. J. Pharm. 2020, 584, 119392. [Google Scholar] [CrossRef]
- Barbosa, J.; Conway, B.; Mechant, H. Going Natural:Using polymers from nature for gastroresistant applications. Br. J. Pharm. 2017, 2, 14–30. [Google Scholar]
- Islam, S.; Khatuna, F.; Bakra, A.; Mondal, I.; Haque, M.; Mahmud, A. A Review on Biodegradable Polymers for Enteric Coating Material. Int. J. Pharm. Pharm. Res. 2016, 6, 141–159. [Google Scholar]
- Yamakita, H.; Matsukawa, Y.; Maejima, T.; Osawa, T. Preparation of controlled release granules of TA-5707F using enteric polymers and ethylcellulose, and their in vivo evaluation. Biol. Pharm. Bull. 1996, 19, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Zhang, Y.; Tang, X. Sustained-release pellets prepared by combination of wax matrices and double-layer coatings for extremely water-soluble drugs. Drug Dev. Ind. Pharm. 2008, 34, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Moreno, P.; Basit, A.W. A novel double-coating approach for improved pH-triggered delivery to the ileo-colonic region of the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lizio, R.; Meier, C.; Petereit, H.U.; Blakey, P.; Basit, A.W. A novel concept in enteric coating: A double-coating system providing rapid drug release in the proximal small intestine. J. Control. Release 2009, 133, 119–124. [Google Scholar] [CrossRef]
- Varum, F.; Freire, A.C.; Fadda, H.M.; Bravo, R.; Basit, A.W. A dual pH and microbiota-triggered coating (Phloral™) for fail-safe colonic drug release. Int. J. Pharm. 2020, 583, 119379. [Google Scholar] [CrossRef]
- Patil, A.T.; Khobragade, D.S.; Chafle, S.A.; Ujjainkar, A.P.; Umathe, S.N.; Lakhotia, C.L. Development and evaluation of a hot-melt coating technique for enteric coating. Braz. J. Pharm. Sci. 2012, 48, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Craig, R.G.; Powers, J.M.; Peyton, F.A. Thermogravimetric Analysis of Waxes. J. Dent. Res. 1971, 50, 450–454. [Google Scholar] [CrossRef]
- USP. <701> Disintegration. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/generalChapter701.pdf (accessed on 19 August 2021).
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Rowe, R.; Shesky, P.; Quinn, M. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- O’Laughlin, R.; Sachs, C.; Brittain, H.; Cohen, E.; Timmins, P.; Varia, S. Effects of variations in physicochemical properties of glyceryl monostearate on the stability of an oil-inwater cream. J. Soc. Cosmet. Chem. 1989, 40, 215–229. [Google Scholar]
- Ravindrakullai Reddy, M.; Manjunath, K. Evaluation of Pectin derived from Orange peel as a Pharmaceutical Excipient. Int. J. Drug Dev. Res. 2013, 5, 283–294. [Google Scholar]
- Virk, B.S.; Sogi, D.S. Extraction and Characterization of Pectin from Apple (Malus Pumila. Cv Amri) Peel Waste. Int. J. Food Prop. 2004, 7, 693–703. [Google Scholar] [CrossRef]
- Patel, S.; Nelson, D.; Gibbs, A. Chemical and physical analyses of wax ester properties. J. Insect Sci. 2001, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Goslinska, M.; Heinrich, S. Characterization of waxes as possible coating material for organic aerogels. Powder Technol. 2019, 357, 223–231. [Google Scholar] [CrossRef]
- Mounica, P.; Pavani, S.; Mounica Rani, P. A review on recent advances in enteric coating and enteric polymers. World J. Pharm. Res. 2018, 7, 475–495. [Google Scholar]
- Sande, S.A. Pectin-based oral drug delivery to the colon. Expert. Opin. Drug Deliv. 2005, 2, 441–450. [Google Scholar] [CrossRef]
- Fadda, H.M.; Merchant, H.A.; Arafat, B.T.; Basit, A.W. Physiological bicarbonate buffers: Stabilisation and use as dissolution media for modified release systems. Int. J. Pharm. 2009, 382, 56–60. [Google Scholar] [CrossRef]
- Liu, F.; Merchant, H.A.; Kulkarni, R.P.; Alkademi, M.; Basit, A.W. Evolution of a physiological pH 6.8 bicarbonate buffer system: Application to the dissolution testing of enteric coated products. Eur. J. Pharm. Biopharm. 2011, 78, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Martin-Dominguez, V.; Estevez, J.; Ojembarrena, F.D.; Santos, V.E.; Ladero, M. Fumaric Acid Production: A Biorefinery Perspective. Fermentation 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- USDA. Available online: https://www.ams.usda.gov/sites/default/files/media/Sodium%20Citrate%20TR%202015.pdf (accessed on 19 September 2018).
- Shibata, H.; Yoshida, H.; Izutsu, K.; Goda, Y. Use of bicarbonate buffer systems for dissolution characterization of enteric-coated proton pump inhibitor tablets. J. Pharm. Pharmacol. 2016, 68, 467–474. [Google Scholar] [CrossRef]
- Krieg, B.J.; Taghavi, S.M.; Amidon, G.L.; Amidon, G.E. In Vivo Predictive Dissolution: Comparing the Effect of Bicarbonate and Phosphate Buffer on the Dissolution of Weak Acids and Weak Bases. J. Pharm. Sci. 2015, 104, 2894–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef] [PubMed]





| Coating | Wax (F1) | Alg (F2) | Alg-wax (F3) | Wax-Alg (F4) | Alg-wax-Alg (F5) | Fum-Alg (F6) | Citr-Alg (F7) |
|---|---|---|---|---|---|---|---|
| Description | 5% WG wax | 10% WG Alg | Wax 5% WG as SC + Alg 10% as OC | Alg 10% WG as SC + Wax 5% WG as OC | Alg 7% WG as SC + Wax 5% WG as mid-layer + Alg 7% WG as OC | Alg 10% WG as SC + 5% fumaric acid as OC | Alg 10% WG as SC + 5% citric acid as OC |
| Material Name | Wax (F1) | Alg (F2) | Alg-wax (F3) | Wax-Alg (F4) | Alg-wax-Alg (F5) | Fum-Alg (F6) | Citr-Alg (F7) |
|---|---|---|---|---|---|---|---|
| Gastric Phase | |||||||
| Acid uptake (%) | Opened | 5 ± 1.49% | 4.6 ± 1.2% | 4.9 ± 1.6% | 3.5 ± 0.78% | 6 ± 1.9% | 2.9 ± 0.48% |
| Acid medium resistance | Opened after 20 min | Resisted 2 h | Opened after 10 min | Resisted 1h then ruptured | Resisted 2 h | Resisted 2 h | Resisted 2 h |
| Drug release in acid medium (120 min) | 100% | 4.75% | 100% | 4.9% | 0% | 6.8% | 4.88% |
| Lag time in intestinal stage (min) | Opened | - | - | Opened | 25 | 15 | 15 |
| Intestinal Phase | |||||||
| 80% release time in buffer stage (min) | - | 30 | - | - | 85 | 35 | 70 |
| Disintegration test | Open after 20 min | Resisted 2 h | Resisted 1 h then ruptured | Resisted 1 h then dislodge of the coat | Resisted 2 h | Resisted 2 h | Resisted 2 h |
| Disintegration time of all tablets in SIF (min) | NA | 10.2 ± 1.1 | NA (opened in acid phase) | 11.3 ± 0.84 | 22.5 ± 3.0 | 9.8 ± 0.89 | 10.4 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habashy, R.; Khoder, M.; Isreb, A.; Alhnan, M.A. A Novel Multilayer Natural Coating for Fed-State Gastric Protection. Pharmaceutics 2022, 14, 283. https://doi.org/10.3390/pharmaceutics14020283
Habashy R, Khoder M, Isreb A, Alhnan MA. A Novel Multilayer Natural Coating for Fed-State Gastric Protection. Pharmaceutics. 2022; 14(2):283. https://doi.org/10.3390/pharmaceutics14020283
Chicago/Turabian StyleHabashy, Rober, Mouhamad Khoder, Abdullah Isreb, and Mohamed A. Alhnan. 2022. "A Novel Multilayer Natural Coating for Fed-State Gastric Protection" Pharmaceutics 14, no. 2: 283. https://doi.org/10.3390/pharmaceutics14020283







