RNA-Based Strategies for Cell Reprogramming toward Pluripotency
Abstract
:1. Introduction
2. Reprogramming
2.1. History
2.2. Somatic Cell Sources
2.3. Reprogramming Factors
2.4. Reprogramming Enhancers
2.5. Reprogramming Strategies
2.5.1. Integrative Strategies
2.5.2. Nonintegrative Strategies
3. RNA-Based Reprogramming
3.1. RNA Technologies
3.1.1. Synthetic RNAs
3.1.2. Self-Replicative RNAs
3.1.3. MicroRNAs
3.1.4. CRISPR-Cas9
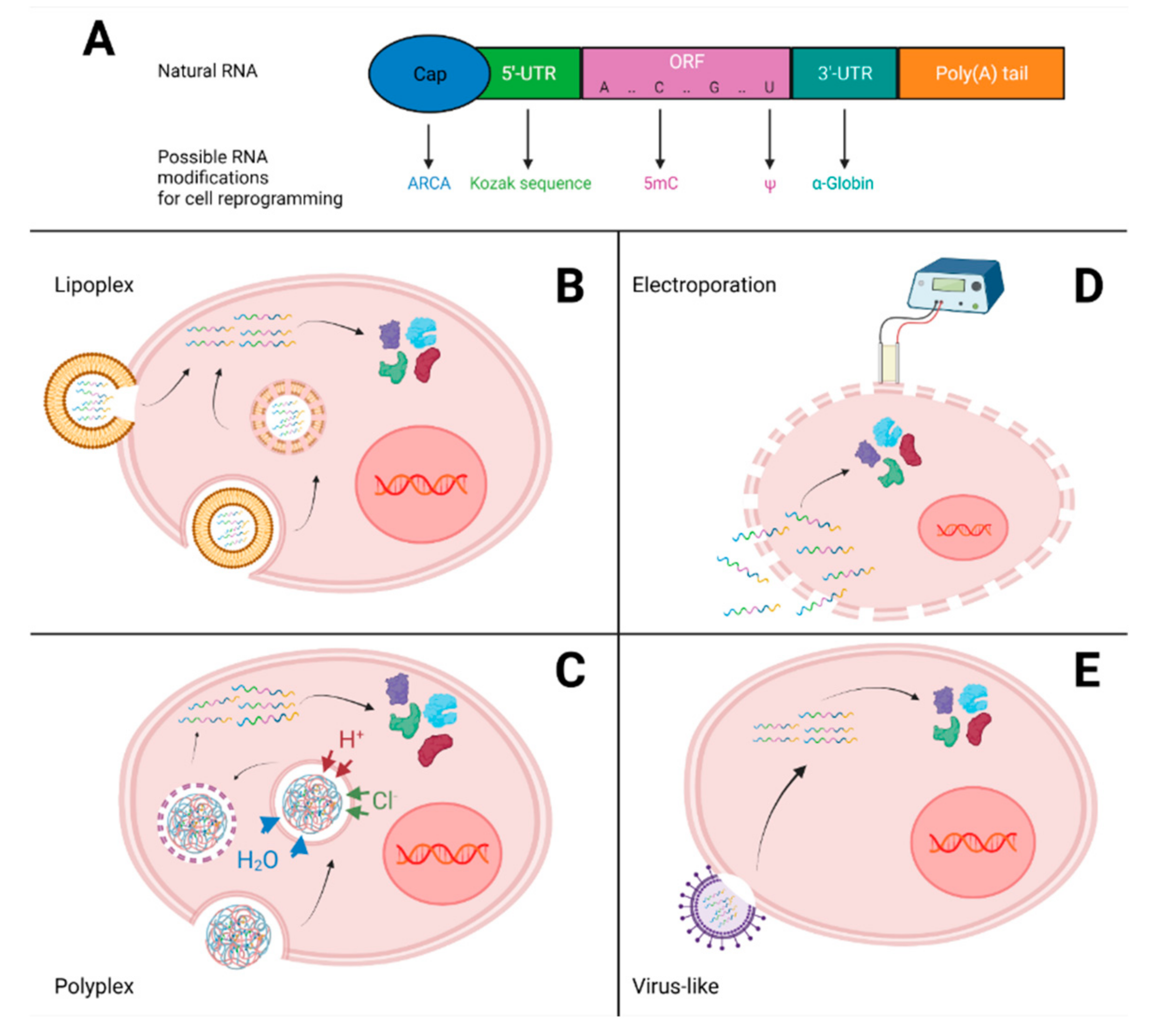
3.2. RNA Delivery
3.2.1. Lipoplex
3.2.2. Polyplex
3.2.3. Electroporation
3.2.4. Virus-Like Particles
4. Transient Reprogramming
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gurdon, J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962, 10, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Wilmut, I.; Schnieke, A.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Takahama, Y.; Abe, K.; Nakatsuji, N.; Tada, T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 2001, 11, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Nobel Prize in Physiology or Medicine 2012. Available online: https://www.nobelprize.org/prizes/medicine/2012/summary/ (accessed on 12 October 2021).
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Lowry, W.E.; Richter, L.; Yachechko, R.; Pyle, A.D.; Tchieu, J.; Sridharan, R.; Clark, A.T.; Plath, K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 2883–2888. [Google Scholar] [CrossRef] [Green Version]
- Si-Tayeb, K.; Noto, F.K.; Sepac, A.; Sedlic, F.; Bosnjak, Z.J.; Lough, J.W.; Duncan, S.A. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev. Biol. 2010, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Utikal, J.; Maherali, N.; Kulalert, W.; Hochedlinger, K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J. Cell Sci. 2009, 122, 3502–3510. [Google Scholar] [CrossRef] [Green Version]
- Loh, Y.-H.; Agarwal, S.; Park, I.-H.; Urbach, A.; Huo, H.; Heffner, G.C.; Kim, K.; Miller, J.D.; Ng, K.; Daley, G.Q. Generation of induced pluripotent stem cells from human blood. Blood 2009, 113, 5476–5479. [Google Scholar] [CrossRef]
- Ye, Z.; Zhan, H.; Mali, P.; Dowey, S.; Williams, N.M.; Jang, Y.-Y.; Dang, C.V.; Spivak, J.L.; Moliterno, A.R.; Cheng, L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 2009, 114, 5473–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, A.A.; Kroboth, S.; Rajesh, D.; Wang, W.B. Generation of Induced Pluripotent Stem Cells from CD34+ Cells across Blood Drawn from Multiple Donors with Non-Integrating Episomal Vectors. PLoS ONE 2011, 6, e27956. [Google Scholar] [CrossRef] [PubMed]
- Dowey, S.N.; Huang, X.; Chou, B.-K.; Ye, Z.; Cheng, L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat. Protoc. 2012, 7, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Staerk, J.; Dawlaty, M.M.; Gao, Q.; Maetzel, D.; Hanna, J.H.; Sommer, C.A.; Mostoslavsky, G.; Jaenisch, R. Reprogramming of Human Peripheral Blood Cells to Induced Pluripotent Stem Cells. Cell Stem Cell 2010, 7, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Yuasa, S.; Oda, M.; Egashira, T.; Yae, K.; Kusumoto, D.; Nakata, H.; Tohyama, S.; Hashimoto, H.; Kodaira, M.; et al. Generation of Induced Pluripotent Stem Cells from Human Terminally Differentiated Circulating T Cells. Cell Stem Cell 2010, 7, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Seki, T.; Yuasa, S.; Fukuda, K. Generation of induced pluripotent stem cells from a small amount of human peripheral blood using a combination of activated T cells and Sendai virus. Nat. Protoc. 2012, 7, 718–728. [Google Scholar] [CrossRef]
- Sun, N.; Panetta, N.J.; Gupta, D.M.; Wilson, K.D.; Lee, A.; Jia, F.; Hu, S.; Cherry, A.M.; Robbins, R.C.; Longaker, M.T.; et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15720–15725. [Google Scholar] [CrossRef] [Green Version]
- Jia, F.; Wilson, K.D.; Sun, N.; Gupta, D.M.; Huang, M.; Li, Z.; Panetta, N.J.; Chen, Z.Y.; Robbins, R.C.; Kay, M.A.; et al. A nonviral minicircle vector for deriving human iPS cells. Nat. Methods 2010, 7, 197–199. [Google Scholar] [CrossRef] [Green Version]
- Narsinh, K.H.; Jia, F.; Robbins, R.C.; Kay, M.A.; Longaker, M.T.; Wu, J.C. Generation of adult human induced pluripotent stem cells using nonviral minicircle DNA vectors. Nat. Protoc. 2010, 6, 78–88. [Google Scholar] [CrossRef]
- Yan, X.; Qin, H.; Qu, C.; Tuan, R.S.; Shi, S.; Huang, G.T.-J. iPS Cells Reprogrammed From Human Mesenchymal-Like Stem/Progenitor Cells of Dental Tissue Origin. Stem Cells Dev. 2010, 19, 469–480. [Google Scholar] [CrossRef]
- Giorgetti, A.; Montserrat, N.; Aasen, T.; Gonzalez, F.; Rodríguez-Pizà, I.; Vassena, R.; Raya, A.; Boue, S.; Barrero, M.; Corbella, B.A.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood Using OCT4 and SOX2. Cell Stem Cell 2009, 5, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Neises, A.; Su, R.-J.; Payne, K.; Ritter, L.; Gridley, D.S.; Wang, J.; Sheng, M.; Lau, K.-H.; Baylink, D.J.; et al. Efficient Reprogramming of Human Cord Blood CD34+ Cells Into Induced Pluripotent Stem Cells With OCT4 and SOX2 Alone. Mol. Ther. 2012, 20, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An Efficient Nonviral Method to Generate Integration-Free Human-Induced Pluripotent Stem Cells from Cord Blood and Peripheral Blood Cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Olmer, R.; Schwanke, K.; Wunderlich, S.; Merkert, S.; Hess, C.; Zweigerdt, R.; Gruh, I.; Meyer, J.; Wagner, S.; et al. Generation of Induced Pluripotent Stem Cells from Human Cord Blood. Cell Stem Cell 2009, 5, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Benda, C.; Duzinger, S.; Huang, Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L.; et al. Generation of Induced Pluripotent Stem Cells from Urine. J. Am. Soc. Nephrol. 2011, 22, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef]
- Xue, Y.; Cai, X.; Wang, L.; Liao, B.; Zhang, H.; Shan, Y.; Chen, Q.; Zhou, T.; Li, X.; Hou, J.; et al. Generating a Non-Integrating Human Induced Pluripotent Stem Cell Bank from Urine-Derived Cells. PLoS ONE 2013, 8, e70573. [Google Scholar] [CrossRef]
- Kim, J.B.; Greber, B.; Araúzo-Bravo, M.J.; Meyer, J.; Park, K.I.; Zaehres, H.; Schöler, H. Direct reprogramming of human neural stem cells by OCT4. Nature 2009, 461, 649–653. [Google Scholar] [CrossRef]
- Liu, H.; Ye, Z.; Kim, Y.-H.; Sharkis, S.; Jang, Y.-Y. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 2010, 51, 1810–1819. [Google Scholar] [CrossRef]
- Aasen, T.; Raya, A.; Barrero, M.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef]
- Aasen, T.; Belmonte, J.C.I. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat. Protoc. 2010, 5, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Hung, S.S.-C.; Lim, S.Y.; Wong, R.C.-B.; Ko, M.S. Efficient Generation of Integration-Free Human Induced Pluripotent Stem Cells From Keratinocytes by Simple Transfection of Episomal Vectors. Stem Cells Transl. Med. 2014, 3, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.P.; Nemes, C.; Varga, E.; Freund, C.; Kosmidis, G.; Gkatzis, K.; de Jong, D.; Szuhai, K.; Dinnyés, A.; Mummery, C.L. Generation of induced pluripotent stem cells from human foetal fibroblasts using the Sleeping Beauty transposon gene delivery system. Differentiation 2013, 86, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Stadtfeld, M.; Nagaya, M.; Utikal, J.; Weir, G.; Hochedlinger, K. Induced Pluripotent Stem Cells Generated Without Viral Integration. Science 2008, 322, 945–949. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Wu, Z.; Wang, Y.; Cheng, L.; Cui, C.; Gao, Y.; Chen, T.; Rao, L.; Chen, S.; Jia, N.; et al. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Res. 2008, 18, 600–603. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Aït-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.-M.; De Vos, J.; et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Collado, M.; Villasante, A.; Strati, K.; Ortega, S.; Cañamero, M.; Blasco, M.A.; Serrano, M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 2009, 460, 1136–1139. [Google Scholar] [CrossRef] [Green Version]
- Marión, R.M.; Strati, K.; Li, H.; Murga, M.; Blanco, R.; Ortega, S.; Fernandez-Capetillo, O.; Serrano, M.; Blasco, M.A. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature 2009, 460, 1149–1153. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, T.; Suzuki, J.; Wang, Y.V.; Menendez, S.; Morera, L.B.; Raya, A.; Wahl, G.M.; Belmonte, J.C.I. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 2009, 460, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Kanagawa, O.; Nakagawa, M.; Okita, K.; Yamanaka, S. Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature 2009, 460, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Utikal, J.; Polo, J.M.; Stadtfeld, M.; Maherali, N.; Kulalert, W.; Walsh, R.M.; Khalil, A.; Rheinwald, J.G.; Hochedlinger, K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 2009, 460, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Banito, A.; Rashid, S.T.; Acosta, J.C.; Li, S.; Pereira, C.F.; Geti, I.; Pinho, S.; Silva, J.C.; Azuara, V.; Walsh, M.; et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009, 23, 2134–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velychko, S.; Adachi, K.; Kim, K.-P.; Hou, Y.; MacCarthy, C.M.; Wu, G.; Schöler, H. Excluding Oct4 from Yamanaka Cocktail Unleashes the Developmental Potential of iPSCs. Cell Stem Cell 2019, 25, 737–753. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, T.S.; Hanna, J.; Zhang, X.; Ku, M.; Wernig, M.; Schorderet, P.; Bernstein, B.E.; Jaenisch, R.; Lander, E.S.; Meissner, A. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008, 454, 49–55. [Google Scholar] [CrossRef]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; A Melton, D. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat. Biotechnol. 2008, 26, 1269–1275. [Google Scholar] [CrossRef]
- Mali, P.; Chou, B.-K.; Yen, J.; Ye, Z.; Zou, J.; Dowey, S.; Brodsky, R.A.; Ohm, J.E.; Yu, W.; Baylin, S.B.; et al. Butyrate Greatly Enhances Derivation of Human Induced Pluripotent Stem Cells by Promoting Epigenetic Remodeling and the Expression of Pluripotency-Associated Genes. Stem Cells 2010, 28, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhou, H.; Abujarour, R.; Zhu, S.; Joo, J.Y.; Lin, T.; Hao, E.; Schöler, H.R.; Hayek, A.; Ding, S. Generation of Human-Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells 2009, 27, 2992–3000. [Google Scholar] [CrossRef] [Green Version]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C Enhances the Generation of Mouse and Human Induced Pluripotent Stem Cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, S.; Ojala, M.; Hyysalo, A.; Ilmarinen, T.; Rajala, K.; Pekkanen-Mattila, M.; Äänismaa, R.; Lundin, K.; Palgi, J.; Weltner, J.; et al. Comparative Analysis of Targeted Differentiation of Human Induced Pluripotent Stem Cells (hiPSCs) and Human Embryonic Stem Cells Reveals Variability Associated With Incomplete Transgene Silencing in Retrovirally Derived hiPSC Lines. Stem Cells Transl. Med. 2013, 2, 83–93. [Google Scholar] [CrossRef]
- Maherali, N.; Ahfeldt, T.; Rigamonti, A.; Utikal, J.; Cowan, C.; Hochedlinger, K. A High-Efficiency System for the Generation and Study of Human Induced Pluripotent Stem Cells. Cell Stem Cell 2008, 3, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockemeyer, D.; Soldner, F.; Cook, E.G.; Gao, Q.; Mitalipova, M.; Jaenisch, R. A Drug-Inducible System for Direct Reprogramming of Human Somatic Cells to Pluripotency. Cell Stem Cell 2008, 3, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernig, M.; Lengner, C.; Hanna, J.H.; Lodato, M.A.; Steine, E.J.; Foreman, R.K.; Staerk, J.; Markoulaki, S.; Jaenisch, R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat. Biotechnol. 2008, 26, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-W.; Lai, Y.-S.; Pawlik, K.M.; Liu, K.; Sun, C.-W.; Li, C.; Schoeb, T.R.; Townes, T.M. Polycistronic Lentiviral Vector for “Hit and Run” Reprogramming of Adult Skin Fibroblasts to Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 1042–1049. [Google Scholar] [CrossRef]
- Mali, P.; Ye, Z.; Hommond, H.H.; Yu, X.; Lin, J.; Chen, G.; Zou, J.; Cheng, L. Improved Efficiency and Pace of Generating Induced Pluripotent Stem Cells from Human Adult and Fetal Fibroblasts. Stem Cells 2008, 26, 1998–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaji, K.; Norrby, K.; Paca, A.; Mileikovsky, M.; Mohseni, P.; Woltjen, K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 2009, 458, 771–775. [Google Scholar] [CrossRef] [Green Version]
- Woltjen, K.; Michael, I.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef]
- Yusa, K.; Rad, R.; Takeda, J.; Bradley, A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat. Methods 2009, 6, 363–369. [Google Scholar] [CrossRef]
- Grabundzija, I.; Wang, J.; Sebe, A.; Erdei, Z.; Kajdi, R.; Devaraj, A.; Steinemann, D.; Szuhai, K.; Stein, U.; Cantz, T.; et al. Sleeping Beauty transposon-based system for cellular reprogramming and targeted gene insertion in induced pluripotent stem cells. Nucleic Acids Res. 2012, 41, 1829–1847. [Google Scholar] [CrossRef]
- Zhou, W.; Freed, C.R. Adenoviral Gene Delivery Can Reprogram Human Fibroblasts to Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 2667–2674. [Google Scholar] [CrossRef]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Muench, M.O.; Fusaki, N.; Beyer, A.I.; Wang, J.; Qi, Z.; Yu, J.; Kan, Y.W. Blood Cell-Derived Induced Pluripotent Stem Cells Free of Reprogramming Factors Generated by Sendai Viral Vectors. Stem Cells Transl. Med. 2013, 2, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Dai, Q.; Guo, T.; Xu, J.; Dai, Q. Efficient generation of transgene- and feeder-free induced pluripotent stem cells from human dental mesenchymal stem cells and their chemically defined differentiation into cardiomyocytes. Biochem. Biophys. Res. Commun. 2018, 495, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, W.; Fujita, Y.; Nakayama, C.; Shinkuma, S.; Suzuki, S.; Nomura, T.; Abe, R.; Shimizu, H. Establishment of integration-free induced pluripotent stem cells from human recessive dystrophic epidermolysis bullosa keratinocytes. J. Dermatol. Sci. 2018, 89, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Ban, H.; Nishishita, N.; Fusaki, N.; Tabata, T.; Saeki, K.; Shikamura, M.; Takada, N.; Inoue, M.; Hasegawa, M.; Kawamata, S.; et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc. Natl. Acad. Sci. USA 2011, 108, 14234–14239. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Ohtaka, M.; Takada, H.; Kurisaki, A.; Tran, N.V.K.; Tran, Y.T.H.; Hisatake, K.; Sano, M.; Nakanishi, M. Simple and effective generation of transgene-free induced pluripotent stem cells using an auto-erasable Sendai virus vector responding to microRNA-302. Stem Cell Res. 2017, 23, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Hu, K.; Smuga-Otto, K.; Tian, S.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science 2009, 324, 797–801. [Google Scholar] [CrossRef] [Green Version]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.-I.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wu, S.; Joo, J.Y.; Zhu, S.; Han, D.W.; Lin, T.; Trauger, S.; Bien, G.; Yao, S.; Zhu, Y.; et al. Generation of Induced Pluripotent Stem Cells Using Recombinant Proteins. Cell Stem Cell 2009, 4, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.-J.; Lee, C.-S.; Kwon, Y.-W.; Paek, J.S.; Lee, S.-H.; Hur, J.; Lee, E.J.; Roh, T.-Y.; Chu, I.-S.; Leem, S.-H.; et al. Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood 2010, 116, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Narayanan, K.; Lu, H.; Choo, Y.; Du, C.; Wiradharma, N.; Yang, C.; Wan, A.C. Delivery of reprogramming factors into fibroblasts for generation of non-genetic induced pluripotent stem cells using a cationic bolaamphiphile as a non-viral vector. Biomaterials 2013, 34, 5336–5343. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ge, J.; Zhang, X.; Cheng, L.; Zhang, Z.; Zhengyuan, Z.; Wang, Y.; Lin, C.; Yang, W.; Liu, J.; et al. Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Res. 2016, 26, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Wang, M.; Gu, H.; Xie, X. Bromodeoxyuridine promotes full-chemical induction of mouse pluripotent stem cells. Cell Res. 2015, 25, 1171–1174. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, A.; Robb, G.B.; Chan, S.-H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. [Google Scholar] [CrossRef]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.-Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef]
- Devarkar, S.C.; Wang, C.; Miller, M.T.; Ramanathan, A.; Jiang, F.; Khan, A.G.; Patel, S.S.; Marcotrigiano, J. Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl. Acad. Sci. USA 2016, 113, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Jalkanen, A.L.; Coleman, S.; Wilusz, J. Determinants and implications of mRNA poly(A) tail size – Does this protein make my tail look big? Semin. Cell Dev. Biol. 2014, 34, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaacs, A.; Cox, R.; Rotem, Z. Foreign nucleic acids as the stimulus to make interferon. Lancet 1963, 282, 113–116. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis E Sousa, C. Innate Antiviral Responses by Means of TLR7-Mediated Recognition of Single-Stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [Green Version]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.-K.; Schlee, M.; et al. 5’-Triphosphate RNA Is the Ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barral, P.M.; Sarkar, D.; Su, Z.-Z.; Barber, G.N.; DeSalle, R.; Racaniello, V.R.; Fisher, P.B. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: Key regulators of innate immunity. Pharmacol. Ther. 2009, 124, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Loo, Y.-M.; Gale, M., Jr. Immune Signaling by RIG-I-like Receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.A.; Paoletti, E.; Moss, B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J. Biol. Chem. 1975, 250, 9322–9329. [Google Scholar] [CrossRef]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl(3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar]
- Mockey, M.; Gonçalves, C.; Dupuy, F.P.; Lemoine, F.M.; Pichon, C.; Midoux, P. mRNA transfection of dendritic cells: Synergistic effect of ARCA mRNA capping with Poly(A) chains in cis and in trans for a high protein expression level. Biochem. Biophys. Res. Commun. 2006, 340, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Tureci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Karikó, K.; Muramatsu, H.; A Welsh, F.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Kormann, M.S.D.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; E Mays, L.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar] [CrossRef]
- Uchida, S.; Kataoka, K.; Itaka, K. Screening of mRNA Chemical Modification to Maximize Protein Expression with Reduced Immunogenicity. Pharmaceutics 2015, 7, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Luo, X.; Dong, Y. Effects of Chemically Modified Messenger RNA on Protein Expression. Bioconj. Chem. 2016, 27, 849–853. [Google Scholar] [CrossRef]
- Durbin, A.F.; Wang, C.; Marcotrigiano, J.; Gehrke, L. RNAs Containing Modified Nucleotides Fail To Trigger RIG-I Conformational Changes for Innate Immune Signaling. Mbio 2016, 7, e00833-16. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; Pan, T.; Kalsotra, A. RNA modifications and structures cooperate to guide RNA–protein interactions. Nat. Rev. Mol. Cell Biol. 2017, 18, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.; Smith, Z.D.; Meissner, A.; et al. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, L.; Ni, Y.; Wang, J.; Guo, X. Feeder-Free Derivation of Human Induced Pluripotent Stem Cells with Messenger RNA. Sci. Rep. 2012, 2, 657. [Google Scholar] [CrossRef]
- Hirai, H.; Tani, T.; Katoku-Kikyo, N.; Kellner, S.; Karian, P.; Firpo, M.; Kikyo, N. Radical Acceleration of Nuclear Reprogramming by Chromatin Remodeling with the Transactivation Domain of MyoD. Stem Cells 2011, 29, 1349–1361. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Katoku-Kikyo, N.; Karian, P.; Firpo, M.; Kikyo, N. Efficient iPS Cell Production with the MyoD Transactivation Domain in Serum-Free Culture. PLoS ONE 2012, 7, e34149. [Google Scholar] [CrossRef]
- Kogut, I.; McCarthy, S.M.; Pavlova, M.; Astling, D.P.; Chen, X.; Jakimenko, A.; Jones, K.L.; Getahun, A.; Cambier, J.C.; Pasmooij, A.M.G.; et al. High-efficiency RNA-based reprogramming of human primary fibroblasts. Nat. Commun. 2018, 9, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, P.S.; McGarvey, S.S.; Kogut, I.; Bilousova, G. Efficient RNA-Based Reprogramming of Disease-Associated Primary Human Fibroblasts into Induced Pluripotent Stem Cells. Springer Protoc. Handb. 2020, 2117, 271–284. [Google Scholar] [CrossRef]
- Sjogren, A.-K.M.; Liljevald, M.; Glinghammar, B.; Sagemark, J.; Li, X.; Jonebring, A.; Cotgreave, I.; Brolén, G.; Andersson, T.B. Critical differences in toxicity mechanisms in induced pluripotent stem cell-derived hepatocytes, hepatic cell lines and primary hepatocytes. Arch. Toxicol. 2014, 88, 1427–1437. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Yang, J.-Y.; Tien, K.T.; McKinley, T.R.; Bocard, B.R.; Maijub, J.G.; Burchell, P.O.; Williams, S.K.; Morris, M.E.; Hoying, J.B.; et al. Restoration of Physiologically Responsive Low-Density Lipoprotein Receptor-Mediated Endocytosis in Genetically Deficient Induced Pluripotent Stem Cells. Sci. Rep. 2015, 5, 13231. [Google Scholar] [CrossRef] [Green Version]
- Preskey, D.; Allison, T.F.; Jones, M.; Mamchaoui, K.; Unger, C. Synthetically modified mRNA for efficient and fast human iPS cell generation and direct transdifferentiation to myoblasts. Biochem. Biophys. Res. Commun. 2016, 473, 743–751. [Google Scholar] [CrossRef]
- Mandal, P.; Rossi, D.J. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat. Protoc. 2013, 8, 568–582. [Google Scholar] [CrossRef]
- Poleganov, M.A.; Eminli, S.; Beissert, T.; Herz, S.; Moon, J.-I.; Goldmann, J.; Beyer, A.; Heck, R.; Burkhart, I.; Roldan, D.B.; et al. Efficient Reprogramming of Human Fibroblasts and Blood-Derived Endothelial Progenitor Cells Using Nonmodified RNA for Reprogramming and Immune Evasion. Hum. Gene Ther. 2015, 26, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Fiacco, E.; Alowaysi, M.; Astro, V.; Adamo, A. Generation of an iPSC cohort of isogenic iPSC lines (46-XY and 47-XXY) from a non-mosaic Klinefelter Syndrome patient (47-XXY) (KAUSTi008-A, KAUSTi008-B, KAUSTi008-C, KAUSTi008-D, KAUSTi008-E, KAUSTi008-F, KAUSTi008-G). Stem Cell Res. 2020, 50, 102119. [Google Scholar] [CrossRef] [PubMed]
- Alowaysi, M.; Fiacco, E.; Astro, V.; Adamo, A. Establishment of iPSC lines from a high-grade Klinefelter Syndrome patient (49-XXXXY) and two genetically matched healthy relatives (KAUSTi003-A, KAUSTi004-A, KAUSTi004-B, KAUSTi005-A, KAUSTi005-B, KAUSTi005-C). Stem Cell Res. 2020, 49, 102008. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Klug, K.; Henkel, L.; Kwok, C.K.; Edenhofer, F.; Klopocki, E.; Kurth, I.; Üçeyler, N. Generation of two induced pluripotent stem cell lines from skin fibroblasts of sisters carrying a c.1094C>A variation in the SCN10A gene potentially associated with small fiber neuropathy. Stem Cell Res. 2019, 35, 101396. [Google Scholar] [CrossRef]
- Sacco, A.M.; Belviso, I.; Romano, V.; Carfora, A.; Schonauer, F.; Nurzynska, D.; Montagnani, S.; Di Meglio, F.; Castaldo, C. Diversity of dermal fibroblasts as major determinant of variability in cell reprogramming. J. Cell. Mol. Med. 2019, 23, 4256–4268. [Google Scholar] [CrossRef]
- Yakubov, E.; Rechavi, G.; Rozenblatt, S.; Givol, D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem. Biophys. Res. Commun. 2010, 394, 189–193. [Google Scholar] [CrossRef]
- Plews, J.R.; Li, J.; Jones, M.; Moore, H.D.; Mason, C.; Andrews, P.W.; Na, J. Activation of Pluripotency Genes in Human Fibroblast Cells by a Novel mRNA Based Approach. PLoS ONE 2010, 5, e14397. [Google Scholar] [CrossRef]
- Arnold, A.; Naaldijk, Y.M.; Fabian, C.; Wirth, H.; Binder, H.; Nikkhah, G.; Armstrong, L.; Stolzing, A. Reprogramming of Human Huntington Fibroblasts Using mRNA. ISRN Cell Biol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Heng, B.C.; Heinimann, K.; Miny, P.; Iezzi, G.; Glatz, K.; Scherberich, A.; Zulewski, H.; Fussenegger, M. mRNA transfection-based, feeder-free, induced pluripotent stem cells derived from adipose tissue of a 50-year-old patient. Metab. Eng. 2013, 18, 9–24. [Google Scholar] [CrossRef]
- Durruthy-Durruthy, J.; Briggs, S.F.; Awe, J.; Ramathal, C.Y.; Karumbayaram, S.; Lee, P.C.; Heidmann, J.D.; Clark, A.; Karakikes, I.; Loh, K.M.; et al. Rapid and Efficient Conversion of Integration-Free Human Induced Pluripotent Stem Cells to GMP-Grade Culture Conditions. PLoS ONE 2014, 9, e94231. [Google Scholar] [CrossRef] [PubMed]
- Varela, I.; Karagiannidou, A.; Oikonomakis, V.; Tzetis, M.; Tzanoudaki, M.; Siapati, E.-K.; Vassilopoulos, G.; Graphakos, S.; Kanavakis, E.; Goussetis, E. Generation of Human β-Thalassemia Induced Pluripotent Cell Lines by Reprogramming of Bone Marrow–Derived Mesenchymal Stromal Cells Using Modified mRNA. Cell. Reprogram. 2014, 16, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-I.; Lee, S.-Y.; Hwang, D.-Y. Extracellular Matrix-Dependent Generation of Integration- and Xeno-Free iPS Cells Using a Modified mRNA Transfection Method. Stem Cells Int. 2016, 2016, 6853081. [Google Scholar] [CrossRef] [Green Version]
- Rohani, L.; Fabian, C.; Holland, H.; Naaldijk, Y.; Dressel, R.; Löffler-Wirth, H.; Binder, H.; Arnold, A.; Stolzing, A. Generation of human induced pluripotent stem cells using non-synthetic mRNA. Stem Cell Res. 2016, 16, 662–672. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.Y.; Lee, T.-J.; Yang, G.-M.; Oh, J.; Won, J.; Han, J.; Jeong, G.-J.; Kim, J.; Kim, J.-H.; Kim, B.-S.; et al. Efficient mRNA delivery with graphene oxide-polyethylenimine for generation of footprint-free human induced pluripotent stem cells. J. Control. Release 2016, 235, 222–235. [Google Scholar] [CrossRef]
- Velasquez-Mao, A.J.; Tsao, C.J.M.; Monroe, M.N.; Legras, X.; Bissig-Choisat, B.; Bissig, K.-D.; Ruano, R.; Jacot, J.G. Differentiation of spontaneously contracting cardiomyocytes from non-virally reprogrammed human amniotic fluid stem cells. PLoS ONE 2017, 12, e0177824. [Google Scholar] [CrossRef]
- Jeriha, J.; Kolundzic, N.; Khurana, P.; Perez-Dominguez, A.; Ilic, D. mRNA-Based Reprogramming Under Xeno-Free and Feeder-Free Conditions. Methods Mol. Biol. 2020, 1–10. [Google Scholar] [CrossRef]
- Yoshioka, N.; Gros, E.; Li, H.-R.; Kumar, S.; Deacon, D.C.; Maron, C.; Muotri, A.R.; Chi, N.C.; Fu, X.-D.; Yu, B.; et al. Efficient Generation of Human iPSCs by a Synthetic Self-Replicative RNA. Cell Stem Cell 2013, 13, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, N.; Dowdy, S.F. Enhanced generation of iPSCs from older adult human cells by a synthetic five-factor self-replicative RNA. PLoS ONE 2017, 12, e0182018. [Google Scholar] [CrossRef]
- Umrath, F.; Steinle, H.; Weber, M.; Wendel, H.-P.; Reinert, S.; Alexander, D.; Avci-Adali, M. Generation of iPSCs from Jaw Periosteal Cells Using Self-Replicating RNA. Int. J. Mol. Sci. 2019, 20, 1648. [Google Scholar] [CrossRef] [Green Version]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; von Ohle, C.; Popov, A.-F.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol. Ther. Nucleic Acids 2019, 17, 907–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouma, M.J.; Arendzen, C.H.; Mummery, C.L.; Mikkers, H.; Freund, C. Reprogramming Urine-Derived Cells Using Commercially Available Self-Replicative RNA and a Single Electroporation. Curr. Protoc. Stem Cell Biol. 2020, 55, 124. [Google Scholar] [CrossRef] [PubMed]
- Leeson, H.C.; Hunter, Z.; Chaggar, H.K.; Lavin, M.F.; Mackay-Sim, A.; Wolvetang, E.J. Ataxia Telangiectasia iPSC line generated from a patient olfactory biopsy identifies novel disease-causing mutations. Stem Cell Res. 2021, 56, 102528. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yourick, J.J.; Sprando, R.L. Comparative transcriptomic analysis of endothelial progenitor cells derived from umbilical cord blood and adult peripheral blood: Implications for the generation of induced pluripotent stem cells. Stem Cell Res. 2017, 25, 202–212. [Google Scholar] [CrossRef]
- Gao, X.; Yourick, J.J.; Sprando, R.L. Generation of nine induced pluripotent stem cell lines as an ethnic diversity panel. Stem Cell Res. 2018, 31, 193–196. [Google Scholar] [CrossRef]
- Eminli, S.; Kwieder, B.; Yi, K.; Huang, C.J.; Moon, J.-I.; Chang, C.-H.; Kiskin, F.N.; Morrell, N.W.; Hamilton, B.; Rana, A.A. Clinically compatible advances in blood-derived endothelial progenitor cell isolation and reprogramming for translational applications. New Biotechnol. 2021, 63, 1–9. [Google Scholar] [CrossRef]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Generation of iPSCs by Nonintegrative RNA-Based Reprogramming Techniques: Benefits of Self-Replicating RNA versus Synthetic mRNA. Stem Cells Int. 2019, 2019, 7641767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-L.; Chang, D.C.; Chang-Lin, S.; Lin, C.-H.; Wu, D.T.; Chen, D.T.; Ying, S.-Y. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 2008, 14, 2115–2124. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-L.; Chang, D.C.; Lin, C.-H.; Ying, S.-Y.; Leu, D.; Wu, D.T.S. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011, 39, 1054–1065. [Google Scholar] [CrossRef] [Green Version]
- Anokye-Danso, F.; Trivedi, C.M.; Juhr, D.; Gupta, M.; Cui, Z.; Tian, Y.; Zhang, Y.; Yang, W.; Gruber, P.J.; Epstein, J.A.; et al. Highly Efficient miRNA-Mediated Reprogramming of Mouse and Human Somatic Cells to Pluripotency. Cell Stem Cell 2011, 8, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Card, D.A.G.; Hebbar, P.B.; Li, L.; Trotter, K.W.; Komatsu, Y.; Mishina, Y.; Archer, T.K. Oct4/Sox2-Regulated miR-302 Targets Cyclin D1 in Human Embryonic Stem Cells. Mol. Cell. Biol. 2008, 28, 6426–6438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, B.; Bao, X.; Liu, L.; Feng, S.; Zovoilis, A.; Liu, W.; Xue, Y.; Cai, J.; Guo, X.; Qin, B.; et al. MicroRNA Cluster 302–367 Enhances Somatic Cell Reprogramming by Accelerating a Mesenchymal-to-Epithelial Transition. J. Biol. Chem. 2011, 286, 17359–17364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanyam, D.; Lamouille, S.; Judson, R.L.; Liu, J.Y.; Bucay, N.; Derynck, R.; Blelloch, R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat. Biotechnol. 2011, 29, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wilson, K.D.; Ghosh, Z.; Han, L.; Wang, Y.; Lan, F.; Ransohoff, K.J.; Burridge, P.; Wu, J.C. MicroRNA-302 Increases Reprogramming Efficiency via Repression of NR2F2. Stem Cells 2012, 31, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xiang, D.; Heriyanto, F.; Gao, Y.; Qian, Z.; Wu, W.-S. Dissecting the Roles of miR-302/367 Cluster in Cellular Reprogramming Using TALE-based Repressor and TALEN. Stem Cell Rep. 2013, 1, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, N.; Ishii, H.; Nagano, H.; Haraguchi, N.; Dewi, D.L.; Kano, Y.; Nishikawa, S.; Tanemura, M.; Mimori, K.; Tanaka, F.; et al. Reprogramming of Mouse and Human Cells to Pluripotency Using Mature MicroRNAs. Cell Stem Cell 2011, 8, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.; Davis, M.P.A.; Abreu-Goodger, C.; Wang, W.; Campos, L.S.; Siede, J.; Vigorito, E.; Skarnes, W.C.; Dunham, I.; Enright, A.J.; et al. MiR-25 Regulates Wwp2 and Fbxw7 and Promotes Reprogramming of Mouse Fibroblast Cells to iPSCs. PLoS ONE 2012, 7, e40938. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Prasain, N.; Chae, H.-D.; Kim, Y.-J.; Mantel, C.; Yoder, M.C.; Broxmeyer, H.E. Epigenetic Regulation of Nanog by MiR-302 Cluster-MBD2 Completes Induced Pluripotent Stem Cell Reprogramming. Stem Cells 2013, 31, 666–681. [Google Scholar] [CrossRef] [Green Version]
- Barta, T.; Peskova, L.; Collin, J.; Montaner, D.; Neganova, I.; Armstrong, L.; Lako, M. Brief Report: Inhibition of miR-145 Enhances Reprogramming of Human Dermal Fibroblasts to Induced Pluripotent Stem Cells. Stem Cells 2015, 34, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Hysolli, E.; Tanaka, Y.; Su, J.; Kim, K.-Y.; Zhong, T.; Janknecht, R.; Zhou, X.-L.; Geng, L.; Qiu, C.; Pan, X.; et al. Regulation of the DNA Methylation Landscape in Human Somatic Cell Reprogramming by the miR-29 Family. Stem Cell Rep. 2016, 7, 43–54. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Cao, Y.; Wang, L.; Han, Y.; Zhong, X.; Zhou, G.; Cai, Y.; Zhang, H.; Gao, P. Human fibroblast reprogramming to pluripotent stem cells regulated by the miR19a/b-PTEN axis. PLoS ONE 2014, 9, e95213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.; Choo, K.B.; Huang, C.J.; Sugii, S.; Cheong, S.K.; Kamarul, T. miR-524-5p of the primate-specific C19MC miRNA cluster targets TP53IPN1- and EMT-associated genes to regulate cellular reprogramming. Stem Cell Res. Ther. 2017, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Guo, W.T.; Tian, S.; He, X.; Wang, X.W.; Liu, X.; Gu, K.L.; Ma, X.; Huang, D.; Hu, L.; et al. miR-290/371-Mbd2-Myc circuit regulates glycolytic metabolism to promote pluripotency. EMBO J. 2015, 34, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.R.; Mantel, C.; Lee, S.A.; Moon, S.H.; Broxmeyer, H.E. MiR-31/SDHA Axis Regulates Reprogramming Efficiency through Mitochondrial Metabolism. Stem Cell Rep. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.-N.; Chang, Y.-H.; Truong, V.A.; Lai, P.-L.; Nguyen, T.K.N.; Hu, Y.-C. CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 2019, 37, 107447. [Google Scholar] [CrossRef]
- Shakirova, K.M.; Ovchinnikova, V.Y.; Dashinimaev, E.B. Cell Reprogramming With CRISPR/Cas9 Based Transcriptional Regulation Systems. Front. Bioeng. Biotechnol. 2020, 8, 882. [Google Scholar] [CrossRef]
- Liu, P.; Chen, M.; Liu, Y.; Qi, L.S.; Ding, S. CRISPR-Based Chromatin Remodeling of the Endogenous Oct4 or Sox2 Locus Enables Reprogramming to Pluripotency. Cell Stem Cell 2018, 22, 252–261.e4. [Google Scholar] [CrossRef] [Green Version]
- Weltner, J.; Balboa, D.; Katayama, S.; Bespalov, M.; Krjutškov, K.; Jouhilahti, E.-M.; Trokovic, R.; Kere, J.; Otonkoski, T. Human pluripotent reprogramming with CRISPR activators. Nat. Commun. 2018, 9, 2643. [Google Scholar] [CrossRef] [Green Version]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; DeVine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M.; et al. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef]
- Höbel, S.; Koburger, I.; John, M.; Czubayko, F.; Hadwiger, P.; Vornlocher, H.-P.; Aigner, A. Polyethylenimine/small interfering RNA-mediated knockdown of vascular endothelial growth factorin vivoexerts anti-tumor effects synergistically with Bevacizumab. J. Gene Med. 2010, 12, 287–300. [Google Scholar] [CrossRef]
- Prel, A.; Caval, V.; Gayon, R.; Ravassard, P.; Duthoit, C.; Payen, E.; Chretien, L.; Creneguy, A.; Nguyen, T.H.; Martin, N.; et al. Highly efficient in vitro and in vivo delivery of functional RNAs using new versatile MS2-chimeric retrovirus-like particles. Mol. Ther. Methods Clin. Dev. 2015, 2, 15039. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [Green Version]
- Abad, M.; Mosteiro, L.; Pantoja, C.; Cañamero, M.; Rayon, T.; Ors, I.; Graña, O.; Megias, D.; Domínguez, O.; Martínez, D.; et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 2013, 502, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Ocampo, A.; Reddy, P.; Redondo, P.M.; Luengo, A.P.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733.e12. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Matellán, A.; Alcazar, N.; Hernández, F.; Serrano, M.; Ávila, J. In Vivo Reprogramming Ameliorates Aging Features in Dentate Gyrus Cells and Improves Memory in Mice. Stem Cell Rep. 2020, 15, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Doeser, M.C.; Schöler, H.R.; Wu, G. Reduction of Fibrosis and Scar Formation by Partial Reprogramming In Vivo. Stem Cells 2018, 36, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- De Lázaro, I.; Yilmazer, A.; Nam, Y.; Qubisi, S.; Razak, F.M.A.; Degens, H.; Cossu, G.; Kostarelos, K. Non-viral, Tumor-free Induction of Transient Cell Reprogramming in Mouse Skeletal Muscle to Enhance Tissue Regeneration. Mol. Ther. 2019, 27, 59–75. [Google Scholar] [CrossRef] [Green Version]
- Alle, Q.; Borgne, E.L.; Bensadoun, P.; Lemey, C.; Béchir, N.; Gabanou, M.; Estermann, F.; Bertrand-Gaday, C.; Pessemesse, L.; Toupet, K.; et al. A single short reprogramming early in life improves fitness and increases lifespan in old age. bioRxiv 2021. [Google Scholar] [CrossRef]
- David, L.; Polo, J.M. Phases of reprogramming. Stem Cell Res. 2014, 12, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Olova, N.; Simpson, D.J.; Marioni, R.E.; Chandra, T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 2019, 18, e12877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 2020, 11, 1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef] [PubMed]

| Transcription Factors | Transfection Regent | Starting Cell Type | mARN Features | References | Authors |
|---|---|---|---|---|---|
| OSNL | Lipofectamine 2000 | Human foreskin fibroblasts | Anti-reverse cap analog (ARCA) IRES sequence Poly(A) tail | [118] | Yakubov |
| OSKM OSKML | RNAiMAX | Human fibroblasts | ARCA 5mC and ψ5′-UTR containing Kozak sequence α-Globin 3′-UTR Poly(A) tail | [103] | Warren |
| OSKMT | Electroporation | Human fibroblasts | 5′ and 3′ UTRs of Xenopus β-globin Poly(A) tail | [119] | Plews |
| OSK OSN ONhT OMN OKN OSKMNhT | Electroporation and FuGENE HD | Human fibroblasts | Cap Poly(A) tail | [120] | Arnold |
| M3OSKMaL M3OSKMaLN | RNAiMAX or Stemfect | Human fibroblasts | ARCA 5mC and ψ 5′-UTR containing Kozak sequence α-Globin 3′-UTR Poly(A) tail | [104] | Warren |
| OSKML | RNAiMAX or Stemfect | Human fibroblasts | ARCA 5mC and ψ 5′-UTR 3′-UTR Poly(A) tail | [112] | Mandal |
| OSKML | RNAiMAX | Adipose tissue-derived mesenchymal stem cells | Synthetic modified mRNA (5mC and ψ) from Stemgent | [121] | Heng |
| OSKML | RNAiMAX | Human fibroblasts | ARCA 5mC and ψ | [122] | Durruthy-Durruthy |
| OSKML | RNAiMAX | Newborn foreskin fibroblasts | Stemgent mRNA Reprogramming Kit | [109] | Sjogren |
| OSKML | RNAiMAX | Bone marrow–derived mesenchymal stromal cells | Synthetic modified mRNA (5mC and ψ) from Stemgent | [123] | Varela |
| OSKMLN +EKB +miR302a–d +miR367 | RNAiMAX | Human fibroblasts and blood-derived endothelial progenitor cells | ARCA 5′-UTR containing Kozak sequence 5mC and ψ or not α-Globin 3′-UTR 3′-human-β-globin-UTR Poly(A) tail | [113] | Poleganov |
| OSKML | RNAiMAX | Human fibroblasts | ARCA 5mC and ψ5 ′-UTR containing Kozak sequence α-Globin 3′-UTR Poly(A) tail | [110] | Ramakrishnan |
| OSKML +miR302a–d +miR367 | Stemfect | Human adult dermal fibroblasts | Synthetic modified mRNA (5mC and ψ) from Stemgent | [124] | Lee |
| ONhT OSK OSKMNhT | jetPEI | Human fibroblatsts | Cap 5′-UTR containing Kozak sequence Poly(A) tail | [125] | Rohani |
| OSKMLN | Stemfect | Human fibroblasts | 6F mRNA Reprogramming Premix – Allele Biotechnology | [111] | Preskey |
| Natural mRNA extracted from HEK 293T or OSKM | Graphene oxide-polyethylenimine (Graphene oxide -PEI) | Human adipose tissue-derived fibroblasts | Natural mRNA extracted from HEK 293T Or Cap 5′-UTR 3′UTR Poly(A) tail | [126] | Choi |
| OSKML | RNAiMAX | Human amniotic fluid-derived stem cells | TriLink Biotechnologies Inc | [127] | Velasquez-Mao |
| M3OSKMLN +miRNA-367/302s | RNAiMAX | Human fibroblasts | ARCA 5mC and ψ Poly(A) tail | [107] | Kogut |
| M3OSKMLN +miRNA-367/302s | RNAiMAX | Human fibroblasts | ARCA 5mC and ψ 5′-UTR containing Kozak sequence α-Globin 3′-UTR Poly(A) tail | [108] | McGrath |
| OSKMLN +EKB +miR from miR302/367 cluster | RNAiMAX | Human Mesenchymal Stromal/Stem Cells | StemRNATM 3rd Gen Reprogramming Kit | [128] | Jeriha |
| miRNAs | Starting Cell Types | Reprogramming Factors | Reference | Authors |
|---|---|---|---|---|
| miR-302 cluster (without miR-367) | Human adipose stem cells | OSKM | [145] | Hu |
| miR-302 cluster (without miR-367) | Human CD34+ cord blood cells | OSKM | [149] | Lee |
| miR-302-367 cluster | Human primary neonatal fibroblasts | OSKMLN | [107] | Kogut |
| miR-302-367 cluster | Human fibroblasts (CRL-2097) | OSK | [143] | Liao |
| miR-302b or/and miR-372 | Human foreskin (BJs) or lung (MRC-5) fibroblasts | OSKM or OSK | [144] | Subramanyam |
| miR-17-92 cluster or only miR-19a and miR-19b | Human fibroblasts (IMR90) | OSKM or OSK | [152] | He |
| miR-524-5p | Human foreskin fibroblasts (HFF-1) | OSKM | [153] | Nguyen |
| miR-371 cluster | Human fibroblasts (IMR90) | OSK | [154] | Cao |
| miR-31 | Human CD34+ cord blood cells | OSKM | [155] | Lee |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, A.; Milhavet, O.; Lemaitre, J.-M. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics 2022, 14, 317. https://doi.org/10.3390/pharmaceutics14020317
Bailly A, Milhavet O, Lemaitre J-M. RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics. 2022; 14(2):317. https://doi.org/10.3390/pharmaceutics14020317
Chicago/Turabian StyleBailly, Anaëlle, Ollivier Milhavet, and Jean-Marc Lemaitre. 2022. "RNA-Based Strategies for Cell Reprogramming toward Pluripotency" Pharmaceutics 14, no. 2: 317. https://doi.org/10.3390/pharmaceutics14020317
APA StyleBailly, A., Milhavet, O., & Lemaitre, J.-M. (2022). RNA-Based Strategies for Cell Reprogramming toward Pluripotency. Pharmaceutics, 14(2), 317. https://doi.org/10.3390/pharmaceutics14020317







