Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Implementation of Quality by Design (QbD)
2.3. Preparation of FAV-THP Cocrystal
2.4. Single Crystal X-ray Diffraction
2.5. Powder X-ray Diffraction (PXRD)
2.6. Thermal Analysis
2.7. Dynamic Vapor Sorption (DVS)
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. Particle Size Distribution Measurement by Laser Diffraction
2.10. Scanning Electron Microscopy (SEM)
2.11. High Performance Liquid Chromatography (HPLC)
2.12. In Vitro Aerosol Performance Evaluation
2.13. Dissolution Study
2.14. Solubility Study
2.15. Stability Study
2.16. MTT Cell Viability Assay
2.17. Statistical Analysis
3. Results and Discussion
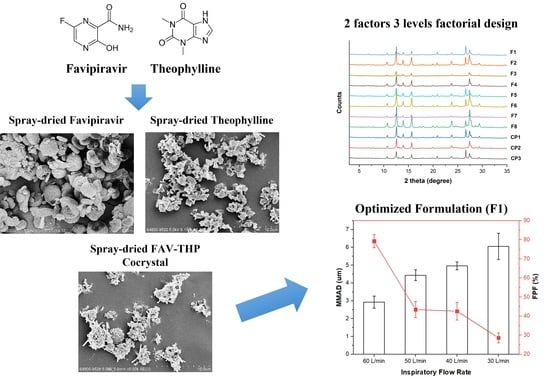
3.1. Cocrystallization of Favipiravir with Theophylline
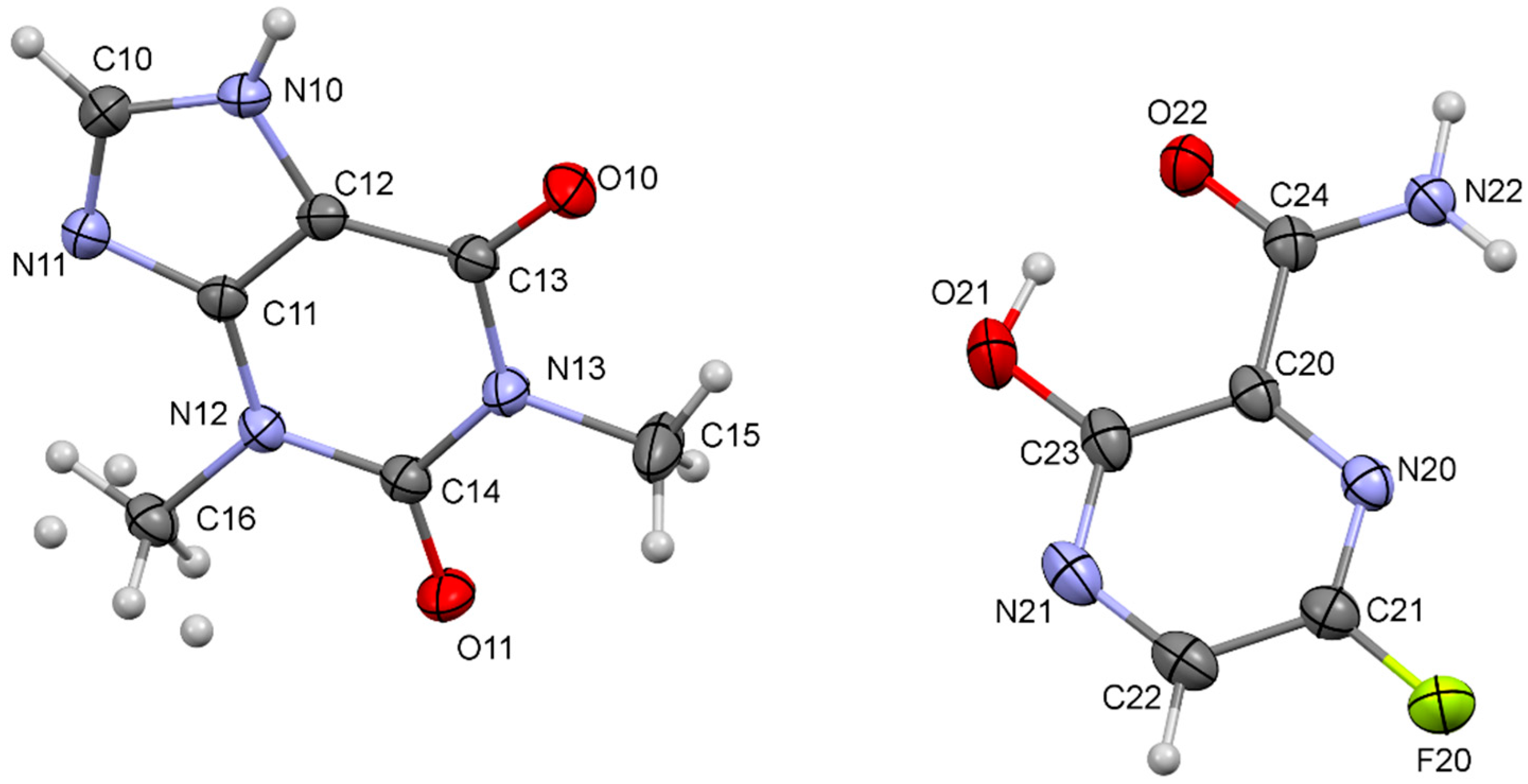
3.2. Single Crystal X-ray Diffraction
3.3. Application of DoE to the Process Optimization of Spray-Dried Pharmaceutical Cocrystal Dry Powder Formulation
3.3.1. In-Vitro Aerosol Performance of Inhalable FAV-THP Cocrystal Powders
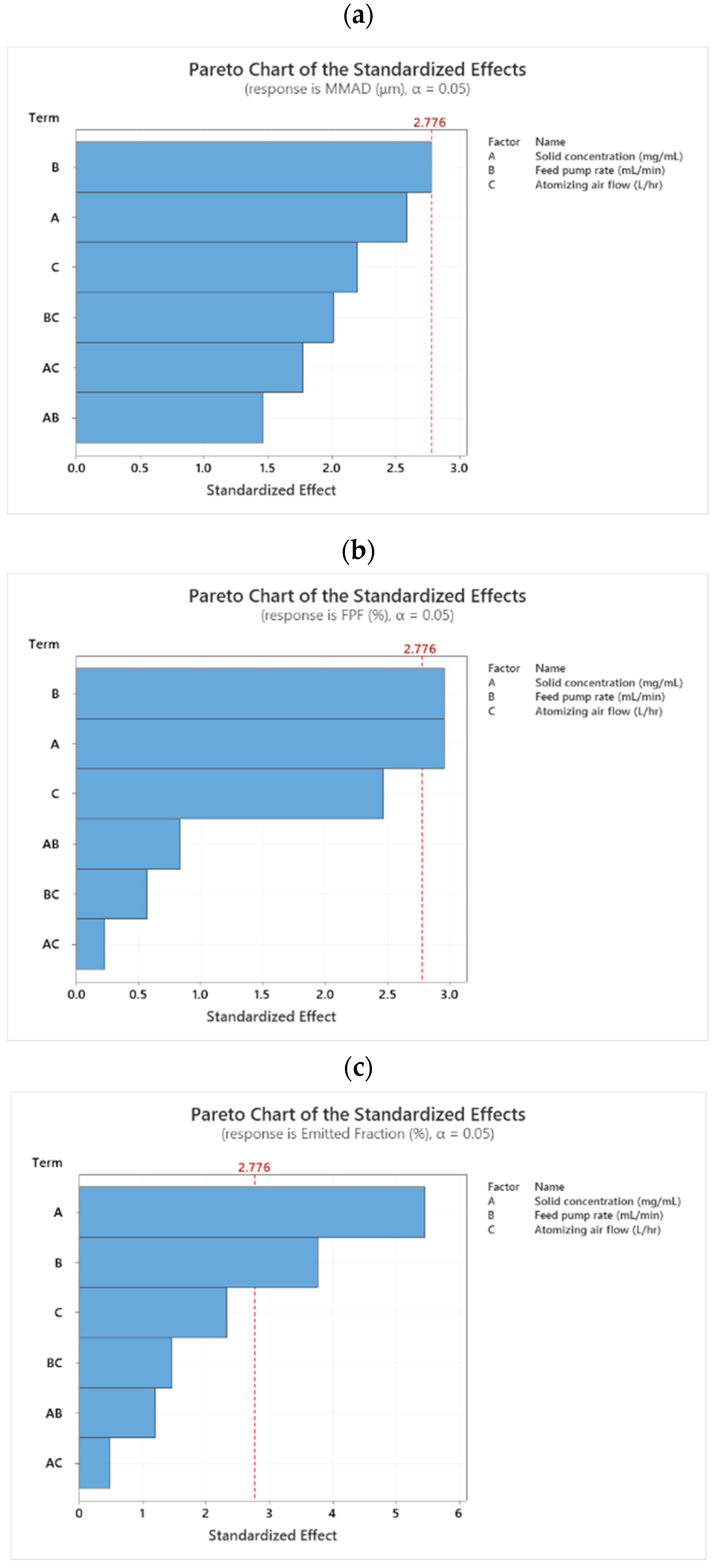
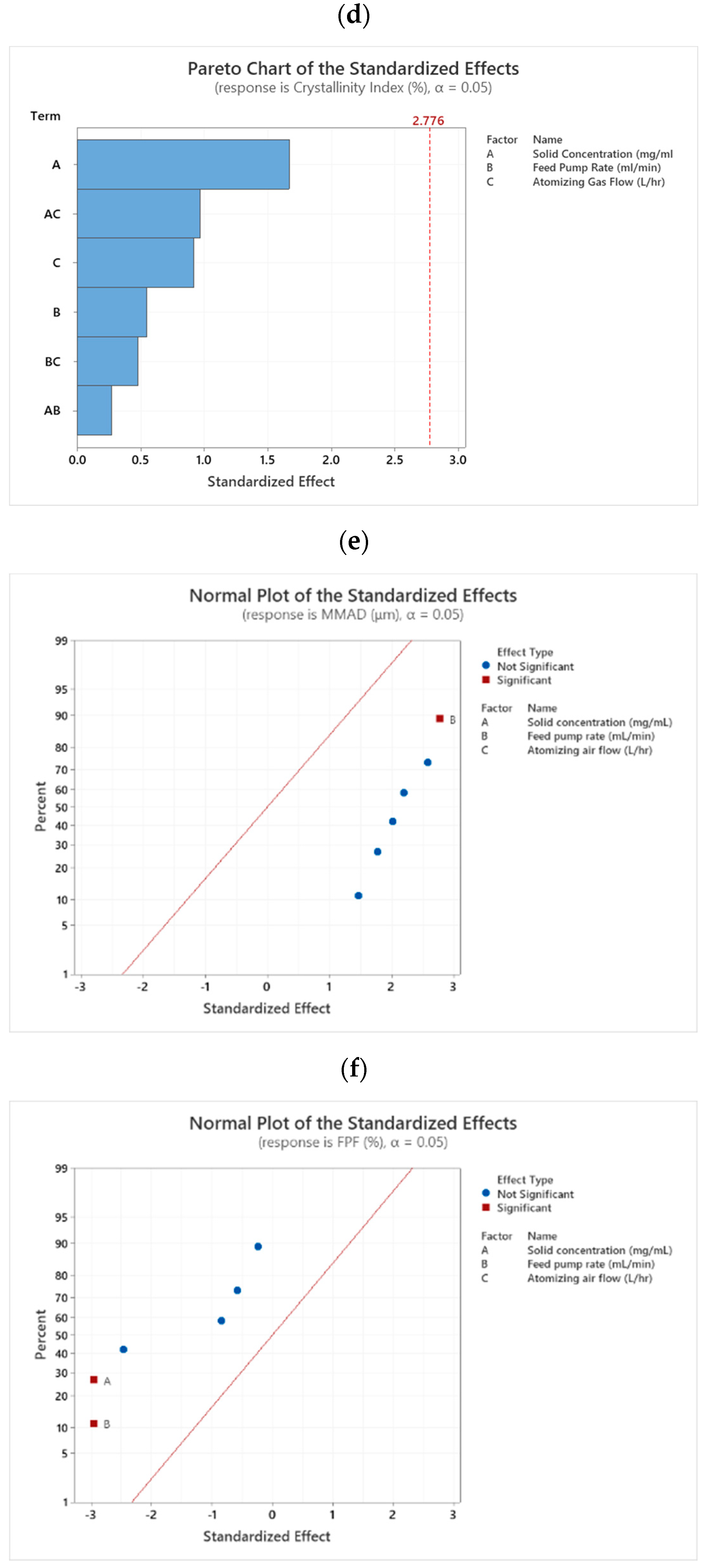
3.3.2. Identification of Influential Factors for the Critical Quality Attributes in the Spray Drying Process
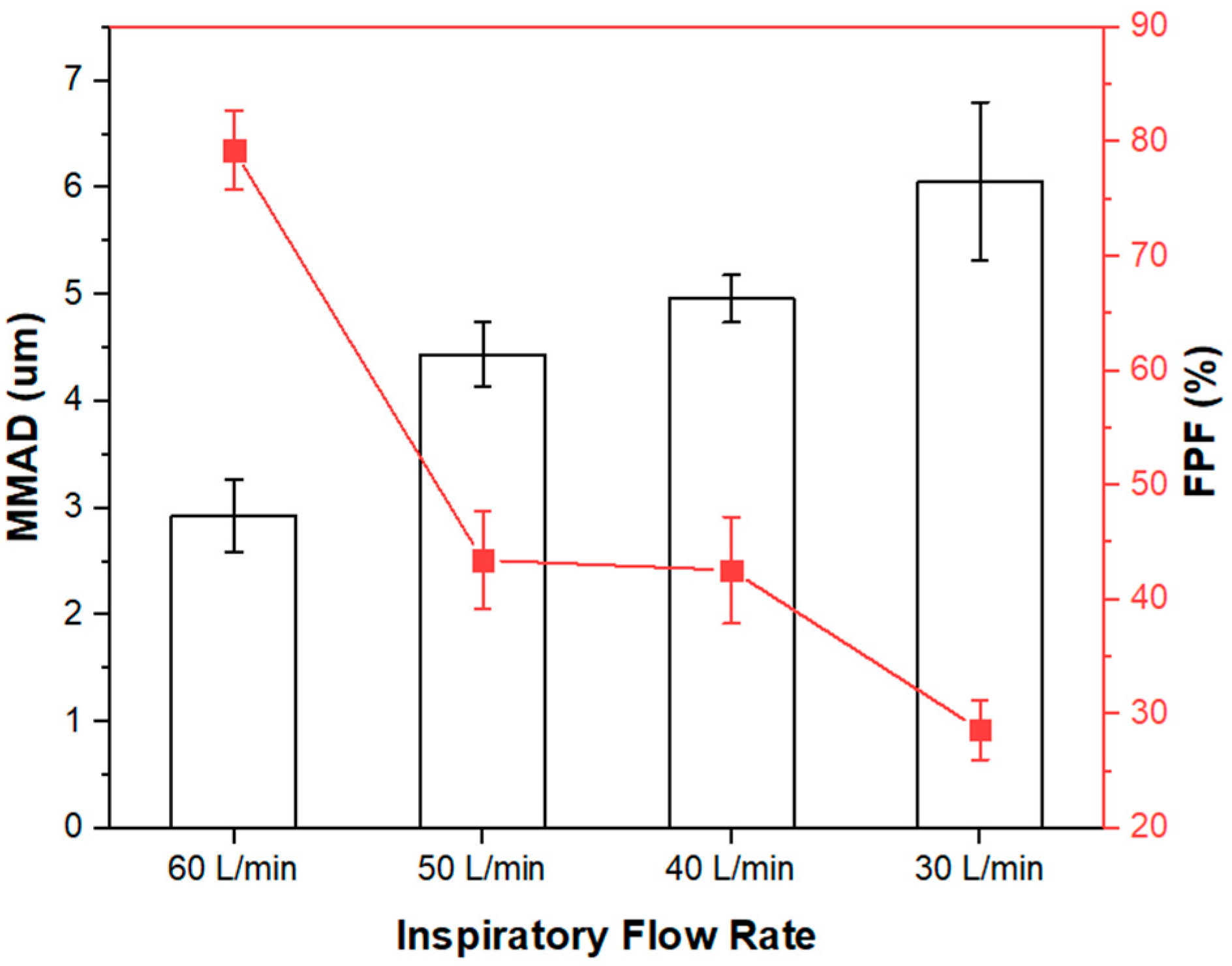
Mass Median Aerodynamic Diameter (MMAD)
Fine Particle Fraction (FPF) and Emitted Fraction (EF)
Crystallinity
3.4. Characterization of the Optimized Spray-Dried Cocrystal Particles
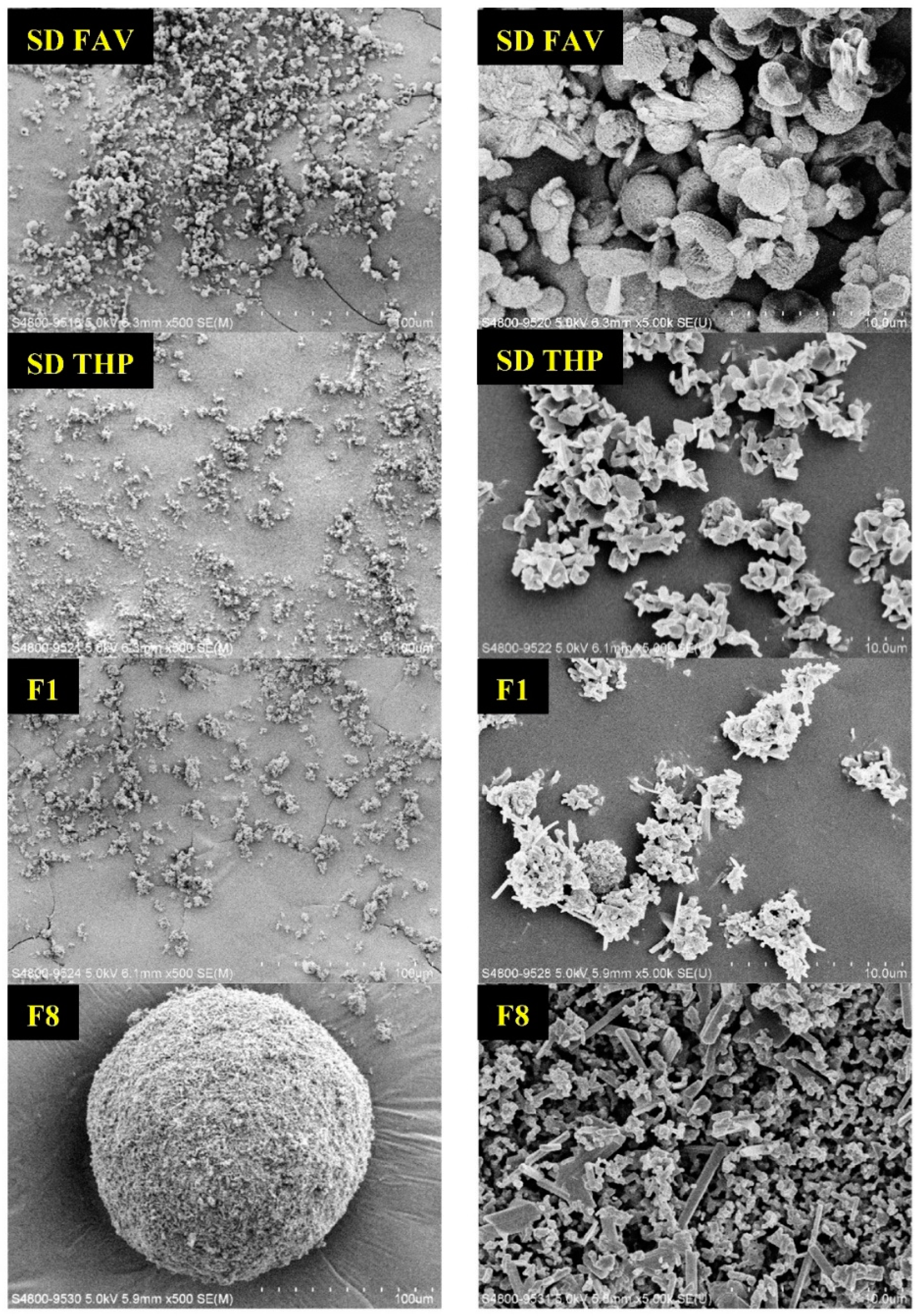
3.4.1. Morphology
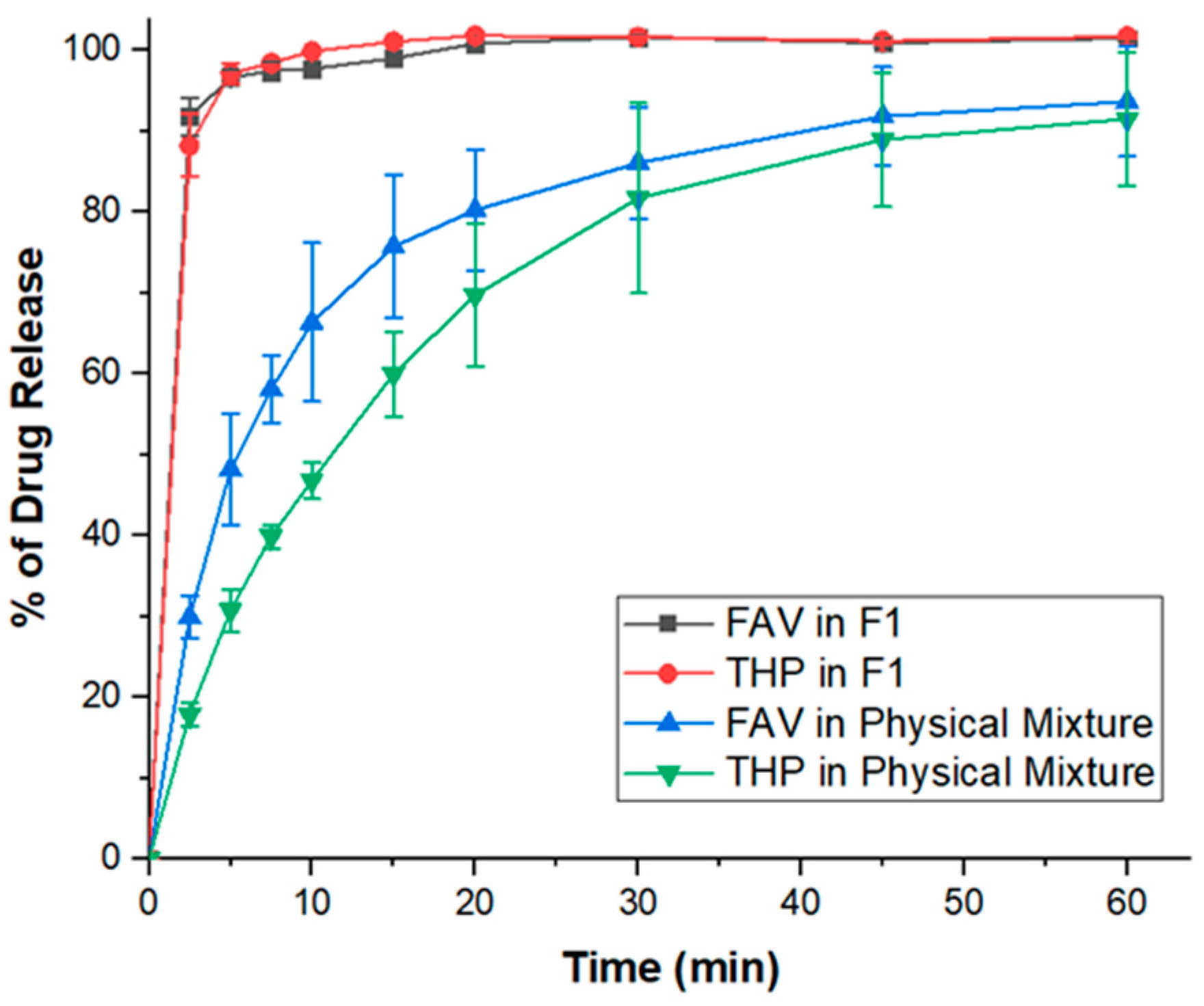
3.4.2. Dissolution Performance
3.4.3. Cytotoxicity
3.5. Significance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 December 2021).
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 1 December 2021).
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hasegawa, K.; Ma, B.; Fujiogi, M.; Camargo, C.A., Jr.; Liang, L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020, 146, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Koh, H.Y.; Moon, S.Y.; Yoo, I.K.; Ha, E.K.; You, S.; Kim, S.Y.; Yon, D.K.; Lee, S.W. Allergic disorders and susceptibility to and severity of COVID-19: A nationwide cohort study. J. Allergy Clin. Immunol. 2020, 146, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Fazio-Eynullayeva, E.; Lane, D.A.; Underhill, P.; Lip, G.Y. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 2020, 17, e1003321. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Z.-W.; Wang, L.; Yuan, M.-L.; Liu, K.; Zhou, L.; Wei, S.; Deng, Y.; Liu, J.; Liu, H.-G. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. 2020, 133, 1032. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, M.; Foster, K.J.; Jauregui, E.; Moore, D.; Adnan, D.; Andy-Nweye, A.B.; Khan, S.; Bishehsari, F. Asthma prolongs intubation in COVID-19. J. Allergy Clin. Immunol. Pract. 2020, 8, 2388–2391. [Google Scholar] [CrossRef]
- Garg, S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, 1–30 March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020, 92, 1915–1921. [Google Scholar] [CrossRef] [Green Version]
- Inoue, H.; Jinno, M.; Ohta, S.; Kishino, Y.; Kawahara, T.; Mikuni, H.; Sato, H.; Yamamoto, M.; Sato, Y.; Onitsuka, C.; et al. Combination treatment of short-course systemic corticosteroid and favipiravir in a successfully treated case of critically ill COVID-19 pneumonia with COPD. Respir. Med. Case Rep. 2020, 31, 101200. [Google Scholar] [CrossRef]
- Liang, W.; Pan, H.W.; Vllasaliu, D.; Lam, J.K.W. Pulmonary Delivery of Biological Drugs. Pharmaceutics 2020, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, B.; Zhao, Y.-Y. Dry Powder for Pulmonary Delivery: A Comprehensive Review. Pharmaceutics 2020, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, H.; Granger, A.; Hibbard, T.; Opesanwo, S. Pulmonary Drug Delivery of Antimicrobials and Anticancer Drugs Using Solid Dispersions. Pharmaceutics 2021, 13, 1056. [Google Scholar] [CrossRef] [PubMed]
- Valiulin, S.; Onischuk, A.; Baklanov, A.; Dubtsov, S.; An’Kov, S.; Shkil, N.; Nefedova, E.; Plokhotnichenko, M.; Tolstikova, T.; Dolgov, A.; et al. Aerosol inhalation delivery of cefazolin in mice: Pharmacokinetic measurements and antibacterial effect. Int. J. Pharm. 2021, 607, 121013. [Google Scholar] [CrossRef]
- Valiulin, S.V.; Onischuk, A.A.; Dubtsov, S.N.; Baklanov, A.M.; An’Kov, S.V.; Plokhotnichenko, M.E.; Tolstikova, T.G.; Dultseva, G.G.; Rusinov, V.L.; Charushin, V.N.; et al. Aerosol Inhalation Delivery of Triazavirin in Mice: Outlooks for Advanced Therapy Against Novel Viral Infections. J. Pharm. Sci. 2020, 110, 1316–1322. [Google Scholar] [CrossRef]
- Onischuk, A.; Tolstikova, T.; Baklanov, A.; Khvostov, M.; Sorokina, I.; Zhukova, N.; An’Kov, S.; Borovkova, O.; Dultseva, G.; Boldyrev, V.; et al. Generation, inhalation delivery and anti-hypertensive effect of nisoldipine nanoaerosol. J. Aerosol Sci. 2014, 78, 41–54. [Google Scholar] [CrossRef]
- Chvatal, A.; Ambrus, R.; Party, P.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Fattal, E.; Tsapis, N. Formulation and comparison of spray dried non-porous and large porous particles containing meloxicam for pulmonary drug delivery. Int. J. Pharm. 2019, 559, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Kadota, K.; Yanagawa, Y.; Tachikawa, T.; Deki, Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. Development of porous particles using dextran as an excipient for enhanced deep lung delivery of rifampicin. Int. J. Pharm. 2018, 555, 280–290. [Google Scholar] [CrossRef]
- Ogienko, A.; Bogdanova, E.; Trofimov, N.; Myz, S.; Kolesov, B.; Yunoshev, A.; Zubikov, N.; Manakov, A.; Boldyrev, V.; Boldyreva, E. Large porous particles for respiratory drug delivery. Glycine-based formulations. Eur. J. Pharm. Sci. 2017, 110, 148–156. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Tanaka, M.; Nishiyama, H.; Tse, J.Y.; Uchiyama, H.; Shirakawa, Y.; Tozuka, Y. An effective approach to modify the inhalable betamethasone powders based on morphology and surface control using a biosurfactant. Powder Technol. 2020, 376, 517–526. [Google Scholar] [CrossRef]
- AVIGAN Tablets 200 mg. Available online: https://www.cdc.gov.tw/File/Get/ht8jUiB_MI-aKnlwstwzvw (accessed on 1 December 2021).
- Kaptein, S.J.; Jacobs, S.; Langendries, L.; Seldeslachts, L.; Ter Horst, S.; Liesenborghs, L.; Hens, B.; Vergote, V.; Heylen, E.; Barthelemy, K. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl. Acad. Sci. USA 2020, 117, 26955–26965. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef]
- Khambholja, K.; Asudani, D. Potential repurposing of Favipiravir in COVID-19 outbreak based on current evidence. Travel Med. Infect. Dis. 2020, 35, 101710. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.S.; Lai, W. Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options. ChemBioChem 2020, 22, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2020, 102, 501–508. [Google Scholar] [CrossRef]
- Rattanaumpawan, P.; Jirajariyavej, S.; Lerdlamyong, K.; Palavutitotai, N.; Saiyarin, J. Real-world Experience with Favipiravir for Treatment of COVID-19 in Thailand: Results from a Multi-center Observational Study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin. Pharmacol. Ther. 2020, 108, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloy, P.; Solas, C.; Touret, F.; Mentré, F.; Malvy, D.; de Lamballerie, X.; Guedj, J. Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin. Pharmacol. Ther. 2020, 108, 188. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.W.; Barnard, D.L.; Day, C.W.; Smee, D.F.; Bailey, K.W.; Wong, M.-H.; Morrey, J.D.; Furuta, Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 2007, 51, 845–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Report on the Deliberation Results (Avigan Tablet 200 mg). Available online: https://www.pmda.go.jp/files/000210319.pdf (accessed on 1 December 2021).
- Driouich, J.-S.; Cochin, M.; Lingas, G.; Moureau, G.; Touret, F.; Petit, P.R.; Piorkowski, G.; Barthélémy, K.; Coutard, B.; Guedj, J. Favipiravir and severe acute respiratory syndrome coronavirus 2 in hamster model. bioRxiv 2020. [Google Scholar] [CrossRef]
- Telko, M.J.; Hickey, A.J. Dry powder inhaler formulation. Respir. Care 2005, 50, 1209–1227. [Google Scholar]
- Malcolmson, R.J.; Embleton, J.K. Dry powder formulations for pulmonary delivery. Pharm. Sci. Technol. Today 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Larhrib, H.; Zeng, X.M.; Martin, G.P.; Marriott, C.; Pritchard, J. The use of different grades of lactose as a carrier for aerosolised salbutamol sulphate. Int. J. Pharm. 1999, 191, 1–14. [Google Scholar] [CrossRef]
- Zeng, X.M.; Pandhal, K.H.; Martin, G.P. The influence of lactose carrier on the content homogeneity and dispersibility of beclomethasone dipropionate from dry powder aerosols. Int. J. Pharm. 2000, 197, 41–52. [Google Scholar] [CrossRef]
- Biddiscombe, M.F.; Usmani, O.S. Is there room for further innovation in inhaled therapy for airways disease? Breathe 2018, 14, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sahakijpijarn, S.; Moon, C.; Ma, X.; Su, Y.; Koleng, J.J.; Dolocan, A.; Williams III, R.O. Using thin film freezing to minimize excipients in inhalable tacrolimus dry powder formulations. Int. J. Pharm. 2020, 586, 119490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; MacKenzie, B.; Koleng, J.J.; Maier, E.; Warnken, Z.N.; Williams III, R.O. Development of an Excipient-Free Peptide Dry Powder Inhalation for the Treatment of Pulmonary Fibrosis. Mol. Pharm. 2020, 17, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Quarta, E.; Chierici, V.; Flammini, L.; Tognolini, M.; Barocelli, E.; Cantoni, A.M.; Dujovny, G.; Ecenarro, S.; Sonvico, F.; Colombo, G. Excipient-free pulmonary insulin dry powder: Pharmacokinetic and pharmacodynamics profiles in rats. J. Control. Release 2020, 323, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Motherwell, W.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical cocrystals: Along the path to improved medicines. Chem. Commun. 2016, 52, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.; Sun, C.C. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef]
- Wong, S.N.; Hu, S.; Ng, W.W.; Xu, X.; Lai, K.L.; Lee, W.Y.T.; Chow, A.H.L.; Sun, C.C.; Chow, S.F. Cocrystallization of curcumin with benzenediols and benzenetriols via rapid solvent removal. Cryst. Growth Des. 2018, 18, 5534–5546. [Google Scholar] [CrossRef]
- Xuan, B.; Wong, S.N.; Zhang, Y.; Weng, J.; Tong, H.H.; Wang, C.; Sun, C.C.; Chow, S.F. Extended release of highly water soluble isoniazid attained through cocrystallization with curcumin. Cryst. Growth Des. 2020, 20, 1951–1960. [Google Scholar] [CrossRef]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.; Sun, C.C.; Chow, S.F. Cocrystal Engineering of Pharmaceutical Solids: Therapeutic Potentials and Challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Ogienko, A.G.; Myz, S.A.; Ogienko, A.A.; Nefedov, A.A.; Stoporev, A.S.; Mel’gunov, M.S.; Yunoshev, A.S.; Shakhtshneider, T.P.; Boldyrev, V.V.; Boldyreva, E.V. Cryosynthesis of Co-Crystals of Poorly Water-Soluble Pharmaceutical Compounds and Their Solid Dispersions with Polymers. The “Meloxicam–Succinic Acid” System as a Case Study. Cryst. Growth Des. 2018, 18, 7401–7409. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Aslaksen, A.; Bakke, O.; Vigander, T. Comparative pharmacokinetics of theophylline and aminophylline in man. Br. J. Clin. Pharmacol. 1981, 11, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emala, C.W. Pulmonary Pharmacology. In Pharmacology and Physiology for Anesthesia; Elsevier: Amsterdam, The Netherlands, 2019; pp. 613–628. [Google Scholar]
- Barnes, P.J. Theophylline for COPD; BMJ Publishing Group Ltd.: London, UK, 2006. [Google Scholar]
- Newhouse, M.T. Is theophylline obsolete? Chest 1990, 98, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, J.E. Inhaled phosphodiesterase 4 (PDE4) inhibitors for inflammatory respiratory diseases. Front. Pharmacol. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddleston, M.D.; Arhangelskis, M.; Fábián, L.; Tizzard, G.J.; Coles, S.J.; Jones, W. Investigation of an amide-pseudo amide hydrogen bonding motif within a series of theophylline: Amide cocrystals. Cryst. Growth Des. 2016, 16, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Alhalaweh, A.; Kaialy, W.; Buckton, G.; Gill, H.; Nokhodchi, A.; Velaga, S.P. Theophylline cocrystals prepared by spray drying: Physicochemical properties and aerosolization performance. Aaps Pharmscitech 2013, 14, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, J.; Wong, S.N.; Xu, X.; Xuan, B.; Wang, C.; Chen, R.; Sun, C.C.; Lakerveld, R.; Kwok, P.C.L.; Chow, S.F. Cocrystal engineering of itraconazole with suberic acid via rotary evaporation and spray drying. Cryst. Growth Des. 2019, 19, 2736–2745. [Google Scholar] [CrossRef]
- Liao, Q.; Lam, I.C.; Lin, H.H.; Wan, L.T.; Lo, J.C.; Tai, W.; Kwok, P.C.; Lam, J.K. Effect of formulation and inhaler parameters on the dispersion of spray freeze dried voriconazole particles. Int. J. Pharm. 2020, 584, 119444. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Kachrimanis, K.; Bloxham, M.; Taylor, K.M.; Buckton, G. Preparation of theophylline inhalable microcomposite particles by wet milling and spray drying: The influence of mannitol as a co-milling agent. Int. J. Pharm. 2016, 514, 200–211. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Q.; Yuan, S.; Cao, J.; Tang, K.; Qiu, Y.; Seow, H.C.; Man, R.C.H.; Shao, Z.; Huang, Y.; Liang, R. Inhaled Dry Powder Formulation of Tamibarotene, a Broad-Spectrum Antiviral against Respiratory Viruses Including SARS-CoV-2 and Influenza Virus. Adv. Ther. 2021, 4, 2100059. [Google Scholar] [CrossRef]
- Marques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Mihranyan, A.; Llagostera, A.P.; Karmhag, R.; Strømme, M.; Ek, R. Moisture sorption by cellulose powders of varying crystallinity. Int. J. Pharm. 2004, 269, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Bobrovs, R.; Seton, L.; Dempster, N. The reluctant polymorph: Investigation into the effect of self-association on the solvent mediated phase transformation and nucleation of theophylline. CrystEngComm 2015, 17, 5237–5251. [Google Scholar] [CrossRef] [Green Version]
- Larsen, A.S.; Olsen, M.A.; Moustafa, H.; Larsen, F.H.; Sauer, S.P.; Rantanen, J.; Madsen, A.Ø. Determining short-lived solid forms during phase transformations using molecular dynamics. CrystEngComm 2019, 21, 4020–4024. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Yao, C.; Xie, G.; Song, S.; Li, H.; Qu, Y.; Tao, X. Novel Formulations of the Antiviral Drug Favipiravir: Improving Permeability and Tabletability. Cryst. Growth Des. 2021, 21, 3807–3817. [Google Scholar] [CrossRef]
- Khamar, D.; Pritchard, R.G.; Bradshaw, I.J.; Hutcheon, G.A.; Seton, L. Polymorphs of anhydrous theophylline: Stable form IV consists of dimer pairs and metastable form I consists of hydrogen-bonded chains. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2011, 67, o496–o499. [Google Scholar] [CrossRef]
- Burnett, M.N.; Johnson, C.K. ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations; Citeseer: Princeton, NJ, USA, 1996. [Google Scholar]
- Hadiwinoto, G.D.; Lip Kwok, P.C.; Lakerveld, R. A review on recent technologies for the manufacture of pulmonary drugs. Ther. Deliv. 2018, 9, 47–70. [Google Scholar] [CrossRef]
- Ohtake, S.; Izutsu, K.-I.; Lechuga-Ballesteros, D. Drying Technologies for Biotechnology and Pharmaceutical Applications; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Demoly, P.; Hagedoorn, P.; de Boer, A.H.; Frijlink, H.W. The clinical relevance of dry powder inhaler performance for drug delivery. Respir. Med. 2014, 108, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Altman, P.; Wehbe, L.; Dederichs, J.; Guerin, T.; Ament, B.; Moronta, M.C.; Pino, A.V.; Goyal, P. Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: A randomized cross-over trial. BMC Pulm. Med. 2018, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Johal, B.; Howald, M.; Fischer, M.; Marshall, J.; Venthoye, G. Fine particle profile of fluticasone propionate/formoterol fumarate versus other combination products: The DIFFUSE study. Comb. Prod. Ther. 2013, 3, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Leung, S.S.; Parumasivam, T.; Nguyen, A.; Gengenbach, T.; Carter, E.A.; Carrigy, N.B.; Wang, H.; Vehring, R.; Finlay, W.H.; Morales, S. Effect of storage temperature on the stability of spray dried bacteriophage powders. Eur. J. Pharm. Biopharm. 2018, 127, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yip, L.; Chow, M.Y.; Chow, S.F.; Chan, H.-K.; Kwok, P.C.; Lam, J.K. Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int. J. Pharm. 2019, 560, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Miyamoto, K.; Taga, H.; Akita, T.; Yamashita, C. Simple method to measure the aerodynamic size distribution of porous particles generated on lyophilizate for dry powder inhalation. Pharmaceutics 2020, 12, 976. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Gonnissen, Y.; Verhoeven, E.; Peeters, E.; Remon, J.P.; Vervaet, C. Coprocessing via spray drying as a formulation platform to improve the compactability of various drugs. Eur. J. Pharm. Biopharm. 2008, 69, 320–334. [Google Scholar] [CrossRef] [Green Version]
- Sultana, S.; Talegaonkar, S.; Ali, R.; Mittal, G.; Ahmad, F.J.; Bhatnagar, A. Inhalation of alendronate nanoparticles as dry powder inhaler for the treatment of osteoporosis. J. Microencapsul. 2012, 29, 445–454. [Google Scholar] [CrossRef]
- Chen, R.; Weng, J.; Chow, S.F.; Lakerveld, R. Integrated Continuous Crystallization and Spray Drying of Insulin for Pulmonary Drug Delivery. Cryst. Growth Des. 2020, 21, 501–511. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Chattopadhyay, P.; Tong, H.H.; Chow, A.H. Particle size analysis in pharmaceutics: Principles, methods and applications. Pharm. Res. 2007, 24, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-K.; Nokhodchi, A.; Dastmalchi, S.; Alizadeh, A.A.; Barzegar-Jalali, M.; Adibkia, K.; Hamishehkar, H. A quantitative approach to predicting lung deposition profiles of pharmaceutical powder aerosols. Int. J. Pharm. 2021, 602, 120568. [Google Scholar] [CrossRef]
- Frijlink, H.; De Boer, A. Dry powder inhalers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2004, 1, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, P.T.; Yang, M.; Mulvad, H.; Nielsen, H.M.; Rantanen, J.; Foged, C. Engineering of an inhalable DDA/TDB liposomal adjuvant: A quality-by-design approach towards optimization of the spray drying process. Pharm. Res. 2013, 30, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray drying of mannitol as a drug carrier—the impact of process parameters on product properties. Drying Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Peng, T.; Lin, S.; Niu, B.; Wang, X.; Huang, Y.; Zhang, X.; Li, G.; Pan, X.; Wu, C. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sin. B 2016, 6, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Miao, X.; Shan, Z.; Huang, Y.; Li, L.; Pan, X.; Yao, Q.; Li, G.; Wu, C. Studies on the spray dried lactose as carrier for dry powder inhalation. Asian J. Pharm. Sci. 2014, 9, 336–341. [Google Scholar] [CrossRef]
- Shetty, N.; Park, H.; Zemlyanov, D.; Mangal, S.; Bhujbal, S.; Zhou, Q.T. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int. J. Pharm. 2018, 544, 222–234. [Google Scholar] [CrossRef]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of sample crystallinity determination methods by X-ray diffraction for challenging cellulose I materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef] [Green Version]
- Hermans, P.; Weidinger, A. Quantitative x-ray investigations on the crystallinity of cellulose fibers. A background analysis. J. Appl. Phys. 1948, 19, 491–506. [Google Scholar] [CrossRef]
- Uvarov, V. The influence of X-ray diffraction pattern angular range on Rietveld refinement results used for quantitative analysis, crystallite size calculation and unit-cell parameter refinement. J. Appl. Crystallogr. 2019, 52, 252–261. [Google Scholar] [CrossRef]
- Wong, S.N.; Chan, S.W.S.; Peng, X.; Xuan, B.; Lee, H.W.; Tong, H.H.; Chow, S.F. Effects of the Glass-Forming Ability and Annealing Conditions on Cocrystallization Behaviors via Rapid Solvent Removal: A Case Study of Voriconazole. Pharmaceutics 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Kissi, E.O.; Löbmann, K.; Thanki, K.; Fattal, E.; Rades, T.; Foged, C.; Yang, M. Design of inhalable solid dosage forms of budesonide and theophylline for pulmonary combination therapy. Aaps Pharmscitech 2019, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Tong, H.; Ayers, J. Free energy barrier to nucleation of amorphous-to-crystalline transformation selects the scale of microstructure of crystallized materials. Appl. Phys. Lett. 1995, 67, 350–352. [Google Scholar] [CrossRef]
- Eriksson, J.; Thörn, H.; Sjögren, E.; Holmstén, L.; Rubin, K.; Lennernäs, H. Pulmonary dissolution of poorly soluble compounds studied in an ex vivo rat lung model. Mol. Pharm. 2019, 16, 3053–3064. [Google Scholar] [CrossRef]
- Riley, T.; Christopher, D.; Arp, J.; Casazza, A.; Colombani, A.; Cooper, A.; Dey, M.; Maas, J.; Mitchell, J.; Reiners, M. Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). Aaps Pharmscitech 2012, 13, 978–989. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.T.; Guedj, J.; Anglaret, X.; Laouénan, C.; Madelain, V.; Taburet, A.-M.; Baize, S.; Sissoko, D.; Pastorino, B.; Rodallec, A. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl. Trop. Dis. 2017, 11, e0005389. [Google Scholar] [CrossRef] [Green Version]
- Oestereich, L.; Lüdtke, A.; Wurr, S.; Rieger, T.; Muñoz-Fontela, C.; Günther, S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antivir. Res. 2014, 105, 17–21. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Products—Quality Considerations Guidance for Industry. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/metered-dose-inhaler-mdi-and-dry-powder-inhaler-dpi-drug-products-quality-considerations (accessed on 1 December 2021).







| Independent Processing Variables (CPPs) | Levels | |||
|---|---|---|---|---|
| Low (−1) | Mid-Point (0) | High (+1) | ||
| X1 | Total solute concentration (mg/mL) | 3 | 6 | 9 |
| X2 | Feed pump rate (mL/min) | 1.5 | 3 | 4.5 |
| X3 | Atomizing air flow (L/h) | 357 | 536 | 742 |
| Responses: Dependent Variables (CQAs) | Goal | Acceptable Range | ||
| Y1 | Mass median aerodynamic diameter (MMAD, μm) | 3 | 1–5 | |
| Y2 | Fine particle fraction (FPF, %) | Maximize | ≥30 | |
| Y3 | Emitted fraction (EF, %) | Maximize | ≥60 | |
| Y4 | Crystallinity Index (CI, %) | Maximize | ≥50 | |
| Formulation | Total Solute Concentration (mg/mL) | Feed Pump Rate (mL/min) | Atomizing Air Flow (L/h) |
|---|---|---|---|
| F1 | 3 | 1.5 | 357 |
| F2 | 9 | 1.5 | 357 |
| F3 | 3 | 1.5 | 742 |
| F4 | 9 | 1.5 | 742 |
| F5 | 3 | 4.5 | 357 |
| F6 | 9 | 4.5 | 357 |
| F7 | 3 | 4.5 | 742 |
| F8 | 9 | 4.5 | 742 |
| CP1 | 6 | 3 | 536 |
| CP2 | 6 | 3 | 536 |
| CP3 | 6 | 3 | 536 |
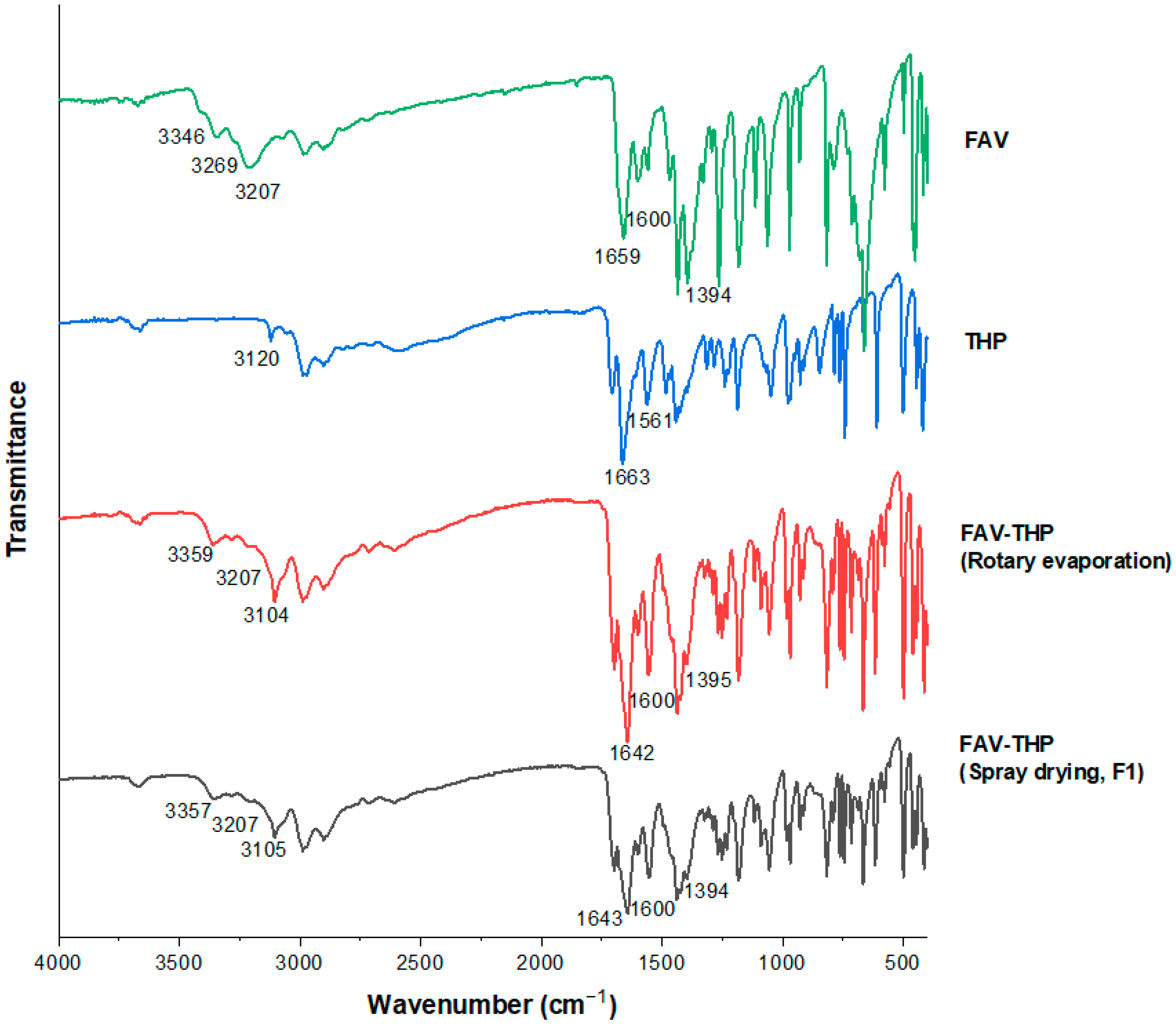
| Peak Assignment | FAV (cm−1) | THP (cm−1) | FAV-THP by Rotary Evaporation (cm−1) | FAV-THP by Spray Drying (cm−1) |
|---|---|---|---|---|
| ν (NH2) | 3346~3269 (broad) | 3120 | 3359~3207 (broad) | 3357~3207 (broad) |
| ν (OH) | 3207 | – | 3104 | 3105 |
| ν (C=O) | 1659 | 1663 | 1642 | 1643 |
| δ (NH2) | 1600 | 1561 | 1600 | 1599 |
| ν (CN)amide | 1394 | – | 1395 | 1394 |
| MMAD (μm) | GSD (μm) | FPF (%) | EF (%) | D10 (μm) | D50 (μm) | D90 (μm) | SPAN | CI (%) | |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 2.93 (0.34) | 1.91 (0.11) | 79.30 (3.44) | 61.26 (2.59) | 1.16 (0.05) | 3.83 (0.03) | 10.64 (0.10) | 2.48 (0.02) | 69.13 |
| F2 | 3.96 (0.09) | 1.98 (0.06) | 51.68 (7.02) | 82.14 (5.31) | 1.84 (0.13) | 5.03 (0.14) | 10.01 (0.19) | 1.62 (0.04) | 74.54 |
| F3 | 3.04 (0.16) | 1.93 (0.11) | 53.76 (3.70) | 66.63 (6.44) | 1.65 (0.18) | 4.20 (0.24) | 9.93 (0.23) | 1.97 (0.10) | 66.36 |
| F4 | 3.87 (0.26) | 2.02 (0.029) | 51.01 (5.66) | 80.24 (2.74) | 1.25 (0.04) | 4.46 (0.08) | 14.20 (0.12) | 2.90 (0.04) | 72.95 |
| F5 | 3.92 (0.28) | 1.99 (0.06) | 54.10 (1.23) | 72.69 (3.01) | 1.51 (0.3) | 4.90 (0.36) | 12.36 (0.45) | 2.21 (0.12) | 71.21 |
| F6 | 4.23 (0.07) | 2.11 (0.05) | 42.64 (3.51) | 82.61 (2.66) | 1.66 (0.21) | 5.40 (0.26) | 10.70 (0.29) | 1.67 (0.06) | 71.83 |
| F7 | 4.22 (0.12) | 2.05 (0.02) | 48.52 (5.81) | 80.90 (4.50) | 1.51 (0.07) | 5.79 (0.08) | 17.88 (0.12) | 2.83 (0.04) | 57.22 |
| F8 | 10.58 (0.64) | 2.48 (0.31) | 5.56 (2.45) | 93.05 (10.68) | 1.36 (0.12) | 6.92 (0.170 | 34.74 (0.36) | 4.82 (0.08) | 73.25 |
| CP1 | 3.81 (0.12) | 2.03 (0.19) | 49.25 (4.48) | 69.66 (5.33) | 1.45 (0.40) | 4.77 (0.39) | 12.98 (0.06) | 2.42 (0.23) | 63.38 |
| CP2 | 3.68 (0.45) | 2.01 (0.37) | 53.54 (5.79) | 77.19 (4.39) | 1.40 (0.13) | 4.86 (0.06) | 14.47 (0.15) | 2.69 (0.03) | 61.89 |
| CP3 | 3.74 (0.22) | 2.00 (0.18) | 54.12 (4.01) | 76.72 (2.23) | 1.49 (0.22) | 4.91 (0.18) | 12.92 (0.09) | 2.33 (0.10) | 61.59 |
| MMAD (μm) | GSD (μm) | FPF (%) | EF (%) | D10 (μm) | D50 (μm) | D90 (μm) | SPAN | |
|---|---|---|---|---|---|---|---|---|
| FAV | 4.91 (0.42) | 2.20 (0.21) | 26.69 (5.81) | 79.07 (7.51) | 1.99 (0.24) | 6.15 (0.26) | 12.92 (0.6) | 1.78 (0.02) |
| THP | 3.66 (0.19) | 1.95 (0.09) | 46.71 (2.36) | 90.95 (4.78) | 1.56 (0.04) | 4.03 (0.10) | 8.91 (0.02) | 1.82 (0.01) |
| F1 | 2.93 (0.34) | 1.91 (0.11) | 79.30 (3.44) | 61.26 (2.59) | 1.16 (0.05) | 3.83 (0.03) | 10.64 (0.10) | 2.48 (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.N.; Weng, J.; Ip, I.; Chen, R.; Lakerveld, R.; Telford, R.; Blagden, N.; Scowen, I.J.; Chow, S.F. Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline. Pharmaceutics 2022, 14, 300. https://doi.org/10.3390/pharmaceutics14020300
Wong SN, Weng J, Ip I, Chen R, Lakerveld R, Telford R, Blagden N, Scowen IJ, Chow SF. Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline. Pharmaceutics. 2022; 14(2):300. https://doi.org/10.3390/pharmaceutics14020300
Chicago/Turabian StyleWong, Si Nga, Jingwen Weng, Ignatius Ip, Ruipeng Chen, Richard Lakerveld, Richard Telford, Nicholas Blagden, Ian J. Scowen, and Shing Fung Chow. 2022. "Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline" Pharmaceutics 14, no. 2: 300. https://doi.org/10.3390/pharmaceutics14020300
APA StyleWong, S. N., Weng, J., Ip, I., Chen, R., Lakerveld, R., Telford, R., Blagden, N., Scowen, I. J., & Chow, S. F. (2022). Rational Development of a Carrier-Free Dry Powder Inhalation Formulation for Respiratory Viral Infections via Quality by Design: A Drug-Drug Cocrystal of Favipiravir and Theophylline. Pharmaceutics, 14(2), 300. https://doi.org/10.3390/pharmaceutics14020300