Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Cell Cultures
2.2. Synthesis of mPEG-hyd-LEV Macroinitiator
2.3. Synthesis of mPEG-hyd-aPHB
2.4. Characterization of Synthetic Copolymers
2.5. Micelles Preparation and Characterization
2.6. In Vitro Drug Loading and Release Studies
2.7. MTT Assay
2.8. Apoptosis and Cell Cycle Analyses
2.9. Cell Microscopy Imaging
2.10. Statistical Analysis
3. Results and Discussion
3.1. Copolymer Synthesis and Characterization
3.2. Self-Assembly of the Copolymers
3.3. Drug Loading and In Vitro Release Studies
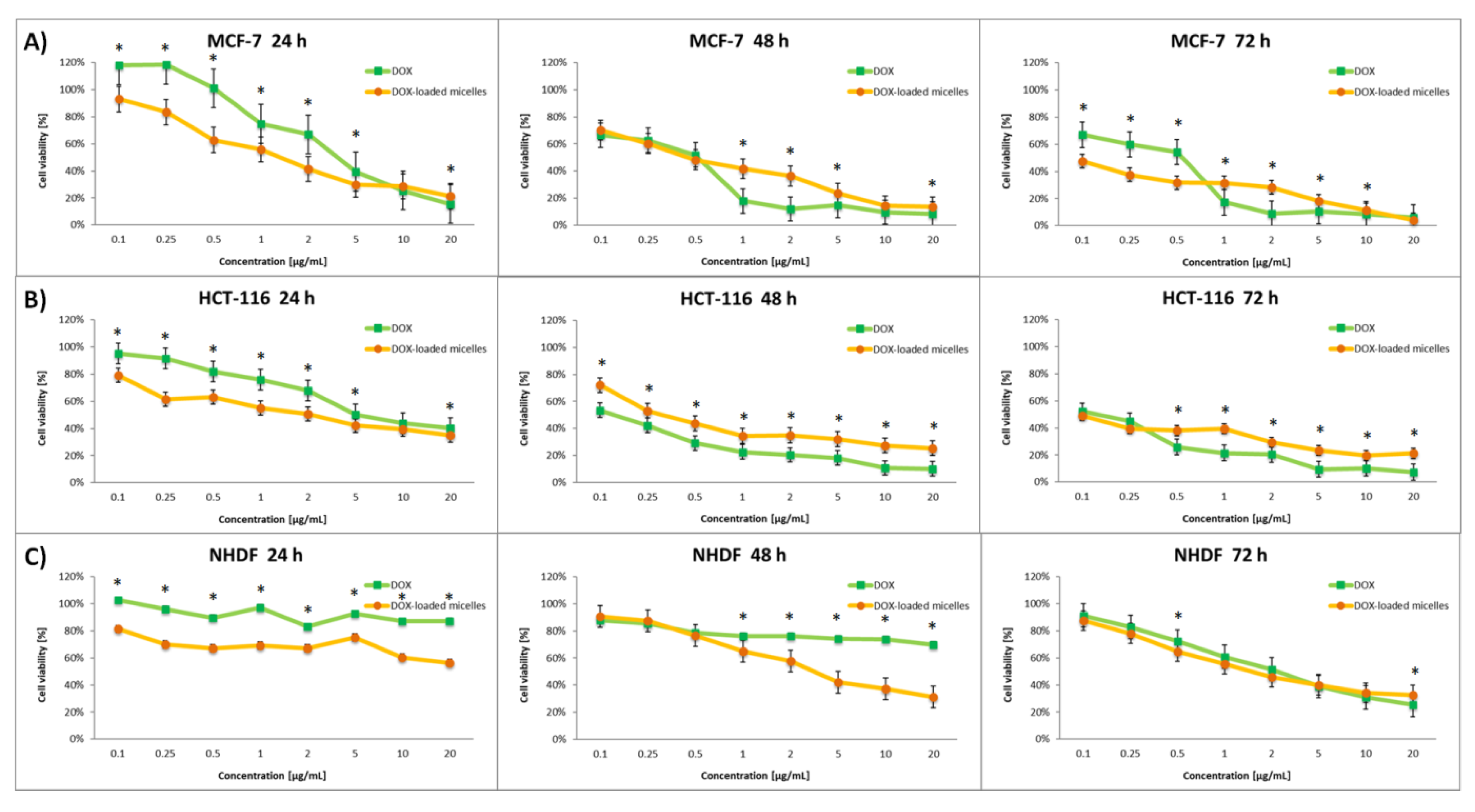
3.4. In Vitro Cytotoxicity Assay
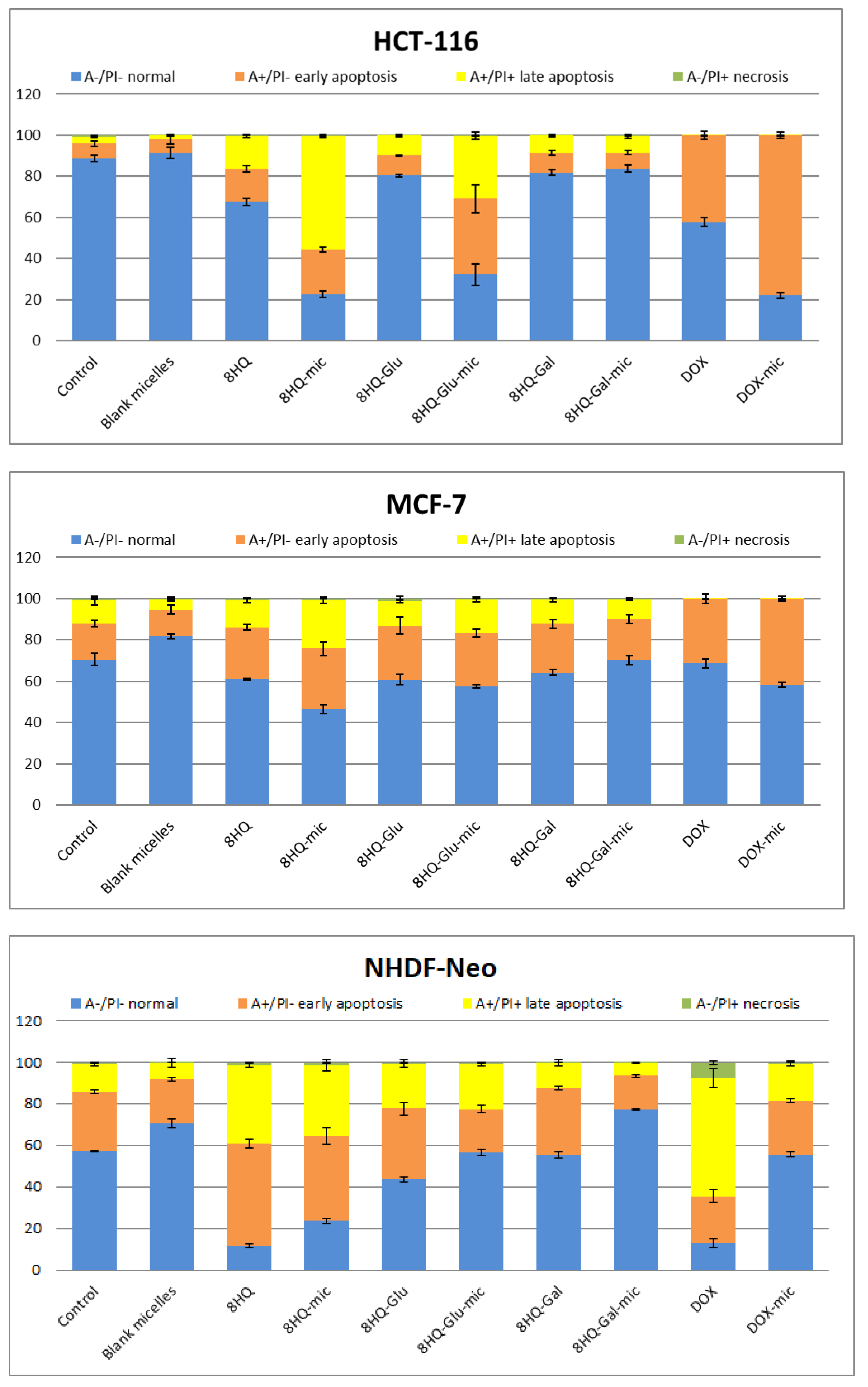
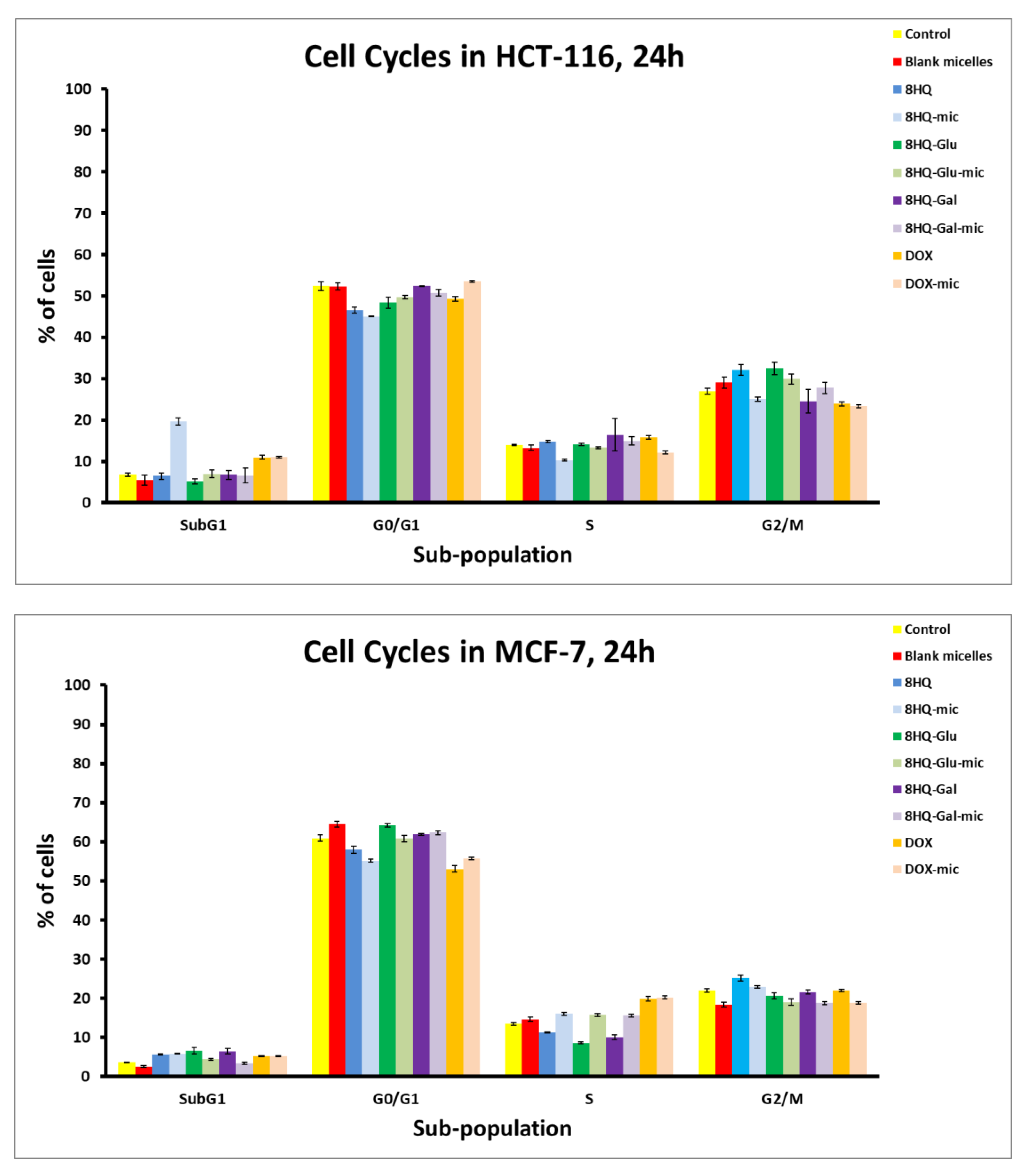
3.5. Apoptosis and Cell Cycle Analyses by Flow Cytometry
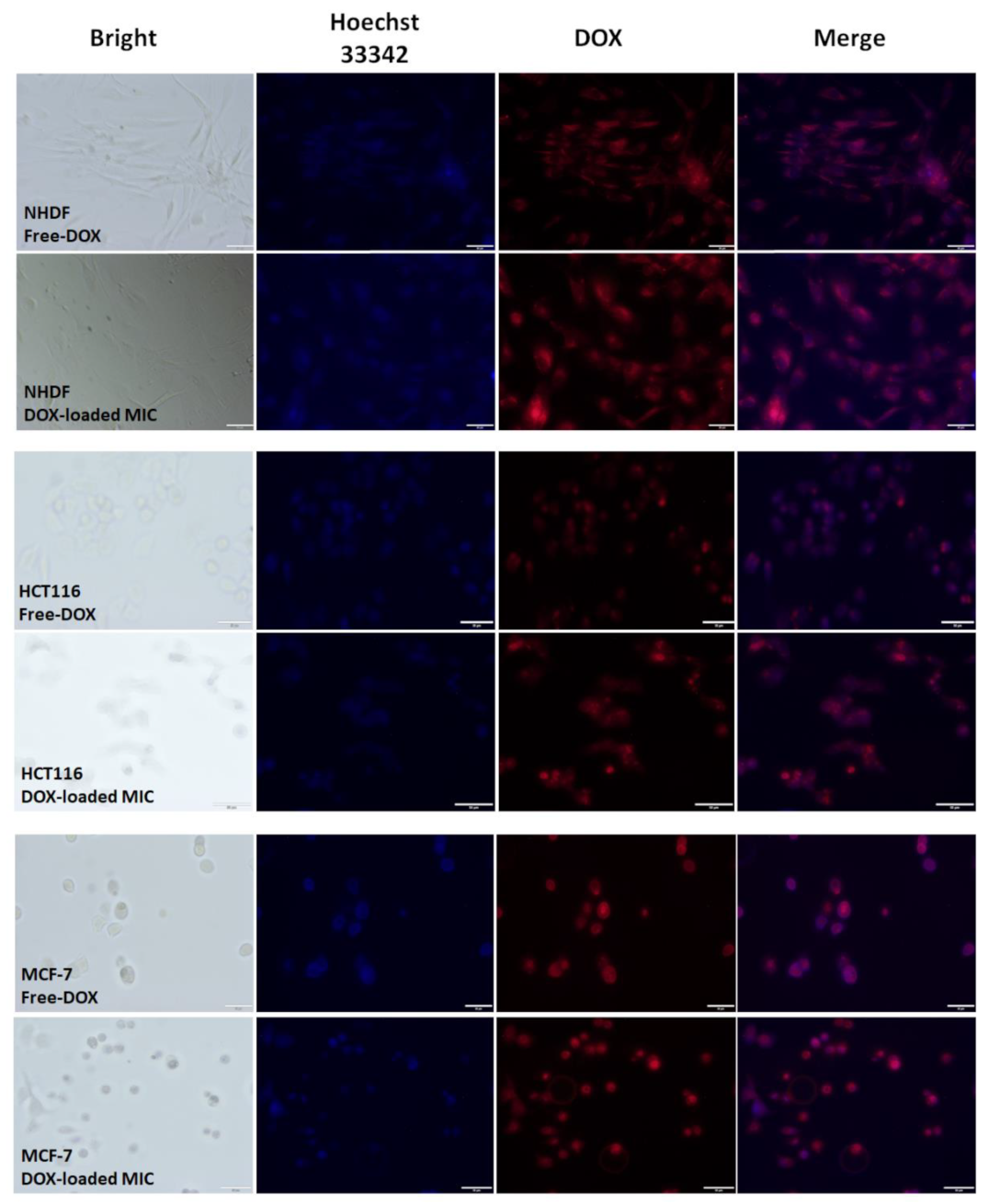
3.6. Cellular Uptake and Intracellular Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Zhong, Z. 100th Anniversary of Macromolecular Science Viewpoint: Biological Stimuli-Sensitive Polymer Prodrugs and Nanoparticles for Tumor-Specific Drug Delivery. ACS Macro Lett. 2020, 9, 1292–1302. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Michalak, M.; Kurcok, P.; Hakkarainen, M. Polyhydroxyalkanoate-based drug delivery systems. Polym. Int. 2016, 66, 617–622. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Duale, K.; Krawczyk, M.; Pastuch-Gawołek, G.; Kurcok, P. Stimuli-Responsive Aliphatic Polycarbonate Nanocarriers for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 2890. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Y.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, e1900283. [Google Scholar] [CrossRef]
- Jedliński, Z.; Kurcok, P.; Lenz, R.W. First Facile Synthesis of Biomimetic Poly-(R)-3-hydroxybutyrate via Regioselective Anionic Polymerization of (S)-β-Butyrolactone. Macromolecules 1998, 31, 6718–6720. [Google Scholar] [CrossRef]
- Kurcok, P.; Dubois, P.; Jerome, R. Polymerization of β-butyrolactone initiated with Al(OiPr)3. Polym. Int. 1996, 41, 479–485. [Google Scholar] [CrossRef]
- Khalil, A.; Cammas-Marion, S.; Coulembier, O. Organocatalysis applied to the ring-opening polymerization of β-lactones: A brief overview. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 657–672. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Zięba, M.; Klim, M.; Kurcok, P. Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism. Polymers 2019, 11, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurcok, P.; Jedlinski, Z.; Kowalczuk, M. Reactions of β-lactones with potassium alkoxides and their complexes with 18-crown-6 in aprotic solvents. J. Org. Chem. 1993, 58, 4219–4220. [Google Scholar] [CrossRef]
- Dong, X.; Robinson, J.R. The role of neutral donor ligands in the isoselective ring-opening polymerization of rac-β-butyrolactone. Chem. Sci. 2020, 11, 8184–8195. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 2018, 9, 2345. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [Green Version]
- Zawidlak-Węgrzyńska, B.; Kawalec, M.; Bosek, I.; Łuczyk-Juzwa, M.; Adamus, G.; Rusin, A.; Filipczak, P.; Głowala-Kosińska, M.; Wolańska, K.; Krawczyk, Z.; et al. Synthesis and antiproliferative properties of ibuprofen–oligo(3-hydroxybutyrate) conjugates. Eur. J. Med. Chem. 2010, 45, 1833–1842. [Google Scholar] [CrossRef]
- Elustondo, P.A.; Angelova, P.R.; Kawalec, M.; Michalak, M.; Kurcok, P.; Abramov, A.Y.; Pavlov, E.V. Polyhydroxybutyrate Targets Mammalian Mitochondria and Increases Permeability of Plasmalemmal and Mitochondrial Membranes. PLoS ONE 2013, 8, e75812. [Google Scholar] [CrossRef]
- Barouti, G.; Jaffredo, C.G.; Guillaume, S.M. Advances in drug delivery systems based on synthetic poly(hydroxybutyrate) (co)polymers. Prog. Polym. Sci. 2017, 73, 1–31. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barron, C.C.; Bilan, P.J.; Tsakiridis, T.; Tsiani, E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism 2015, 65, 124–139. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Sun, X. The Warburg effect and glucose-derived cancer theranostics. Drug Discov. Today 2017, 22, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawołek, G.; Pluta, A.; Erfurt, K.; Domiński, A.; Kurcok, P. 8-Hydroxyquinoline Glycoconjugates: Modifications in the Linker Structure and Their Effect on the Cytotoxicity of the Obtained Compounds. Molecules 2019, 24, 4181. [Google Scholar] [CrossRef] [Green Version]
- Calvaresi, E.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [Green Version]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef] [Green Version]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Domiński, A.; Krawczyk, M.; Konieczny, T.; Kasprów, M.; Foryś, A.; Pastuch-Gawołek, G.; Kurcok, P. Biodegradable pH-responsive micelles loaded with 8-hydroxyquinoline glycoconjugates for Warburg effect-based tumor targeting. Eur. J. Pharm. Biopharm. 2020, 154, 317–329. [Google Scholar] [CrossRef]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive Nanoparticles for Drug Delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y.; et al. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. INNO-206 (DOXO-EMCH), an Albumin-Binding Prodrug of Doxorubicin Under Development for Phase II Studies. Curr. Bioact. Compd. 2011, 7, 33–38. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Jin, Q.; Deng, Y.; Chen, X.; Ji, J. Rational Design of Cancer Nanomedicine for Simultaneous Stealth Surface and Enhanced Cellular Uptake. ACS Nano 2019, 13, 954–977. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol Dilemma: Advantage and Disadvantage of PEGylation of Liposomes for Systemic Genes and Nucleic Acids Delivery to Tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Campbell, F.; Kros, A. DePEGylation strategies to increase cancer nanomedicine efficacy. Nanoscale Horiz. 2018, 4, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Huang, L.; Moingeon, F.; Gauthier, M.; Yang, G. pH-Responsive Poly(Ethylene Glycol)-block-Polylactide Micelles for Tumor-Targeted Drug Delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef] [Green Version]
- Hira, S.K.; Ramesh, K.; Gupta, U.; Mitra, K.; Misra, N.; Ray, B.; Manna, P.P. Methotrexate-Loaded Four-Arm Star Amphiphilic Block Copolymer Elicits CD8+ T Cell Response against a Highly Aggressive and Metastatic Experimental Lymphoma. ACS Appl. Mater. Interfaces 2015, 7, 20021–20033. [Google Scholar] [CrossRef]
- Liu, S.; Ono, R.J.; Yang, C.; Gao, S.; Tan, J.Y.M.; Hedrick, J.L.; Yang, Y.Y. Dual pH-Responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for in Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19355–19364. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-Linked Paclitaxel Prodrug Micellar Nanoparticles as a Versatile and Potent Platform for Cancer Therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Zhang, J.; Meng, F.; Deng, C.; Cheng, R.; Feijen, J.; Zhong, Z. Exogenous vitamin C boosts the antitumor efficacy of paclitaxel containing reduction-sensitive shell-sheddable micelles in vivo. J. Control. Release 2017, 250, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, M.; Coulembier, O.; Gerbaux, P.; Sobota, M.; De Winter, J.; Dubois, P.; Kowalczuk, M.; Kurcok, P. Traces do matter—Purity of 4-methyl-2-oxetanone and its effect on anionic ring-opening polymerization as evidenced by phosphazene superbase catalysis. React. Funct. Polym. 2012, 72, 509–520. [Google Scholar] [CrossRef]
- Liu, K.L.; Goh, S.H.; Li, J. Controlled synthesis and characterizations of amphiphilic poly[(R,S)-3-hydroxybutyrate]-poly(ethylene glycol)-poly[(R,S)-3-hydroxybutyrate] triblock copolymers. Polymers 2007, 49, 732–741. [Google Scholar] [CrossRef]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their β1,4-GalT inhibitory and anti-cancer properties. Bioorg. Chem. 2018, 84, 326–338. [Google Scholar] [CrossRef]
- Won, Y.-Y.; Brannan, A.K.; Davis, H.T.; Bates, F.S. Cryogenic Transmission Electron Microscopy (Cryo-TEM) of Micelles and Vesicles Formed in Water by Poly(ethylene oxide)-Based Block Copolymers. J. Phys. Chem. B 2002, 106, 3354–3364. [Google Scholar] [CrossRef]
- Kawalec, M.; Śmiga-Matuszowicz, M.; Kurcok, P. Counterion and solvent effects on the anionic polymerization of β-butyrolactone initiated with acetic acid salts. Eur. Polym. J. 2008, 44, 3556–3563. [Google Scholar] [CrossRef]
- Cui, C.; Yu, P.; Wu, M.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Reduction-sensitive micelles with sheddable PEG shells self-assembled from a Y-shaped amphiphilic polymer for intracellular doxorubicine release. Colloids Surf. B Biointerfaces 2015, 129, 137–145. [Google Scholar] [CrossRef]
- Wang, D.; Su, Y.; Jin, C.; Zhu, B.; Pang, Y.; Zhu, L.; Liu, J.; Tu, C.; Yan, D.; Zhu, X. Supramolecular Copolymer Micelles Based on the Complementary Multiple Hydrogen Bonds of Nucleobases for Drug Delivery. Biomacromolecules 2011, 12, 1370–1379. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and Their Loading Strategies. Adv. Health Mater. 2018, 8, e1801002. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, C.; Xie, D.; Chen, X.; Wang, P.; Wang, Y.; Song, H.; Wang, W. A hyperbranched amphiphilic acetal polymer for pH-sensitive drug delivery. Polym. Chem. 2017, 9, 169–177. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Gaur, K.; Vázquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.A.; Fernández-Vega, L.; Sarabia, L.C.; García, A.C.; Pérez-Deliz, F.; Román, J.A.M.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2014, 6, 61–74. [Google Scholar] [CrossRef]
- Pastuch-Gawołek, G.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musioł, M.; Serda, M.; Czaplińska, B.; Musioł, R. Small molecule glycoconjugates with anticancer activity. Eur. J. Med. Chem. 2016, 112, 130–144. [Google Scholar] [CrossRef]
- Oliveri, V. Glycoconjugates of Quinolines: Application in Medicinal Chemistry. Mini-Rev. Med. Chem. 2016, 16, 1185–1194. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.S.; Wahl, R.L. Overexpression of Glut-1 Glucose Transporter in Human Breast Cancer. Cancer 1993, 72, 2979–2985. [Google Scholar] [CrossRef]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 Glucose Transporter Expression in Colorectal Carcinoma: A marker for poor prognosis. Cancer 1998, 83, 34–40. [Google Scholar] [CrossRef]
- Schindler, M.; Grabski, S.; Hoff, E.; Simon, S.M. Defective pH Regulation of Acidic Compartments in Human Breast Cancer Cells (MCF-7) Is Normalized in Adriamycin-Resistant Cells (MCF-7adr). Biochemistry 1996, 35, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles that are Responsive to Intracellular pH Change. Angew. Chem. 2003, 115, 4788–4791. [Google Scholar] [CrossRef]
- Skonieczna, M.; Hudy, D.; Poterala-Hejmo, A.; Hejmo, T.; Buldak, R.J.; Dziedzic, A. Effects of Resveratrol, Berberine and Their Combinations on Reactive Oxygen Species, Survival and Apoptosis in Human Squamous Carcinoma (SCC-25) Cells. Anti-Cancer Agents Med. Chem. 2019, 19, 1161–1171. [Google Scholar] [CrossRef]
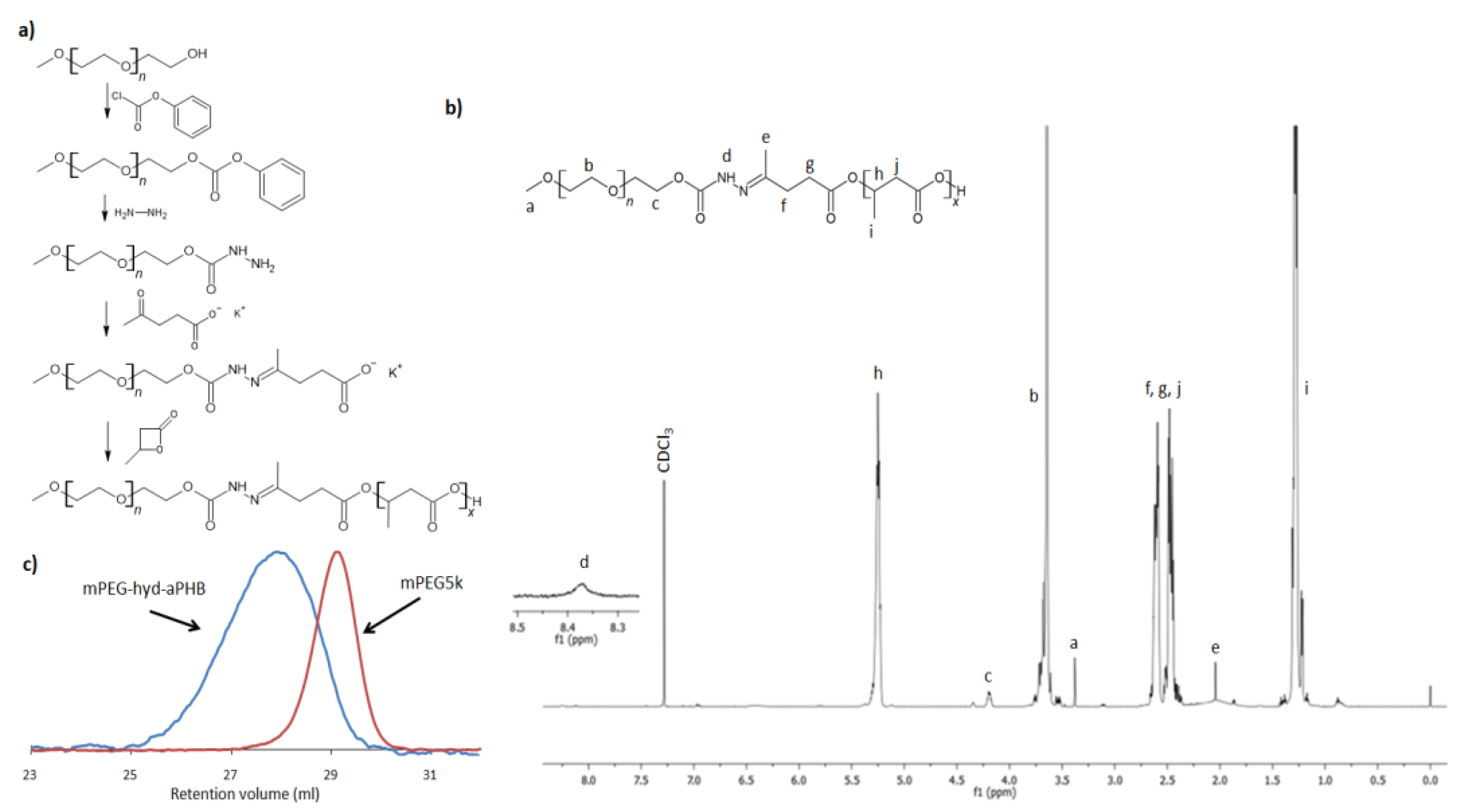

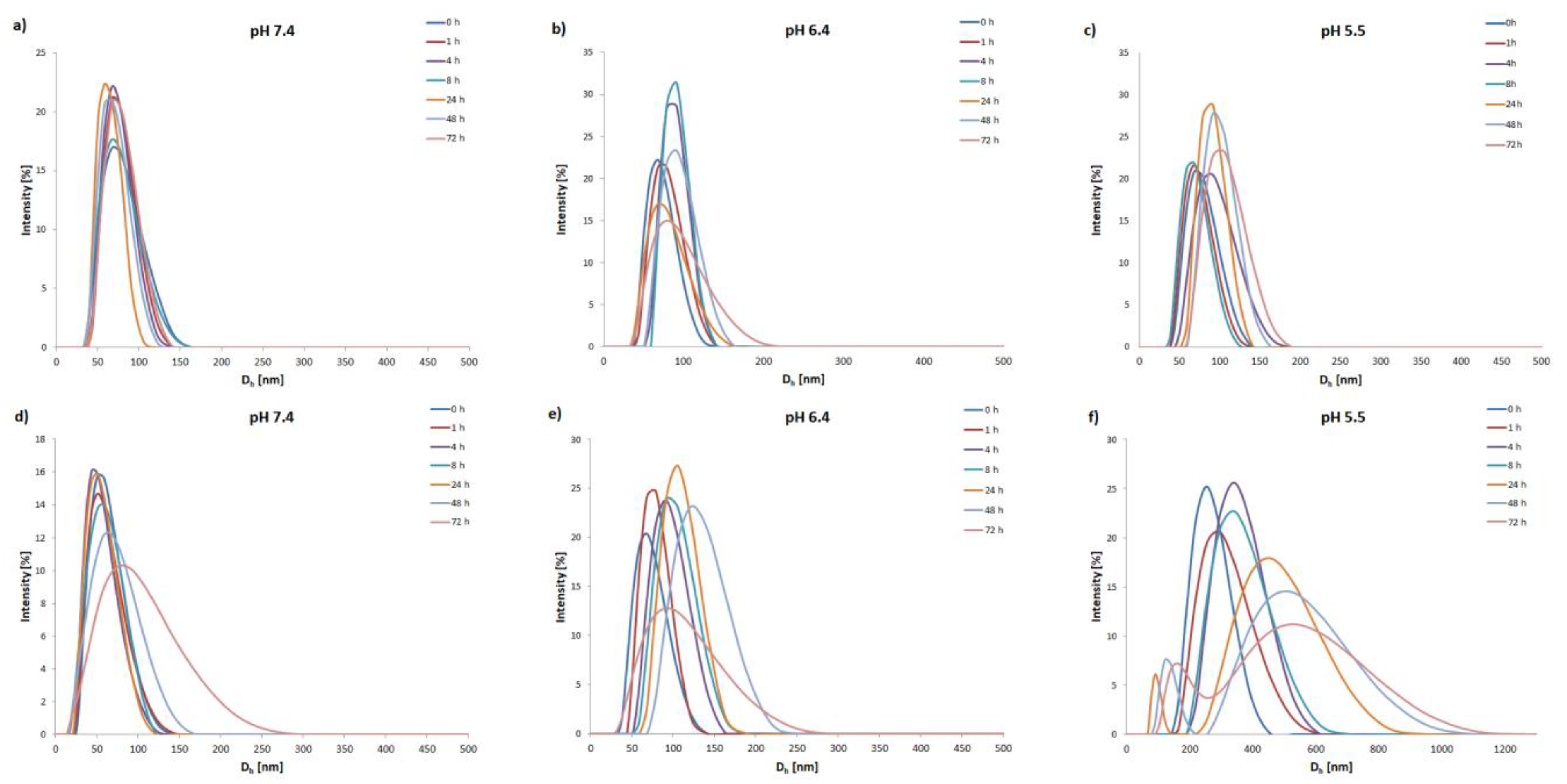

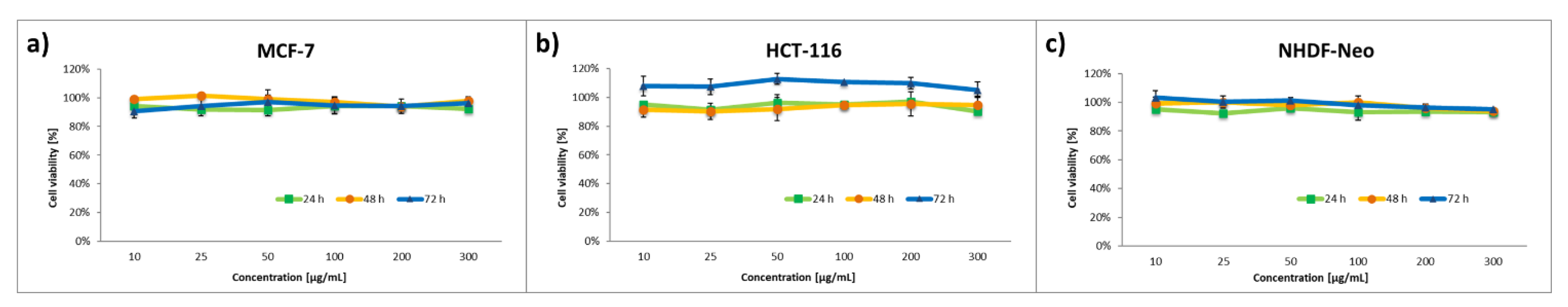


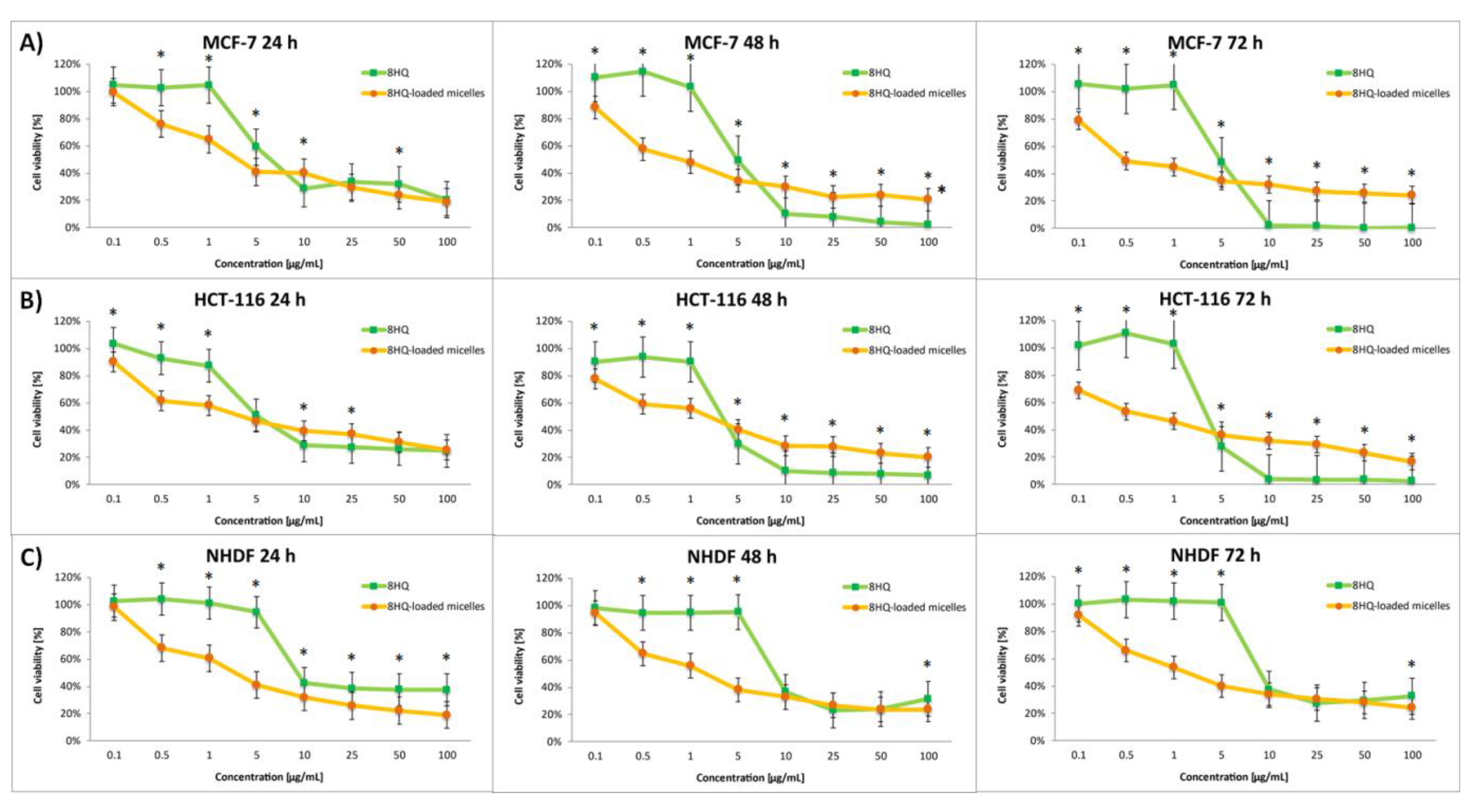

| Block Copolymer | Mn,NMR a [g mol−1] | Mn,SEC b [g mol−1] | Đ | fPEG [%] | CMC c [µg mL−1] |
|---|---|---|---|---|---|
| mPEG-hyd-aPHB | 8600 | 8200 | 1.14 | 58 | 3.6 |
| mPEG-b-aPHB | 8100 | 7900 | 1.08 | 62 | 2.2 |
| Sample | mPEG-hyd-aPHB | mPEG-b-aPHB | ||||||
|---|---|---|---|---|---|---|---|---|
| Size [nm] | PDI | DLC [%] | DLE [%] | Size [nm] | PDI | DLC [%] | DLE [%] | |
| Blank micelles | 55.3 ± 2.8 | 0.14 ± 0.01 | _ | _ | 49.5 ± 4.1 | 0.18 ± 0.02 | _ | _ |
| DOX-loaded micelles | 85.7 ± 5.1 | 0.22 ± 0.03 | 5.3 ± 0.4 | 48.6 ± 3.1 | 82.8 ± 6.2 | 0.2 ± 0.01 | 5.7 ± 0.2 | 54.1 ± 3.8 |
| 8HQ-loaded micelles | 77.7 ± 3.8 | 0.19 ± 0.02 | 6.0 ± 0.1 | 55.1 ± 1.9 | 87.9 ± 2.9 | 0.15 ± 0.02 | 5.8 ± 0.4 | 55.3 ± 2.3 |
| 8HQ-Glu-loaded micelles | 104.9 ± 4.5 | 0.25 ± 0.03 | 5.2 ± 0.2 | 45.6 ± 2.2 | 100.8 ± 3.1 | 0.28 ± 0.04 | 5.1 ± 0.3 | 47.1 ± 2.6 |
| 8HQ-Gal-loaded micelles | 92.5 ± 4.2 | 0.22 ± 0.02 | 5.3 ± 0.3 | 46.4 ± 2.5 | 99.9 ± 5.4 | 0.19 ± 0.02 | 5.3 ± 0.3 | 48.1 ± 3.2 |
| Compound | Activity IC50 [µM] a | ||
| MCF-7 | |||
| 24 h | 48 h | 72 h | |
| DOX | 3.42 ± 0.03 | 0.58 ± 0.02 | 0.57 ± 0.02 |
| DOX-micelles | 1.96 ± 0.01 | 0.52 ± 0.02 | 0.13 ± 0.01 |
| 8HQ | 7.17 ± 0.21 | 2.86 ± 0.09 | 3.01 ± 0.05 |
| 8HQ-mic b | 4.82 ± 0.17 | 2.11 ± 0.11 | 0.75 ± 0.04 |
| 8HQ-Glu | 65.86 ± 2.22 | 62.07 ± 1.84 | 63.57 ± 2.01 |
| 8HQ-Glu-mic c | 37.57 ± 0.92 | 3.85 ± 0.19 | 0.59 ± 0.02 |
| 8HQ-Gal | 26.39 ± 1.28 | 23.15 ± 0.99 | 24.60 ± 0.52 |
| 8HQ-Gal-mic d | 5.59 ± 0.14 | 0.21 ± 0.01 | 0.19 ± 0.01 |
| Compound | Activity IC50 [µM] a | ||
| HCT-116 | |||
| 24 h | 48 h | 72 h | |
| DOX | 6.94 ± 0.33 | 0.095 ± 0.01 | 0.105 ± 0.01 |
| DOX-micelles | 2.36 ± 0.08 | 0.45 ± 0.01 | 0.078 ± 0.01 |
| 8HQ | 9.33 ± 0.22 | 4.12 ± 0.12 | 4.40 ± 0.08 |
| 8HQ-mic b | 4.95 ± 0.16 | 1.80 ± 0.05 | 0.91 ± 0.04 |
| 8HQ-Glu | 49.67 ± 1.32 | 47.20 ± 1.14 | 45.73 ± 1.81 |
| 8HQ-Glu-mic c | 49.60 ± 2.11 | 8.46 ± 0.12 | 0.38 ± 0.02 |
| 8HQ-Gal | 22.10 ± 0.84 | 23.09 ± 0.92 | 22.52 ± 1.02 |
| 8HQ-Gal-mic d | 10.83 ± 0.33 | 2.64 ± 0.12 | 0.017 ± 0.01 |
| Compound | Activity IC50 [µM] a | ||
| NHDF-Neo | |||
| 24 h | 48 h | 72 h | |
| DOX | >20 | >20 | 2.71 ± 0.08 |
| DOX-micelles | >20 | 3.93 ± 0.12 | 2.50 ± 0.06 |
| 8HQ | 9.34 ± 0.25 | 8.97 ± 0.19 | 9.64 ± 0.32 |
| 8HQ-mic b | 5.18 ± 0.26 | 3.96 ± 0.12 | 3.97 ± 0.09 |
| 8HQ-Glu | 41.00 ± 2.01 | 41.11 ± 1.73 | 30.02 ± 0.94 |
| 8HQ-Glu-mic c | 41.70 ± 1.42 | 22.53 ± 0.65 | 13.38 ± 0.39 |
| 8HQ-Gal | 20.14 ± 0.75 | 21.77 ± 0.89 | 18.30 ± 0.74 |
| 8HQ-Gal-mic d | 7.47 ± 0.17 | 5.55 ± 0.17 | 2.79 ± 0.13 |
| Compound | Selectivity Index (SI) a | |
|---|---|---|
| MCF-7 | HCT-116 | |
| DOX | 4.75 | 25.81 |
| DOX-micelles | 19.23 | 32.05 |
| 8HQ | 3.20 | 2.19 |
| 8HQ-mic b | 5.29 | 4.36 |
| 8HQ-Glu | 0.47 | 1.39 |
| 8HQ-Glu-mic c | 22.68 | 35.21 |
| 8HQ-Gal | 0.74 | 0.81 |
| 8HQ-Gal-mic d | 14.68 | 164.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domiński, A.; Domińska, M.; Skonieczna, M.; Pastuch-Gawołek, G.; Kurcok, P. Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics 2022, 14, 290. https://doi.org/10.3390/pharmaceutics14020290
Domiński A, Domińska M, Skonieczna M, Pastuch-Gawołek G, Kurcok P. Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics. 2022; 14(2):290. https://doi.org/10.3390/pharmaceutics14020290
Chicago/Turabian StyleDomiński, Adrian, Monika Domińska, Magdalena Skonieczna, Gabriela Pastuch-Gawołek, and Piotr Kurcok. 2022. "Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System" Pharmaceutics 14, no. 2: 290. https://doi.org/10.3390/pharmaceutics14020290
APA StyleDomiński, A., Domińska, M., Skonieczna, M., Pastuch-Gawołek, G., & Kurcok, P. (2022). Shell-Sheddable Micelles Based on Poly(ethylene glycol)-hydrazone-poly[R,S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics, 14(2), 290. https://doi.org/10.3390/pharmaceutics14020290








