Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems
Abstract
:1. Introduction
1.1. Principles of Photodynamic Therapy
1.2. Mechanism of Photodynamic Therapy
1.3. Photosensitizers
2. Combination of Photodynamic Therapy and Chemotherapy
2.1. Combination of Photosensitizers and Chemo-Drugs without External Carriers
2.1.1. Photosensitizers as Carriers
MXenes
2.1.2. Photosensitizer-Drug Materials
2.2. Combination of Photosensitizers and Chemo-Drugs with External Carriers
2.2.1. Transition Metal Based Nano-Platforms
Synthesis Routes of Transition Metals Nano-Platforms
Application of Transition Metals in PDT
2.2.2. Silica
Synthesis Routes of Silica
Application of Silica in PDT
2.2.3. Graphene
Application of Graphene in PDT
2.2.4. Liposomes
Synthesis Routes of Liposomes
Application of Liposome in PDT
2.2.5. Dendrimers
2.2.6. Preparation Methods of Dendrimers
Application of Dendrimers in PDT
2.2.7. Polymers
Main Synthesizing Methods
Application of Polymers in PDT
2.2.8. Metal–Organic Frameworks
Preparation Method of Metal–Organic Frameworks
Application of Metal–Organic Frameworks in PDT
2.2.9. Biological Nanocarriers
Preparation of Red Blood Cells Membranes-Derived Vesicles
Application of Biological Nanocarriers in PDT
2.2.10. Nano Emulsions
Synthesis Routes of Nano Emulsions
Application of Nano Emulsion in PDT
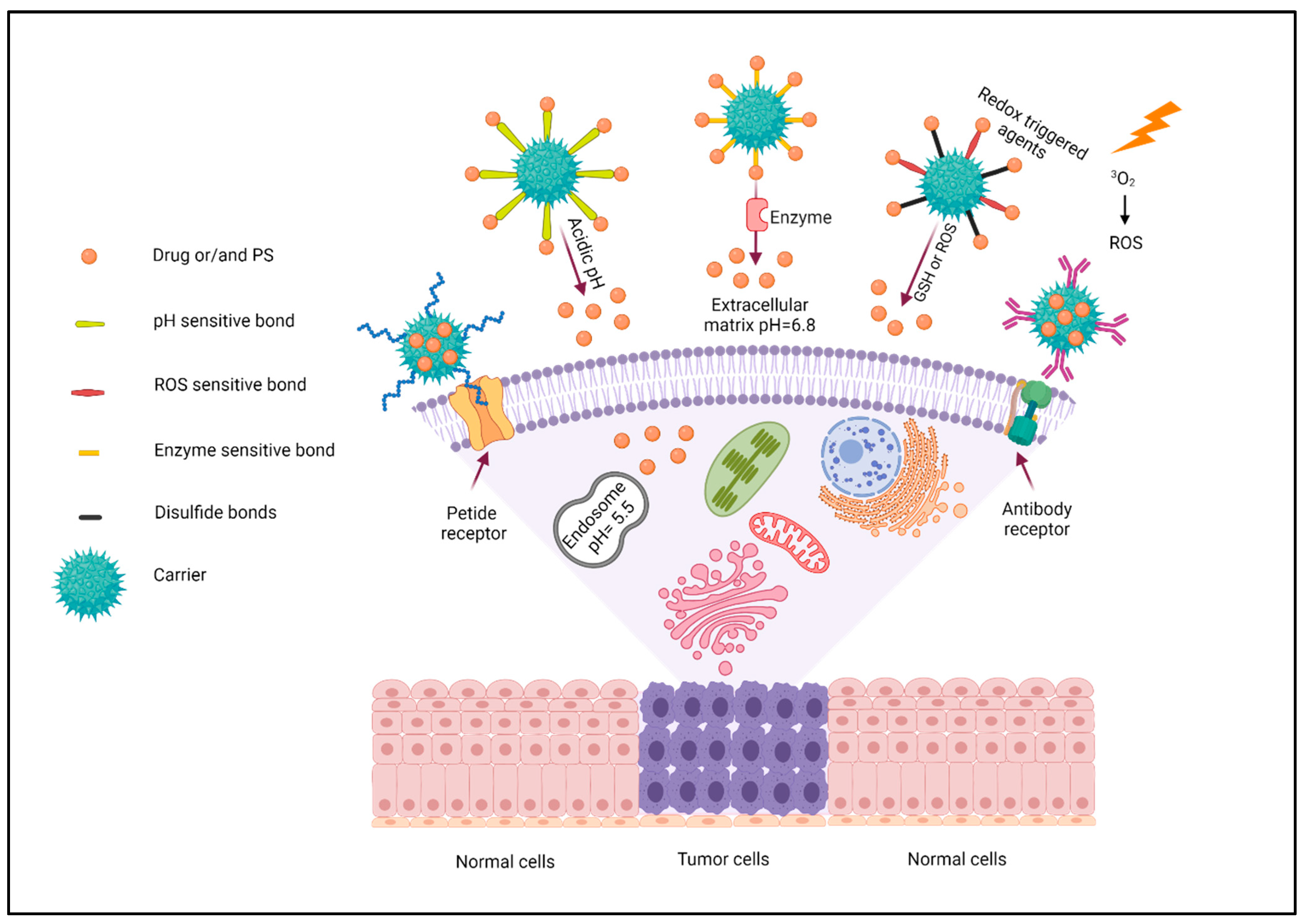
2.3. Targeting Strategy
2.3.1. pH Triggered
2.3.2. Enzyme Triggered
2.3.3. Redox Triggered Agents
2.3.4. Chemical and Biological Targeting Agents
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Names |
| PDT | Photodynamic therapy |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| 1O2 | Singlet oxygen |
| SPNpd | Semiconducting polymer nano-prodrug |
| NIR | Near-infrared |
| 2D | Two dimensional |
| SP | Soybean phospholipids |
| PAI | Photoacoustic imaging |
| IONP | Iron oxide nanoparticles |
| DMSO | Dimethyl sulfoxide |
| TBAOH | Tetrabutylammonium hydroxide |
| TMAOH | Tetramethylammonium hydroxide |
| LSPR | Localized surface plasmon resonance |
| CP | Compound polysaccharide |
| Met | Metformin |
| DOX | Doxorubicin |
| PEG | Polyethylene glycol |
| PNBMA | Poly (4,5-dimethoxy-2-nitrobenzyl methacrylate) |
| HSA | Human serum albumin |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| CVD | Chemical vapor deposition |
| NMP | N-methyl-pyrrolidone |
| SDBS | Sodium dodecylbenzene sulfonate |
| GSH | Glutathione |
| CPT | Camptothecin |
| MB | Methylene blue |
| FA | Folic acid |
| PAMAM | Polyamidoamine |
| LED | Light emitting diode |
| SPN | Semiconducting polymer nanoparticles |
| ZnPc | Zinc(II) phthalocyanine |
| Hp | hematoporphyrin |
| PASP | Polyaspartic acid |
| TPZ | Tirapazamine |
| DTX | Docetaxel |
| LBL | Layer-by-layer |
| Pt | Cis-platinum |
| PTX | Paclitaxel |
| Gem | Gemcitabine |
| Rf | Riboflavin |
| MOF | Metal–organic framework |
| HPPH | Photochlor |
| MDR | Multidrug resistance |
| P-gp | P-glycoprotein |
| mAbs | Monoclonal antibodies |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Leo, A.; Curigliano, G.; Diéras, V.; Malorni, L.; Sotiriou, C.; Swanton, C.; Thompson, A.; Tutt, A.; Piccart, M. New approaches for improving outcomes in breast cancer in Europe. Breas 2015, 24, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242. [Google Scholar]
- Liu, G.; Zhang, S.; Ma, Y.; Wang, Q.; Chen, X.; Zhang, L.; Ma, F. Effects of error on dose of target region and organs at risk in treating nasopharynx cancer with intensity modulated radiation therapy. Pak. J. Med. Sci. 2016, 32, 95. [Google Scholar] [CrossRef] [Green Version]
- Candido, N.M.; De Melo, M.T.; Franchi, L.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Combining photodynamic therapy and chemotherapy: Improving breast cancer treatment with nanotechnology. J. Biomed. Nanotechnol. 2018, 14, 994–1008. [Google Scholar] [CrossRef]
- Skyrme, R.J.; French, A.J.; Datta, S.N.; Allman, R.; Mason, M.D.; Matthews, P.N. A phase-1 study of sequential mitomycin C and 5—aminolaevulinic acid-mediated photodynamic therapy in recurrent superficial bladder carcinoma. BJU Int. 2005, 95, 1206–1210. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA A Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Jin, F.; Qi, J.; Liu, D.; You, Y.; Shu, G.; Du, Y.; Wang, J.; Xu, X.; Ying, X.; Ji, J.; et al. Cancer-cell-biomimetic upconversion nanoparticles combining chemo-photodynamic therapy and cd73 blockade for metastatic triple-negative breast cancer. J. Control. Release 2021, 337, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, E.; Varriale, L.; Iovino, M.; Chiaviello, A.; Veneziani, B.M.; Palumbo, G. Photodynamic therapy with indocyanine green complements and enhances low-dose cisplatin cytotoxicity in MCF-7 breast cancer cells. Mol. Cancer Ther. 2004, 3, 537–544. [Google Scholar] [PubMed]
- Zimmermann, A.; Walt, H.; Haller, U.; Baas, P.; Klein, S.D. Effects of chlorin-mediated photodynamic therapy combined with fluoropyrimidines in vitro and in a patient. Cancer Chemother. Pharmacol. 2003, 51, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.S.; Miao, P.T.; Liu, T.T.; Jia, Y.S.; Liu, X.D. Enhanced antitumor effects of Bpd-Ma-Mediated photodynamic therapy combined with adriamycin on breast cancer in mice. Acta Pharmacol. Sin. 2012, 33, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Von Tappeiner, H. Therapeutische versuche mit fluoreszierenden stoffen. Munch. Med. Wochenschr. 1903, 1, 2042–2044. [Google Scholar]
- Von Tappeiner, H. Ueber wirking der photodynamichen (fluorescierenden) stoffe auf protozoan und enzyme. Dtsch. Arch. Klin. Med. 1904, 80, 427–487. [Google Scholar]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Zhang, J.; Jiang, C.; Longo, J.P.F.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Ding, H.; Yu, H.; Dong, Y.; Tian, R.; Huang, G.; Boothman, D.A.; Sumer, B.D.; Gao, J. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Control. Release 2011, 156, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Junior, J.C.; Sabino, C.P.; Tan, X.; Junqueira, J.C.; Wang, Y.; Fuchs, B.B.; Jorge, A.O.; Tegos, G.P.; Hamblin, M.R.; Mylonakis, E. Selective photoinactivation of candida albicans in the non-vertebrate host infection model galleria mellonella. BMC Microbiol. 2013, 13, 1–9. [Google Scholar]
- Shah, J.; Park, S.; Aglyamov, S.R.; Larson, T.; Ma, L.; Sokolov, K.V.; Johnston, K.P.; Milner, T.E.; Emelianov, S.Y. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008, 13, 034024. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Bilgin, M.D.; Grossweiner, L.I. Singlet oxygen generation by photodynamic agents. J. Photochem. Photobiol. B Biol. 1997, 37, 131–140. [Google Scholar] [CrossRef]
- Yang, M.; Deng, J.; Guo, D.; Zhang, J.; Yang, L.; Wu, F. A folate-conjugated platinum porphyrin complex as a new cancer-targeting photosensitizer for photodynamic therapy. Org. Biomol. Chem. 2019, 17, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, H.; Zhang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Cit/CuS@ Fe3O4-based and enzyme-responsive magnetic nanoparticles for tumor chemotherapy, photothermal, and photodynamic therapy. J. Biomater. Appl. 2017, 31, 1010–1025. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, W.; Jiang, G.; Hou, Y.; Li, C.; Zhang, B.; Zhou, Q.; Wang, X. Fusion of photodynamic therapy and photoactivated chemotherapy: A novel Ru (II) arene complex with dual activities of photobinding and photocleavage toward DNA. Dalton Trans. 2014, 43, 15375–15384. [Google Scholar] [CrossRef]
- Lim, W.Q.; Yang, G.; Phua, S.Z.F.; Chen, H.; Zhao, Y. Self-assembled oxaliplatin (IV) prodrug–porphyrin conjugate for combinational photodynamic therapy and chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 16391–16401. [Google Scholar] [CrossRef]
- Zhang, F.L.; Song, M.R.; Yuan, G.K.; Ye, H.N.; Tian, Y.; Huang, M.D.; Xue, J.P.; Zhang, Z.H.; Liu, J.Y. A molecular combination of Zinc (II) phthalocyanine and tamoxifen derivative for dual targeting photodynamic therapy and hormone therapy. J. Med. Chem. 2017, 60, 6693–6703. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, H.; Tham, H.P.; Phua, S.Z.F.; Liu, J.G.; Zhao, Y. Cyclometalated iridium (III)-complex-based micelles for glutathione-responsive targeted chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 27553–27562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, T.; Liu, H.; Ren, F.; Qiu, W.; Sun, Q.; Yan, F.; Zheng, H.; Li, Z.; Gao, M. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2−XSe nanoparticles. Nanoscale 2019, 11, 7600–7608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Pan, Y.; Tian, Y.; Wang, X.; Ren, W.; Wang, S.; Lu, G.; Wu, A. Doxorubicin-loaded NaYF4: Yb/Tm–TiO2 inorganic photosensitizers for nir-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers. Biomaterials 2015, 57, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Bhardwaj, V.; Nagasetti, A.; Fernandez-Fernandez, A.; McGoron, A.J. Multifunctional surface-enhanced raman spectroscopy-detectable silver nanoparticles for combined photodynamic therapy and PH-triggered chemotherapy. J. Biomed. Nanotechnol. 2016, 12, 2202–2219. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Jiang, H.; Wang, C.; Wang, H.; Wang, X. A strategy for zno nanorod mediated multi-mode cancer treatment. Biomaterials 2011, 32, 1906–1914. [Google Scholar] [CrossRef]
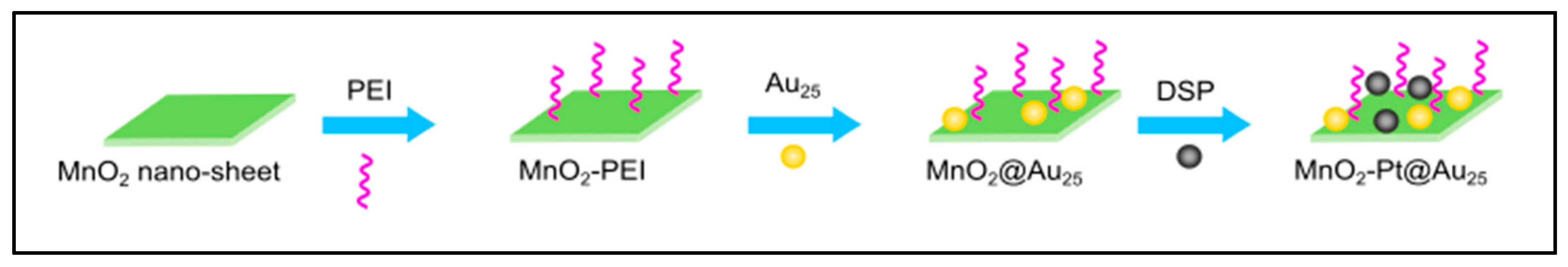
- Bi, H.; Dai, Y.; Yang, P.; Xu, J.; Yang, D.; Gai, S.; He, F.; An, G.; Zhong, C.; Lin, J. Glutathione and H2O2 consumption promoted photodynamic and chemotherapy based on biodegradable MnO2–Pt@ Au25 nanosheets. Chem. Eng. J. 2019, 356, 543–553. [Google Scholar] [CrossRef]
- Huang, L.; Wei, G.; Sun, X.; Jiang, Y.; Huang, Z.; Huang, Y.; Shen, Y.; Xu, X.; Liao, Y.; Zhao, C. A Tumor-targeted ganetespib-Zinc phthalocyanine conjugate for synergistic chemo-photodynamic therapy. Eur. J. Med. Chem. 2018, 151, 294–303. [Google Scholar] [CrossRef]
- Yuan, Y.; Min, Y.; Hu, Q.; Xing, B.; Liu, B. Nir photoregulated chemo-and photodynamic cancer therapy based on conjugated polyelectrolyte–drug conjugate encapsulated upconversion nanoparticles. Nanoscale 2014, 6, 11259–11272. [Google Scholar] [CrossRef]
- Naderi, E.; Aghajanzadeh, M.; Zamani, M.; Hashiri, A.; Sharafi, A.; Kamalianfar, A.; Naseri, M.; Danafar, H. Improving the anti-cancer activity of quercetin-loaded AgFeO2 through UV irradiation: Synthesis, characterization, and in vivo and in vitro biocompatibility study. J. Drug Deliv. Sci. Technol. 2020, 57, 101645. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Naderi, E.; Zamani, M.; Sharafi, A.; Naseri, M.; Danafar, H. In vivo and in vitro biocompatibility study of MnFe2O4 and Cr2Fe6O12 as Photosensitizer for photodynamic therapy and drug delivery of anti-cancer drugs. Drug Dev. Ind. Pharm. 2020, 46, 846–851. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface modified Ti3C2 mxene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yi, W.; Sun, T.; Tian, Y.; Zhang, P.; Si, J.; Hou, X.; Hou, J. Surface modification engineering of two-dimensional titanium carbide for efficient synergistic multitherapy of breast cancer. J. Mater. Chem. B 2020, 8, 6402–6417. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, J.; Ding, P.; Li, C.; Zhang, B.; Chen, W.; Zhao, Y.D.; Cao, Y.; Liu, B. Antitumor immunity triggered by photothermal therapy and photodynamic therapy of a 2D MoS2 nanosheet-incorporated injectable polypeptide-engineered hydrogel combinated with chemotherapy for 4T1 breast tumor therapy. Nanotechnology 2020, 31, 205102. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Peng, H.; Qing, X.; Fan, Z.; Xu, W.; Du, X.; Shi, R.; Zhang, Y. Ph-responsive pluronic F127–lenvatinib-encapsulated halogenated boron-dipyrromethene nanoparticles for combined photodynamic therapy and chemotherapy of liver cancer. ACS Omega 2021, 6, 12331–12342. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xiao, C.; Huang, B.; Wang, C.; Zhang, W. Janus macromolecular brushes for synergistic cascade-amplified photodynamic therapy and enhanced chemotherapy. Acta Biomater. 2020, 101, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Ye, J.H.; Qi, F.; Fang, H.; Lin, F.; Wang, S.; Mu, C.; Zhang, W.; He, W. A pegylated photosensitizer-core pH-responsive polymeric nanocarrier for imaging-guided combination chemotherapy and photodynamic therapy. New J. Chem. 2021, 45, 6180–6185. [Google Scholar] [CrossRef]
- Liu, W.; Song, N.; Li, Y.; Liu, Y.; Chen, L.; Liu, S.; Xie, Z. Cyclometallic iridium-based nanorods for chemotherapy/photodynamic therapy. Mater. Lett. 2020, 266, 127346. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Tan, H.; Xue, C.; He, Y.; Wei, X.; Zha, Y.; Niu, J.; Liu, Y.; Cheng, Y.; et al. Manganese/iron-based nanoprobes for photodynamic/chemotherapy combination therapy of tumor guided by multimodal imaging. Nanoscale 2021, 13, 5383–5399. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Huang, J.; Zhen, X.; Li, J.; Jiang, Y.; Pu, K. A semiconducting polymer nano-prodrug for hypoxia-activated photodynamic cancer therapy. Angew. Chem. 2019, 131, 5981–5985. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Wang, L.; Ruan, X.; Wang, F.; Xu, D.; Zhang, J.; Jia, X.; Liu, D. Evaluation on short-term therapeutic effect of 2 porphyrin photosensitizer-mediated photodynamic therapy for esophageal cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819831989. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hope, M.A.; Forse, A.C.; Griffith, K.J.; Lukatskaya, M.R.; Ghidiu, M.; Gogotsi, Y.; Grey, C.P. Nmr reveals the surface functionalisation of Ti3C2 mxene. Phys. Chem. Chem. Phys. 2016, 18, 5099–5102. [Google Scholar] [CrossRef] [Green Version]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2TX Mxene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Mxene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, X.; Ong, W.J.; Zhao, X.; Li, N. Surface and heterointerface engineering of 2D mxenes and their nanocomposites: Insights into electro-and photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on mxene-Ti3C2. Sens. Actuators B Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D titanium carbide (mxenes) composite nanosheets for pH-responsive mri-guided tumor hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M.; Lin, H.; Dai, C.; Ren, C.; Zhang, S.; Peng, W.; Chen, Y. 2D magnetic titanium carbide mxene for cancer theranostics. J. Mater. Chem. B 2018, 6, 3541–3548. [Google Scholar] [CrossRef]
- Tang, W.; Dong, Z.; Zhang, R.; Yi, X.; Yang, K.; Jin, M.; Yuan, C.; Xiao, Z.; Liu, Z.; Cheng, L. Multifunctional two-dimensional core–shell mxene@ gold nanocomposites for enhanced photo–radio combined therapy in the second biological window. ACS Nano 2018, 13, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, T.; Yoshizawa, K. A theoretical study on the mechanism of camphor hydroxylation by compound I of cytochrome P450. J. Am. Chem. Soc. 2003, 125, 4652–4661. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (mxenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 mxenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The synthesis process and thermal stability of V2C mxene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Yu, C.; Jianlin, S. Insights into 2D mxenes for versatile biomedical applications: Current advances and challenges ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar]
- George, S.M.; Kandasubramanian, B. Advancements in mxene-polymer composites for various biomedical applications. Ceram. Int. 2020, 46, 8522–8535. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. The max phases: Unique new carbide and nitride materials: Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight. Am. Sci. 2001, 89, 334–343. [Google Scholar] [CrossRef]
- Xia, Y.; Mathis, T.S.; Zhao, M.-Q.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline mxenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent advances in layered Ti3C2TX mxene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “mxenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-dimensional transition metal carbides and nitrides (mxenes): Synthesis, properties, and electrochemical energy storage applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-dimensional ultrathin mxene ceramic nanosheets for photothermal conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- He, Z.; Dai, Y.; Li, X.; Guo, D.; Liu, Y.; Huang, X.; Jiang, J.; Wang, S.; Zhu, G.; Zhang, F.; et al. Hybrid nanomedicine fabricated from photosensitizer-terminated metal–organic framework nanoparticles for photodynamic therapy and hypoxia-activated cascade chemotherapy. Small 2019, 15, 1804131. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Wei, X.; Chen, K. Magnetic-luminescent Ybpo4: Er, dy microspheres designed for tumor theranostics with synergistic effect of photodynamic therapy and chemotherapy. Int. J. Nanomed. 2014, 9, 4879. [Google Scholar] [CrossRef] [Green Version]
- Imanparast, A.; Bakhshizadeh, M.; Salek, R.; Sazgarnia, A. Pegylated hollow gold-mitoxantrone nanoparticles combining photodynamic therapy and chemotherapy of cancer cells. Photodiagnosis Photodyn. Ther. 2018, 23, 295–305. [Google Scholar] [CrossRef]
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Peng, W.; Xiao, Q.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous Mr/Ucl imaging. Biomaterials 2014, 35, 8992–9002. [Google Scholar] [CrossRef]
- Peng, C.L.; Lai, P.S.; Lin, F.H.; Wu, S.Y.H.; Shieh, M.J. Dual Chemotherapy and photodynamic therapy in an Ht-29 human colon cancer xenograft model using Sn-38-loaded chlorin-core star block copolymer micelles. Biomaterials 2009, 30, 3614–3625. [Google Scholar] [CrossRef]
- Ai, F.; Sun, T.; Xu, Z.; Wang, Z.; Kong, W.; To, M.W.; Wang, F.; Zhu, G. An upconversion nanoplatform for simultaneous photodynamic therapy and Pt chemotherapy to combat cisplatin resistance. Dalton Trans. 2016, 45, 13052–13060. [Google Scholar] [CrossRef]
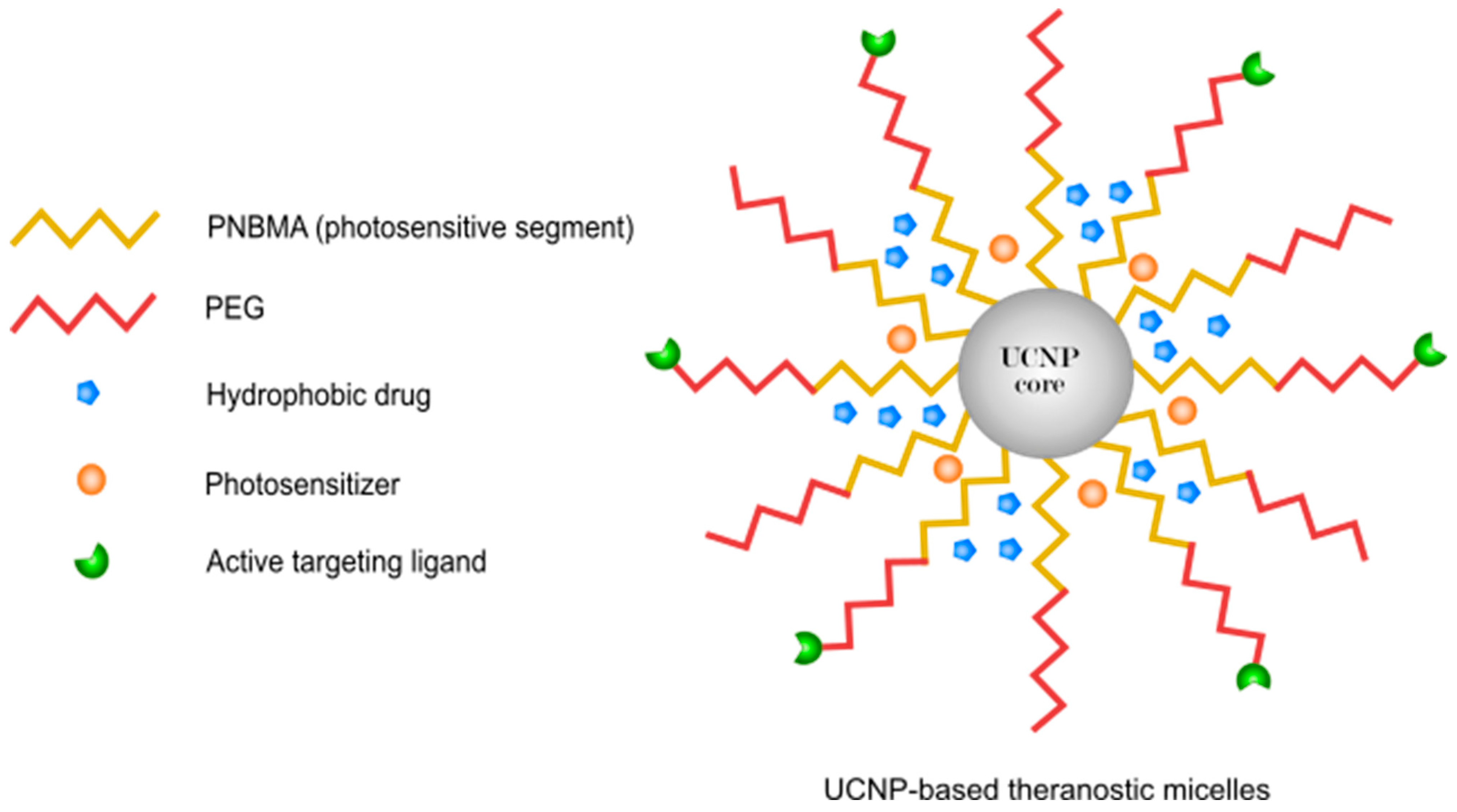
- Chen, G.; Jaskula-Sztul, R.; Esquibel, C.R.; Lou, I.; Zheng, Q.; Dammalapati, A.; Harrison, A.; Eliceiri, K.W.; Tang, W.; Chen, H.; et al. Neuroendocrine tumor-targeted upconversion nanoparticle-based micelles for simultaneous nir-controlled combination chemotherapy and photodynamic therapy, and fluorescence imaging. Adv. Funct. Mater. 2017, 27, 1604671. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, G.; Zhang, S.; Liu, X. A reactive 1O2-responsive combined treatment system of photodynamic and chemotherapy for cancer. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, C.; Alfranca, G.; Yang, Y.; Jiang, X.; Yang, Y.; Pan, F.; de la Fuente, J.M.; Cui, D. Near-infrared light triggered ros-activated theranostic platform based on Ce6-CPt-UCPNS for simultaneous fluorescence imaging and chemo-photodynamic combined therapy. Theranostics 2016, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wu, B.; Hu, X.; Xing, D. Nir-triggered high-efficient photodynamic and chemo-cascade therapy using caspase-3 responsive functionalized upconversion nanoparticles. Biomaterials 2017, 141, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, R.; Yan, L.; Chen, X.; Zhu, G. Combined chemotherapy and photodynamic therapy using a nanohybrid based on layered double hydroxides to conquer cisplatin resistance. Chem. Commun. 2015, 51, 11587–11590. [Google Scholar] [CrossRef]
- Wong, R.C.; Ng, D.K.; Fong, W.P.; Lo, P.C. Encapsulating Ph-responsive doxorubicin–phthalocyanine conjugates in mesoporous silica nanoparticles for combined photodynamic therapy and controlled chemotherapy. Chem.–Eur. J. 2017, 23, 16505–16515. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.M.; Kim, J.; Kim, W.J. Doxorubicin/Ce6-loaded nanoparticle coated with polymer via singlet oxygen-sensitive linker for photodynamically assisted chemotherapy. Nanotheranostics 2017, 1, 196. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Gong, H.; Qian, X.; Tan, P.; Li, Z.; Liu, T.; Liu, J.; Li, Y.; Liu, Z. Mesoporous silica nanorods intrinsically doped with photosensitizers as a multifunctional drug carrier for combination therapy of cancer. Nano Res. 2015, 8, 751–764. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Su, Z.; Wang, C.; Liao, Y.; Fu, Q. Multifunctional hollow mesoporous silica nanocages for cancer cell detection and the combined chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2011, 3, 2479–2486. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Elnagheeb, M. Mesoporous silica nanoparticles loaded with cisplatin and phthalocyanine for combination chemotherapy and photodynamic therapy in vitro. Nanomaterials 2015, 5, 2302–2316. [Google Scholar] [CrossRef]
- Yao, X.; Chen, X.; He, C.; Chen, L.; Chen, X. Dual Ph-responsive mesoporous silica nanoparticles for efficient combination of chemotherapy and photodynamic therapy. J. Mater. Chem. B 2015, 3, 4707–4714. [Google Scholar] [CrossRef] [PubMed]
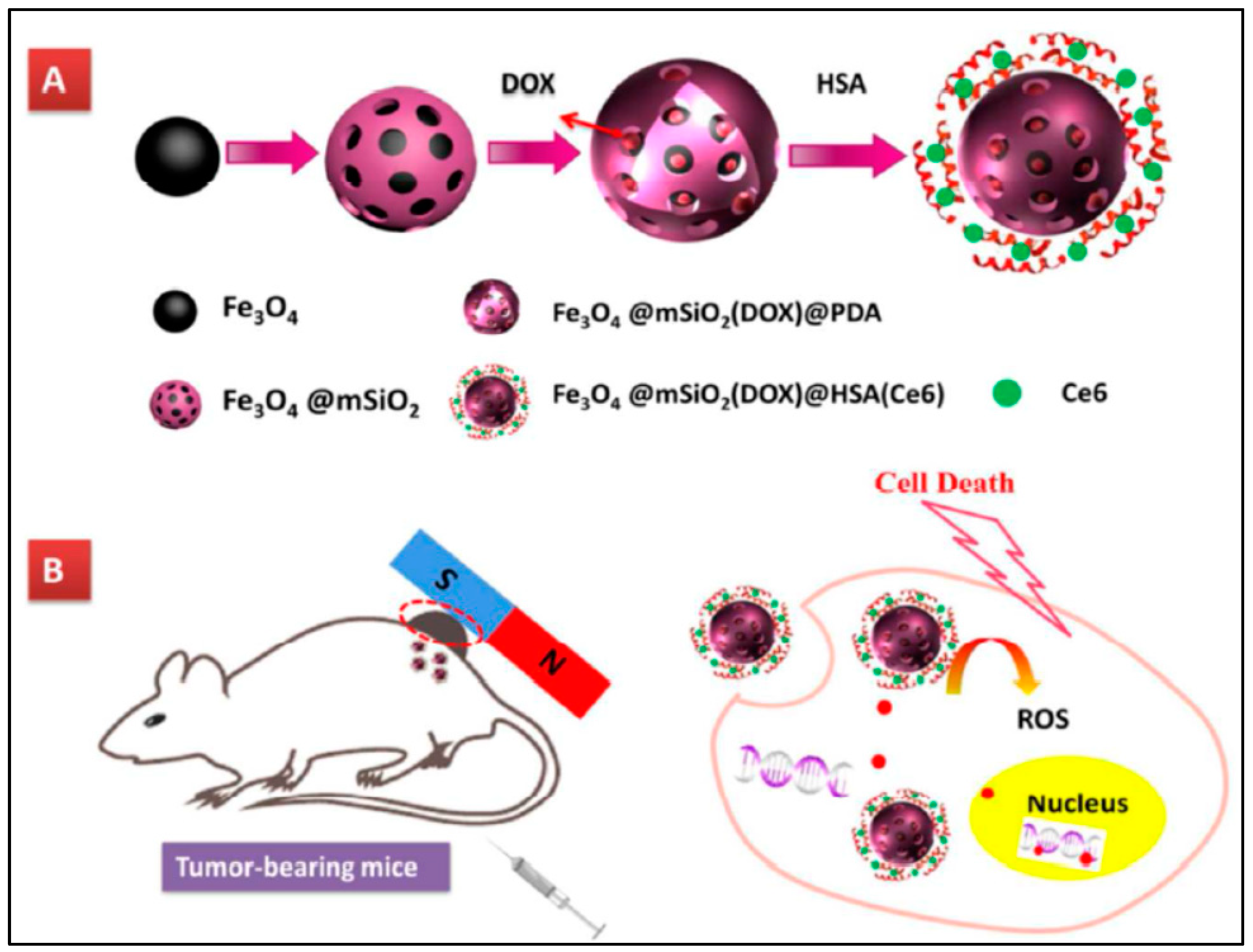
- Tang, X.L.; Jing, F.; Lin, B.L.; Cui, S.; Yu, R.T.; Shen, X.D.; Wang, T.W. Ph-responsive magnetic mesoporous silica-based nanoplatform for synergistic photodynamic therapy/chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, M.; Song, H.; Wang, Y.; Yu, C. Preparation of fluorescent mesoporous hollow silica–fullerene nanoparticles via selective etching for combined chemotherapy and photodynamic therapy. Nanoscale 2015, 7, 11894–11898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, J.; Su, H.; Mu, G.; Sun, J.H.; Tan, C.P.; Liang, X.J.; Ji, L.N.; Mao, Z.W. Co-delivery of cisplatin prodrug and chlorin E6 by mesoporous silica nanoparticles for chemo-photodynamic combination therapy to combat drug resistance. ACS Appl. Mater. Interfaces 2016, 8, 13332–13340. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.W.; Greco, W.R.; Bellnier, D.A.; Vaughan, L.; Henderson, B.W. Photodynamic therapy: A means to enhanced drug delivery to tumors. Cancer Res. 2003, 63, 8126–8131. [Google Scholar] [PubMed]
- Lee, H.; Han, J.; Shin, H.; Han, H.; Na, K.; Kim, H. Combination of chemotherapy and photodynamic therapy for cancer treatment with sonoporation effects. J. Control. Release 2018, 283, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y.; et al. A photosensitive liposome with Nir light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Li, H.; Li, C.; Ding, H.; Zhang, M.; Guo, Y.; Sun, M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf. B Biointerfaces 2019, 173, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Pan, X.; Xu, L.; Xue, Y.; Zhang, W. Doxorubicin-loaded redox-responsive amphiphilic dendritic porphyrin conjugates for chemotherapy and photodynamic therapy. RSC Adv. 2016, 6, 57552–57562. [Google Scholar] [CrossRef]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-activatable red blood cell membrane-camouflaged dimeric prodrug nanoparticles for synergistic photodynamic/chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef]
- Luo, Z.; Tian, H.; Liu, L.; Chen, Z.; Liang, R.; Chen, Z.; Wu, Z.; Ma, A.; Zheng, M.; Cai, L. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Chen, B.; Li, L.; Wang, D.; Shi, S.; Zhang, T.; Wang, Y.; Zhang, L.; Wang, Y. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials 2018, 155, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Naderi, E.; Aghajanzadeh, M.; Zamani, M.; Sharafi, A.; Naseri, M.; Danafar, H. The effect of calcination temperature on the anticancer activity of CaFe2O4@ PVA nanocarriers: Photodynamic therapy and drug delivery study. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5261–5269. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, S.; Ge, X.; Zhou, J.; Jiang, H.; Li, F.; Shen, J. Combination of chemotherapy and photodynamic therapy using graphene oxide as drug delivery system. J. Photochem. Photobiol. B Biol. 2014, 135, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xie, X.; Liu, M.; Hu, S.; Ding, J.; Zhou, W. A smart Mno2-doped graphene oxide nanosheet for enhanced chemo-photodynamic combinatorial therapy via simultaneous oxygenation and glutathione depletion. Acta Pharm. Sin. B 2021, 11, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Rajendran, N.K.; Rajan, M.; Ramu, A.; Marraiki, N.; Elgorban, A.M. A Magnetic nanoparticle functionalized reduced graphene oxide-based drug carrier system for a chemo-photodynamic cancer therapy. New J. Chem. 2020, 44, 5265–5277. [Google Scholar] [CrossRef]
- Liang, J.; Chen, B.; Hu, J.; Huang, Q.; Zhang, D.; Wan, J.; Hu, Z.; Wang, B. Ph and thermal dual-responsive graphene oxide nanocomplexes for targeted drug delivery and photothermal-chemo/photodynamic synergetic therapy. ACS Appl. Bio Mater. 2019, 2, 5859–5871. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Lo, P.C. Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated Zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy. J. Control. Release 2018, 282, 46–61. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Y.; Zhu, X.; Cui, S.; Cao, Y.; Li, R.; Wang, L. The study of killing effect and inducing apoptosis of 630-nm laser on lung adenocarcinoma a549 cells mediated by hematoporphyrin derivatives in vitro. Lasers Med. Sci. 2020, 35, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Peng, J.; Tan, L.; Wu, J.; Shi, K.; Qu, Y.; Wei, X.; Qian, Z. Mild Photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by lyp-1 modified docetaxel/Ir820 co-loaded micelles. Biomaterials 2016, 106, 119–133. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Z.; Liu, L.; Jiang, W.; Li, S.; Wang, Y.; Yan, L. Nir imaging-guided combined photodynamic therapy and chemotherapy by a Ph-responsive amphiphilic polypeptide prodrug. Biomater. Sci. 2017, 5, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Dai, J.; Han, Y.; Xu, M.; Zhang, X.; Zhen, S.; Zhao, Z.; Lou, X.; Xia, F. A high therapeutic efficacy of polymeric prodrug nano-assembly for a combination of photodynamic therapy and chemotherapy. Commun. Biol. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, L.; Zhu, M.; Wan, G.; Li, C.; Zhang, J.; Wang, Y.; Wang, Y. Reactive oxygen species-responsive nanoparticles based on peglated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 29260–29272. [Google Scholar] [CrossRef]
- Zhen, S.; Yi, X.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Drug delivery micelles with efficient near-infrared photosensitizer for combined image-guided photodynamic therapy and chemotherapy of drug-resistant cancer. Biomaterials 2019, 218, 119330. [Google Scholar] [CrossRef]
- Zhu, R.; He, H.; Liu, Y.; Cao, D.; Yan, J.; Duan, S.; Chen, Y.; Yin, L. Cancer-selective bioreductive chemotherapy mediated by dual hypoxia-responsive nanomedicine upon photodynamic therapy-induced hypoxia aggravation. Biomacromolecules 2019, 20, 2649–2656. [Google Scholar] [CrossRef]
- Conte, C.; Ungaro, F.; Maglio, G.; Tirino, P.; Siracusano, G.; Sciortino, M.T.; Leone, N.; Palma, G.; Barbieri, A.; Arra, C. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J. Control. Release 2013, 167, 40–52. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Qian, J.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Sequentially self-assembled polysaccharide-based nanocomplexes for combined chemotherapy and photodynamic therapy of breast cancer. Carbohydr. Polym. 2019, 203, 203–213. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Feng, L.; Chen, Q.; Chao, Y.; Dong, Z.; Liu, Z. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018, 11, 3244–3257. [Google Scholar] [CrossRef]
- Ren, Q.; Liang, Z.; Jiang, X.; Gong, P.; Zhou, L.; Sun, Z.; Xiang, J.; Xu, Z.; Peng, X.; Li, S.; et al. Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy. Int. J. Biol. Macromol. 2019, 130, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, Y.; Wang, L.; Gao, J.; Zhang, J.; Yu, X.; Ma, R.; Liu, R.; Zhang, Z. A tumoral acidic pH-responsive drug delivery system based on a novel photosensitizer (fullerene) for in vitro and in vivo chemo-photodynamic therapy. Acta Biomater. 2014, 10, 1280–1291. [Google Scholar] [CrossRef]
- Hu, D.; Chen, L.; Qu, Y.; Peng, J.; Chu, B.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.; Qian, Z. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin E6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics 2018, 8, 1558. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Cui, J.; Li, X.; Dou, Y.; Su, L.; Chang, J.; Wang, H.; Li, X.; Zhang, B. pH-and Nir light responsive nanocarriers for combination treatment of chemotherapy and photodynamic therapy. Biomater. Sci. 2016, 4, 338–345. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, W.; Di, Y.; Gu, J.; Guo, Z.; Li, H.; Fu, D.; Jin, C. Triple-functional albumin-based nanoparticles for combined chemotherapy and photodynamic therapy of pancreatic cancer with lymphatic metastases. Int. J. Nanomed. 2017, 12, 6771. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Q.; Sun, X.; Zhang, B.; Kang, H.; Zhang, F.; Jin, Y. Doxorubicin-loaded photosensitizer-core pH-responsive copolymer nanocarriers for combining photodynamic therapy and chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 1008–1016. [Google Scholar] [CrossRef]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. Ros-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
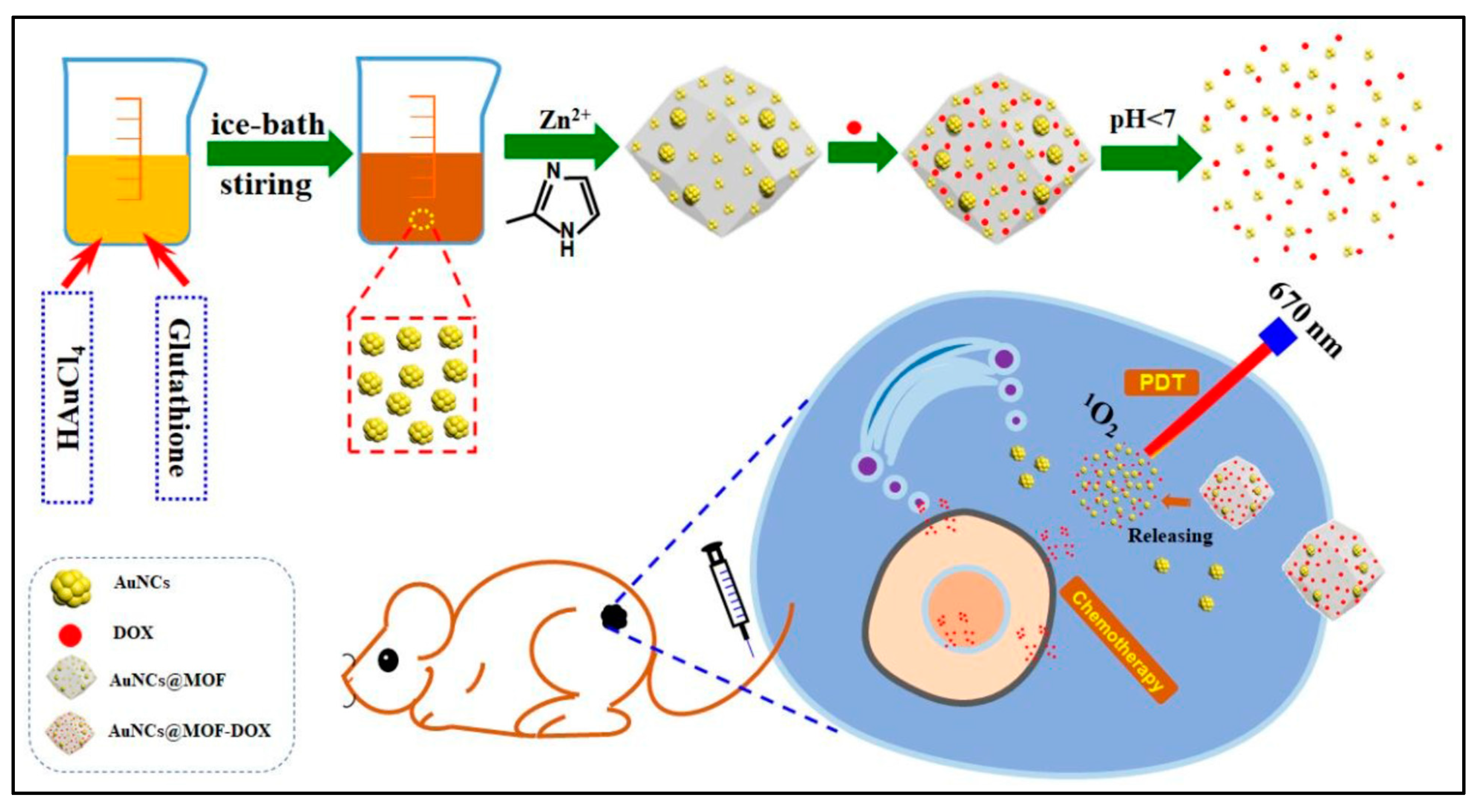
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. pH-responsive metal–organic framework encapsulated gold nanoclusters with modulated release to enhance photodynamic therapy/chemotherapy in breast cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef]
- Ren, S.Z.; Wang, B.; Zhu, X.H.; Zhu, D.; Liu, M.; Li, S.K.; Yang, Y.S.; Wang, Z.C.; Zhu, H.L. Oxygen self-sufficient core–shell metal–organic framework-based smart nanoplatform for enhanced synergistic chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 24662–24674. [Google Scholar] [CrossRef]
- Ihsanullah, K.M.; Kumar, B.N.; Zhao, Y.; Muhammad, H.; Liu, Y.; Wang, L.; Liu, H.; Jiang, W. Stepwise-activatable hypoxia triggered nanocarrier-based photodynamic therapy for effective synergistic bioreductive chemotherapy. Biomaterials 2020, 245, 119982. [Google Scholar] [CrossRef]
- Han, L.; Wang, Y.; Huang, X.; Liu, F.; Ma, C.; Feng, F.; Zhang, J.; Liu, W.; Qu, W.; Pang, H.; et al. Specific-oxygen-supply functionalized core-shell nanoparticles for smart mutual-promotion between photodynamic therapy and gambogic acid-induced chemotherapy. Biomaterials 2020, 257, 120228. [Google Scholar] [CrossRef]
- Zou, R.; Gao, Y.; Zhang, Y.; Jiao, J.; Wong, K.L.; Wang, J. 68Ga-labeled magnetic-nir persistent luminescent hybrid mesoporous nanoparticles for multimodal imaging-guided chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2021, 13, 9667–9680. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Pan, C.; Yuan, W. Light-enhanced hypoxia-responsive and azobenzene cleavage-triggered size-shrinkable micelles for synergistic photodynamic therapy and chemotherapy. Biomater. Sci. 2020, 8, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yin, Z.; Qi, Y.; Liu, S.; Yi, Y.; Tian, X.; Wu, Y.; Zhong, D.; Gu, Z.; Zhang, H.; et al. An intracellular enzyme-responsive polymeric prodrug with synergistic effect of chemotherapy and two-photon photodynamic therapy. Appl. Mater. Today 2021, 23, 100996. [Google Scholar] [CrossRef]
- Lee, D.; Jang, S.Y.; Kwon, S.; Lee, Y.; Park, E.; Koo, H. Optimized combination of photodynamic therapy and chemotherapy using gelatin nanoparticles containing tirapazamine and pheophorbide A. ACS Appl. Mater. Interfaces 2021, 13, 10812–10821. [Google Scholar] [CrossRef]
- Jiang, H.; Su, Y.; Li, N.; Jin, X. Laser-responsive polymeric nanomicelles to subdue tumor multidrug resistance based on mild photodynamic therapy and chemotherapy. ACS Appl. Nano Mater. 2020, 3, 6702–6710. [Google Scholar] [CrossRef]
- Sun, C.; Gao, S.; Tan, Y.; Zhang, Z.; Xu, H. Side-chain selenium-grafted polymers combining antiangiogenesis treatment with photodynamic therapy and chemotherapy. ACS Biomater. Sci. Eng. 2021, 7, 3201–3208. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Cui, Z.; Wang, P.; Chen, S.; Yang, G.; Zhang, W. Mitochondria-targeting and ros-sensitive smart nanoscale supramolecular organic framework for combinational amplified photodynamic therapy and chemotherapy. Acta Biomater. 2021, 130, 447–459. [Google Scholar] [CrossRef]
- Chen, L.; Zhuang, W.; Hu, C.; Yu, T.; Su, X.; Liang, Z.; Li, G.; Wang, Y. pH and singlet oxygen dual-responsive gem prodrug micelles for efficient combination therapy of chemotherapy and photodynamic therapy. J. Mater. Chem. B 2020, 8, 5645–5654. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, W.; Yu, T.; Chen, L.; Liang, Z.; Li, G.; Wang, Y. Multi-stimuli responsive polymeric prodrug micelles for combined chemotherapy and photodynamic therapy. J. Mater. Chem. B 2020, 8, 5267–5279. [Google Scholar] [CrossRef]
- Cong, C.; He, Y.; Zhao, S.; Zhang, X.; Li, L.; Wang, D.; Liu, L.; Gao, D. Diagnostic and therapeutic nanoenzymes for enhanced chemotherapy and photodynamic therapy. J. Mater. Chem. B 2021, 9, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-responsive hyaluronic acid-based nanoparticles for targeted photodynamic therapy/chemotherapy against breast cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Qu, Y.; He, X.; Hao, Y.; Yang, C.; Yang, Y.; Hu, D.; Wang, F.; Qian, Z. Ros-responsive camptothecin prodrug nanoparticles for on-demand drug release and combination of chemotherapy and photodynamic therapy. Adv. Funct. Mater. 2020, 30, 2005918. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Niu, D.; Qin, L.; Li, Y. Upconversion nanoparticle-based organosilica–micellar hybrid nanoplatforms for redox-responsive chemotherapy and nir-mediated photodynamic therapy. ACS Appl. Bio Mater. 2020, 3, 4655–4664. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Li, F.; Sheng, J.; Xu, C.; Li, D.; Yu, H.; Liu, W. Combination of chemotherapy and photodynamic therapy with oxygen self-supply in the form of mutual assistance for cancer therapy. Int. J. Nanomed. 2021, 16, 3679. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Li, F.; Sheng, J.; Xu, C.; Li, D.; Yu, H.; Liu, W. An ros-sensitive tegafur-ppix-heterodimer-loaded in situ injectable thermosensitive hydrogel for photodynamic therapy combined with chemotherapy to enhance the tegafur-based treatment of breast cancer. Biomater. Sci. 2021, 9, 221–237. [Google Scholar]
- Li, Y.; Sutrisno, L.; Hou, Y.; Fei, Y.; Xue, C.; Hu, Y.; Li, M.; Luo, Z. A redox-activatable biopolymer-based micelle for sequentially enhanced mitochondria-targeted photodynamic therapy and hypoxia-dependent chemotherapy. Chem. Commun. 2020, 56, 9978–9981. [Google Scholar] [CrossRef]
- Chen, C.T.; Peng, P.C.; Tsai, T.; Chien, H.F.; Lee, M.J. A novel treatment modality for malignant peripheral nerve sheath tumor using a dual-effect liposome to combine photodynamic therapy and chemotherapy. Pharmaceutics 2020, 12, 317. [Google Scholar] [CrossRef] [Green Version]
- Enling, C. Combination of Photodynamic Therapy and Chemotherapy for Cancer Treatment by Using Paclitaxel-Loaded Porphyrin-Shelled Nanoemulsions. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2021. [Google Scholar]
- Wang, Y.; Zu, M.; Ma, X.; Jia, D.; Lu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Xu, Z. Glutathione-responsive multifunctional “trojan horse” nanogel as a nanotheranostic for combined chemotherapy and photodynamic anticancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 50896–50908. [Google Scholar] [CrossRef]
- Zamani, M.; Naderi, E.; Aghajanzadeh, M.; Naseri, M.; Sharafi, A.; Danafar, H. Co1−XZnxFe2O4 based nanocarriers for dual-targeted anticancer drug delivery: Synthesis, characterization and in vivo and in vitro biocompatibility study. J. Mol. Liq. 2019, 274, 60–67. [Google Scholar] [CrossRef]
- Ayubia, M.; Karimib, M.; Abdpoura, S.; Rostamizadehbc, K.; Parsa, M.; Zamanic, M.; Saedia, A. Magnetic nanoparticles decorated with pegylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef]
- Siefe, C.; Mehlenbacher, R.D.; Peng, C.S.; Zhang, Y.; Fischer, S.; Lay, A.; McLellan, C.A.; Alivisatos, A.P.; Chu, S.; Dionne, J.A. Sub-20 nm core–shell–shell nanoparticles for bright upconversion and enhanced foörster resonant energy transfer. J. Am. Chem. Soc. 2019, 141, 16997–17005. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, R.; Kim, E.; Lee, S.; Park, Y.I. Near-infrared light-triggered photodynamic therapy and apoptosis using upconversion nanoparticles with dual photosensitizers. Front. Bioeng. Biotechnol. 2020, 8, 275. [Google Scholar] [CrossRef]
- Wu, J.; Du, S.; Wang, Y. Photosensitizer coated upconversion nanoparticles for triggering reactive oxygen species under 980 nm near-infrared excitation. J. Mater. Chem. B 2019, 7, 7306–7313. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Song, S.; Sun, Y.; Liu, J.; Chen, K.; Liu, J.; Wang, W. Anticancer effects and cell death pathways in ultralow-power 980 nm laser-triggered photodynamic therapy by Gd2O3: Yb, Tm nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 462–476. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Rana, S.; Kumar, R.; Das, J.; Sil, P.C. Microwave induced synthesis of zno nanorods and their efficacy as a drug carrier with profound anticancer and antibacterial properties. Toxicol. Rep. 2019, 6, 176–185. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Qiu, K.; Liao, X.; Rees, T.W.; Ji, L.; Chao, H. Fabrication of Red blood cell membrane-camouflaged Cu2−xSe nanoparticles for phototherapy in the second near-infrared window. Chem. Commun. 2019, 55, 6523–6526. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Lea-Banks, H.; Abrahao, A.; Davidson, B.; Suppiah, S.; Vecchio, L.M.; Samuel, N.; Mahmud, F.; Hynynen, K.; et al. Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J. Control. Release 2019, 309, 25–36. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Meng, L.B.; Huang, Q.; Li, J.; Zheng, K.; Zhang, F.L.; Liu, J.Y.; Xue, J.P. Synthesis and in vitro anticancer activity of Zinc (II) phthalocyanines conjugated with coumarin derivatives for dual photodynamic and chemotherapy. ChemMedChem 2015, 10, 304–311. [Google Scholar] [CrossRef]
- Calavia, P.G.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [Green Version]
- Mallakpour, S.; Hatami, M. Fabrication and characterization of pH-sensitive Bio-nanocomposite beads havening folic acid intercalated Ldh and chitosan: Drug release and mechanism evaluation. Int. J. Biol. Macromol. 2019, 122, 157–167. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Chen, H.; Zeng, D.; Li, Y.; Zheng, Y.; Li, F.; Ji, X.; Wang, X.; Chen, F.; et al. Engineering inorganic nanoemulsions/nanoliposomes by fluoride-silica chemistry for efficient delivery/co-delivery of hydrophobic agents. Adv. Funct. Mater. 2012, 22, 1586–1597. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Sun, Y.; Zheng, Y.; Zeng, D.; Li, F.; Zhang, S.; Wang, X.; Zhang, K.; Ma, M.; et al. Multifunctional mesoporous composite nanocapsules for highly efficient mri-guided high-intensity focused ultrasound cancer surgery. Angew. Chem. Int. Ed. 2011, 50, 12505–12509. [Google Scholar] [CrossRef]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef]
- Ren, G.; Su, H.; Wang, S. The combined method to synthesis silica nanoparticle by stöber process. J. Sol-Gel Sci. Technol. 2020, 96, 108–120. [Google Scholar] [CrossRef]
- Nayeem, J.; Al-Bari, A.A.; Mahiuddin, A.; Rahman, A.; Mefford, O.T.; Ahmad, H.; Rahman, M. Silica coating of iron oxide magnetic nanoparticles by reverse microemulsion method and their functionalization with cationic polymer P (Nipam-Co-Amptma) for antibacterial vancomycin immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125857. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Chen, X.; Tian, S.; Li, K. Synthesis and characterization of silica nanoparticles preparing by low-temperature vapor-phase hydrolysis of SiCl4. Ind. Eng. Chem. Res. 2014, 53, 11884–11890. [Google Scholar] [CrossRef]
- Nagasawa, H.; Yamamoto, Y.; Tsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Atmospheric-pressure plasma-enhanced chemical vapor deposition of microporous silica membranes for gas separation. J. Membr. Sci. 2017, 524, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Kumar, S.; Chaudhary, R.G. Tuning of structural, optical and toxicological properties of Gd3+ doped Yb2O3 nanoparticles. Ceram. Int. 2019, 45, 19307–19315. [Google Scholar] [CrossRef]
- Magdaong, N.C.M.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Photophysical properties and electronic structure of Zinc(II) porphyrins bearing 0–4 meso-phenyl substituents: Zinc porphine to Zinc tetraphenylporphyrin (Zntpp). J. Phys. Chem. A 2020, 124, 7776–7794. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kihm, K.D.; Kim, H.G.; Shin, S.; Lee, C.; Park, J.S.; Cheon, S.; Kwon, O.M.; Lim, G.; Lee, W. In-plane thermal conductivity of polycrystalline chemical vapor deposition graphene with controlled grain sizes. Nano Lett. 2017, 17, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Vijayaraghavan, A.; Erni, R.; Ariga, K.; Khalakhan, I.; Hill, J.P. High Purity graphenes prepared by a chemical intercalation method. Nanoscale 2010, 2, 2139–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, T.E.; Ellerby, M.; Saxena, S.S.; Smith, R.P.; Skipper, N.T. Superconductivity in the intercalated graphite compounds C6Yb and C6Ca. Nat. Phys. 2005, 1, 39–41. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, C.; Gao, L.; Yu, X.; Lai, J.; Lu, D.; Bao, R.; Wang, Y.; Jia, B.; Wang, F.; et al. Chemotherapy-induced macrophage infiltration into tumors enhances nanographene-based photodynamic therapy. Cancer Res. 2017, 77, 6021–6032. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Iijima, S. Formation of single-wall carbon nanotubes by laser ablation of fullerenes at low temperature. Appl. Phys. Lett. 1999, 75, 3087–3089. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Putri, D.C.A.; Dwiastuti, R.; Marchaban, M.; Nugroho, A.K. Optimization of mixing temperature and sonication duration in liposome preparation. J. Pharm. Sci. Community 2017, 14, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, V.; Usman, M.; Md, R.; Dwivedi, S.; Dubey, R. Preparation and evaluation of itraconazole liposome using ether injection solvent evaporation method. Int. J. Pharm. Life Sci. 2019, 10, 6091–6097. [Google Scholar]
- Marín-Peñalver, D.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Gelling properties of hake muscle with addition of freeze-thawed and freeze-dried soy phosphatidylcholine liposomes protected with trehalose. LWT 2018, 98, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of extrusion technique for nanosizing liposomes. pharmaceutics. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, J.; Shen, Y.; Fan, M.; Tang, H.; Radosz, M. Facile synthesis of polyester dendrimers from sequential click coupling of asymmetrical monomers. J. Am. Chem. Soc. 2009, 131, 14795–14803. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Wang, M.; Xiao, J.; Cheng, Y. Fluorinated poly(propylenimine) dendrimers as gene vectors. Biomaterials 2014, 35, 5407–5413. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Hu, K.; Shao, N.; Cheng, Y. Tumor extracellular acidity activated “off–on” release of bortezomib from a biocompatible dendrimer. Biomater. Sci. 2015, 3, 480–489. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, Q.; Zhang, X.; Nie, Y.; Zhang, Z.; Li, Y.; Cheng, G.; Gu, Z. Virus-inspired mimics: Self-assembly of dendritic lipopeptides into arginine-rich nanovectors for improving gene delivery. J. Mater. Chem. B 2015, 3, 7006–7010. [Google Scholar] [CrossRef]
- Khandare, J.; Mohr, A.; Calderón, M.; Welker, P.; Licha, K.; Haag, R. Structure-biocompatibility relationship of dendritic polyglycerol derivatives. Biomaterials 2010, 31, 4268–4277. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Luo, K.; Li, N.; Guo, C.; Zheng, X.; Gu, Z. Dendrimer–doxorubicin conjugate as enzyme-sensitive and polymeric nanoscale drug delivery vehicle for ovarian cancer therapy. Polym. Chem. 2014, 5, 5227–5235. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Xu, X.; Li, Y.; Li, Y.; Jian, Y.; Gu, Z. Bioinspired therapeutic dendrimers as efficient peptide drugs based on supramolecular interactions for tumor inhibition. Angew. Chem. 2015, 127, 4363–4368. [Google Scholar] [CrossRef]
- Han, M.; Lv, Q.; Tang, X.J.; Hu, Y.L.; Xu, D.H.; Li, F.Z.; Liang, W.Q.; Gao, J.Q. Overcoming drug resistance of Mcf-7/Adr cells by altering intracellular distribution of doxorubicin via mvp knockdown with a novel sirna polyamidoamine-hyaluronic acid complex. J. Control. Release 2012, 163, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Urzua, J.I.; Torneiro, M. Divergent synthesis of porous tetraphenylmethane dendrimers. J. Org. Chem. 2017, 82, 13231–13238. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, J.; Rozhkov, V.; Kachala, V.V.; Fetyukhin, V.; Lukin, O. An optimized divergent synthesis of sulfonimide-based dendrimers achieving the fifth generation. Synth. Commun. 2019, 49, 3536–3545. [Google Scholar] [CrossRef]
- Zamani, M.; Shirinzadeh, A.; Aghajanzadeh, M.; Andalib, S.; Danafar, H. In vivo study of mPEG–PCL as a nanocarriers for anti-inflammatory drug delivery of simvastatin. Pharm. Dev. Technol. 2019, 24, 663–670. [Google Scholar] [CrossRef]
- Senevirathne, S.A.; Washington, K.E.; Biewer, M.C.; Stefan, M.C. Peg based anti-cancer drug conjugated prodrug micelles for the delivery of anti-cancer agents. J. Mater. Chem. B 2016, 4, 360–370. [Google Scholar] [CrossRef]
- Vogus, D.R.; Krishnan, V.; Mitragotri, S. A review on engineering polymer drug conjugates to improve combination chemotherapy. Curr. Opin. Colloid Interface Sci. 2017, 31, 75–85. [Google Scholar] [CrossRef]
- Zamani, M.; Aghajanzadeh, M.; Rostamizadeh, K.; Manjili, H.K.; Fridoni, M.; Danafar, H. In vivo study of poly(ethylene glycol)-poly(caprolactone)-modified folic acid nanocarriers as a ph responsive system for tumor-targeted co-delivery of tamoxifen and quercetin. J. Drug Deliv. Sci. Technol. 2019, 54, 101283. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Andalib, S.; Danafar, H.; Rostamizadeh, K.; Sharafi, A. The effect of baicalein-loaded Y-shaped miktoarm copolymer on spatial memory and hippocampal expression of DHCR24, SELADIN and SIRT6 genes in rat model of alzheimer. Int. J. Pharm. 2020, 586, 119546. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Ghannad, F.; Zamani, M.; Andalib, S.; Danafar, H. Anti-inflammatory effect of rosuvastatin using diblock amphiphilic copolymer: Synthesis, characterization, in vitro and in vivo study. J. Biomater. Appl. 2019, 34, 229–238. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; Lyu, Y.; Huang, J.; Jiang, Y.; Xie, C.; Pu, K. Photoactivatable organic semiconducting pro-nanoenzymes. J. Am. Chem. Soc. 2019, 141, 4073–4079. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhen, X.; Lyu, Y.; Jiang, Y.; Huang, J.; Pu, K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Zeng, J.; Jiang, Y.; Zhen, X.; Wang, T.; Qiu, S.; Lou, X.; Gao, M.; Pu, K. Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Nano 2018, 12, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, J.; Kolotylo, M.; Rozhkov, V.; Burilov, V.; Lukin, O. A convergent approach to sulfonimide-based dendrimers and dendrons. Tetrahedron Lett. 2020, 61, 152011. [Google Scholar] [CrossRef]
- Wehner, M.; Würthner, F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem. 2020, 4, 38–53. [Google Scholar] [CrossRef]
- Fukushima, K.; Honda, K.; Inoue, Y.; Tanaka, M. Synthesis of antithrombotic poly(carbonate-urethane) s through a sequential process of ring-opening polymerization and polyaddition facilitated by organocatalysts. Eur. Polym. J. 2017, 95, 728–736. [Google Scholar] [CrossRef]
- Yamauchi, A.; Sudo, A.; Endo, T. Polymer with zwitterionic structure in main chain via polyaddition of bifunctional cyclic amidine and diisothiocyanate. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2145–2148. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lv, S.; Song, Z.; Dang, J.; Li, X.; He, H.; Xu, X.; Zhou, Z.; Yin, L. Photodynamic therapy-mediated remote control of chemotherapy toward synergistic anticancer treatment. Nanoscale 2018, 10, 14554–14562. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liu, D.; Lin, W. Self-assembled core–shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Liu, G.; Hu, J.; Liu, S. Near-infrared light-activated photochemical internalization of reduction-responsive polyprodrug vesicles for synergistic photodynamic therapy and chemotherapy. Biomacromolecules 2017, 18, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with bodipy dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Silva, V.L.; Kaassis, A.; Dehsorkhi, A.; Koffi, C.R.; Severic, M.; Abdelhamid, M.; Nyimanu, D.; Morris, C.J. Enhanced selectivity, cellular uptake, and in vitro activity of an intrinsically fluorescent copper–tirapazamine nanocomplex for hypoxia targeted therapy in prostate cancer. Biomater. Sci. 2020, 8, 2420–2433. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.A. Hypoxia-activated prodrugs in cancer therapy: Progress to the clinic. Future Oncol. 2010, 6, 419–428. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Semiconducting polymer nanomaterials as near-infrared photoactivatable protherapeutics for cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Ariga, K.; Ahn, E.; Park, M.; Kim, B. Layer-by-layer assembly: Recent progress from layered assemblies to layered nanoarchitectonics. Chem.–Asian J. 2019, 14, 2553–2566. [Google Scholar] [CrossRef]
- Fan, F.; Wang, L.; Li, F.; Fu, Y.; Xu, H. Stimuli-responsive layer-by-layer tellurium-containing polymer films for the combination of chemotherapy and photodynamic therapy. ACS Appl. Mater. Interfaces 2016, 8, 17004–17010. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, S.; Liu, Y.; Sun, C.; Chang, M.; Zhao, X.; Hu, C.; Pang, M. Facile fabrication of nanoscale porphyrinic covalent organic polymers for combined photodynamic and photothermal cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 12321–12326. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Liu, J.; Dong, Z.; Liu, Z. pH-responsive nanoscale covalent organic polymers as a biodegradable drug carrier for combined photodynamic chemotherapy of cancer. ACS Appl. Mater. Interfaces 2018, 10, 14475–14482. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Zhang, T.; Wan, G.; Chen, B.; Xiong, Q.; Zhang, J.; Zhang, W.; Wang, Y. Pullulan-coated phospholipid and pluronic F68 complex nanoparticles for carrying Ir780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int. J. Nanomed. 2017, 12, 8649. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.; Qu, X.; Ding, S.; Lv, J.; Li, X.; Gao, P.; Liu, Y.; Yuan, Z. Improved Ir780 derivatives bearing morpholine group as tumor-targeted therapeutic agent for near-infrared fluorescence imaging and photodynamic therapy. Dye. Pigment. 2020, 177, 107979. [Google Scholar] [CrossRef]
- Wan, G.; Cheng, Y.; Song, J.; Chen, Q.; Chen, B.; Liu, Y.; Ji, S.; Chen, H.; Wang, Y. Nucleus-Targeting near-infrared nanoparticles based on tat peptide-conjugated Ir780 for photo-chemotherapy of breast cancer. Chem. Eng. J. 2020, 380, 122458. [Google Scholar] [CrossRef]
- Holden, A.; Varcoe, R.L.; Jaff, M.R.; Schneider, P.A.; Tepe, G.; Zeller, T. Paclitaxel and Mortality: The Dose Argument Is Critical; SAGE Publications Sage CA: Los Angeles, CA, USA, 2019. [Google Scholar]
- Liu, L.; Wang, R.; Wang, C.; Wang, J.; Chen, L.; Cheng, J. Light-triggered release of drug conjugates for an efficient combination of chemotherapy and photodynamic therapy. Biomater. Sci. 2018, 6, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.; Chen, D.; Patil, Y.; Ma, L.; Dou, Q.P.; Shekhar, M.; Panyam, J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance. J. Control. Release 2010, 141, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongrapipat, J.; Kopečková, P.; Liu, J.; Prakongpan, S.; Kopeček, J. Combination chemotherapy and photodynamic therapy with fab′ fragment targeted hpma copolymer conjugates in human ovarian carcinoma cells. Mol. Pharm. 2008, 5, 696–709. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Liu, Z.; Wang, S.; Zheng, B.; Guo, W.; Yang, W.; Gong, X.; Wu, X.; Wang, H.; Chang, J. A protein–polymer bioconjugate-coated upconversion nanosystem for simultaneous tumor cell imaging, photodynamic therapy, and chemotherapy. ACS Appl. Mater. Interfaces 2016, 8, 32688–32698. [Google Scholar] [CrossRef]
- Monroe, J.D.; Belekov, E.; Er, A.O.; Smith, M.E. Anticancer Photodynamic therapy properties of sulfur-doped graphene quantum dot and methylene blue preparations in MCF-7 breast cancer cell culture. Photochem. Photobiol. 2019, 95, 1473–1481. [Google Scholar] [CrossRef]
- Narumi, A.; Rachi, R.; Yamazaki, H.; Kawaguchi, S.; Kikuchi, M.; Konno, H.; Osaki, T.; Okamoto, Y.; Shen, X.; Kakuchi, T.; et al. Maltotriose–chlorin E6 conjugate linked via tetraethyleneglycol as an advanced photosensitizer for photodynamic therapy. synthesis and antitumor activities against canine and mouse mammary carcinoma cells. ACS Omega 2021, 6, 7023–7033. [Google Scholar] [CrossRef]
- Pan, Z.; He, X.; Song, N.; Fang, D.; Li, Z.; Li, J.; Luo, F.; Li, J.; Tan, H.; Fu, Q. Albumin-modified cationic nanocarriers to potentially create a new platform for drug delivery systems. ACS Appl. Mater. Interfaces 2019, 11, 16421–16429. [Google Scholar] [CrossRef]
- Liu, X.; Mohanty, R.P.; Maier, E.Y.; Peng, X.; Wulfe, S.; Looney, A.P.; Aung, K.L.; Ghosh, D. Controlled loading of albumin-drug conjugates ex vivo for enhanced drug delivery and antitumor efficacy. J. Control. Release 2020, 328, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhou, N.; Yuan, P.; Su, Y.; Shao, M.; Chi, C.; Pan, F. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon 2017, 118, 752–764. [Google Scholar] [CrossRef]
- Klepka, M.T.; Nedelko, N.; Greneche, J.-M.; Lawniczak-Jablonska, K.; Demchenko, I.N.; Slawska-Waniewska, A.; Rodrigues, C.A.; Debrassi, A.; Bordini, C. Local atomic structure and magnetic ordering of iron in Fe-chitosan complexes. Biomacromolecules 2008, 9, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Nanomedicine applications of hybrid nanomaterials built from metal–ligand coordination bonds: Nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem. Rev. 2015, 115, 11079–11108. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, I.A.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. selective surface pegylation of Uio-66 nanoparticles for enhanced stability, cell uptake, and pH-responsive drug delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemzadeh, A.; Amerizadeh, F.; Asgharzadeh, F.; Darroudi, M.; Avan, A.; Hassanian, S.M.; Landarani, M.; Khazaei, M. Delivery of oxaliplatin to colorectal cancer cells by folate-targeted Uio-66-Nh2. Toxicol. Appl. Pharmacol. 2021, 423, 115573. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Basir, M.H.; Shirini, F.; Tajik, H.; Ghasemzadeh, M.A. Zn (Bdc)-(MOF): Introduction of a new catalyst for the synthesis pyrimido [4, 5−d] pyrimidine derivatives under ultrasound irradiation in the absence of solvent. Polycycl. Aromat. Compd. 2019, 41, 1–10. [Google Scholar] [CrossRef]
- Abdollahi-Basir, M.H.; Shirini, F.; Tajik, H.; Ghasemzadeh, M.A. A facile and regioselective synthesis of some new pyrimido [4,5−d][1,2,4] triazolo [1,5−a] pyrimidinediones catalyzed by Zn(BDC)-mof under ultrasound irradiation. J. Mol. Struct. 2019, 1195, 302–308. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Zhang, W.; Cao, Y.; Huang, S.S.; Li, Y.Z.; Guo, D.; Wang, X.Y.; Ran, H.T. A cleverly designed novel lipid nanosystem: Targeted retention, controlled visual drug release, and cascade amplification therapy for mammary carcinoma in vitro. Int. J. Nanomed. 2020, 15, 3953. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.H.; Anselmo, A.; Mitragotri, S.; Muzykantov, V. Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. 2016, 106, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Tang, S.; Zhang, P.; Chen, Y.; Zhang, Z.; Yu, H.; Li, Y. Long circulation red-blood-cell-mimetic nanoparticles with peptide-enhanced tumor penetration for simultaneously inhibiting growth and lung metastasis of breast cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Wang, H.; Xu, M.; Xiong, M.; Cheng, J. Reduction-responsive dithiomaleimide-based nanomedicine with high drug loading and fret-indicated drug release. Chem. Commun. 2015, 51, 4807–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Gan, N.; Meng, L.H.; Wang, F. Amperometric immunosensor for α-fetoprotein antigen in human serum based on co-immobilizing dinuclear copper complex and gold nanoparticle doped chitosan film. J. Phys. Conf. Ser. 2009, 188, 012047. [Google Scholar] [CrossRef]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT 2020, 128, 109388. [Google Scholar] [CrossRef]
- Rocha, M.S.T.; Lucci, C.M.; Longo, J.P.F.; Galera, P.D.; Simioni, A.R.; Lacava, Z.G.M.; Tedesco, A.; Azevedo, R.B. Aluminum-chloride-phthalocyanine encapsulated in liposomes: Activity against naturally occurring dog breast cancer cells. J. Biomed. Nanotechnol. 2012, 8, 251–257. [Google Scholar] [CrossRef]
- Lopes, S.C.; Silva, R.A.; Novais, M.V.; Coelho, L.D.; Ferreira, L.A.; Souza, P.E.; Tedesco, A.; Azevedo, R.B.; Aguiar, M.G.; Oliveira, M.C. Topical photodynamic therapy with chloroaluminum phthalocyanine liposomes is as effective as systemic pentavalent antimony in the treatment of experimental cutaneous leishmaniasis. Photodiagnosis Photodyn. Ther. 2019, 28, 210–215. [Google Scholar] [CrossRef]
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.; Valic, M.S.; Cheng, M.H.; Rosilio, V.; Chen, J.; Zheng, G. Porphyrin-lipid stabilized paclitaxel nanoemulsion for combined photodynamic therapy and chemotherapy. J. Nanobiotechnol. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Drug Deliv. 2010, 197, 3–53. [Google Scholar]
- Zamani, M.; Rostamizadeh, K.; Manjili, H.K.; Danafar, H. In vitro and in vivo biocompatibility study of folate-lysine-peg-pcl as nanocarrier for targeted breast cancer drug delivery. Eur. Polym. J. 2018, 103, 260–270. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Rashidzadeh, H.; Rostamizadeh, K.; Sharafi, A.; Danafar, H. Amphiphilic Y shaped miktoarm star copolymer for anticancer hydrophobic and hydrophilic drugs codelivery: Synthesis, characterization, in vitro, and in vivo biocompatibility study. J. Biomed. Mater. Res. Part A 2018, 106, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Aghajanzadeh, M.; Sharafi, A.; Rostamizadeh, K.; Danafar, H. Targeted drug delivery via folate decorated nanocarriers based on linear polymer for treatment of breast cancer. Pharm. Dev. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, M.; Gong, K.; Peng, H.; Ge, L.; Zhao, L.; Chen, J. PH-triggered degradable polymeric micelles for targeted anti-tumor drug delivery. Mater. Sci. Eng. C 2017, 78, 912–922. [Google Scholar] [CrossRef]
- Xie, P.; Liu, P. Core-shell-corona chitosan-based micelles for tumor intracellular PH-triggered drug delivery: Improving performance by grafting polycation. Int. J. Biol. Macromol. 2019, 141, 161–170. [Google Scholar] [CrossRef]
- Aghajanzadeh, M.; Zamani, M.; Rostamizadeh, K.; Sharafi, A.; Danafar, H. The role of miktoarm star copolymers in drug delivery systems. J. Macromol. Sci. Part A 2018, 55, 559–571. [Google Scholar] [CrossRef]
- Hamblin, M.R. Fullerenes as photosensitizers in photodynamic therapy: Pros and cons. Photochem. Photobiol. Sci. 2018, 17, 1515–1533. [Google Scholar] [CrossRef]
- Kumar, V.; Koyasseril-Yehiya, T.M.; Thayumanavan, S. Enzyme-triggered nanomaterials and their applications. Mol. Assem. Charact. Appl. 2020, 1355, 95–107. [Google Scholar]
- Li, R.; Peng, F.; Cai, J.; Yang, D.; Zhang, P. Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects. Asian J. Pharm. Sci. 2020, 15, 311–325. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials 2013, 34, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of Active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meihua, Y.; Jambhrunkar, S.; Thorn, P.; Jiezhong, C.; Wenyi, G.; Chengzhong, Y. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to Cd44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar]
- Zhou, H.; Xu, H.; Li, X.; Lv, Y.; Ma, T.; Guo, S.; Huang, Z.; Wang, X.; Xu, P. Dual targeting hyaluronic acid-Rgd mesoporous silica coated gold nanorods for chemo-photothermal cancer therapy. Mater. Sci. Eng. C 2017, 81, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.; Mulholland, P.; Chester, K. Antibody-targeted nanoparticles for cancer treatment. Immunotherapy 2016, 8, 941–958. [Google Scholar] [CrossRef]
- Krakovičová, H.; Etrych, T.; Ulbrich, K. Hpma-based polymer conjugates with drug combination. Eur. J. Pharm. Sci. 2009, 37, 405–412. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Liu, C.; Zou, Y.; Huang, L.; Liang, Y.; Ren, J.; Liu, Y.; Lin, Q. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial peptide nisin. Food Chem. 2021, 336, 127669. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-Her2-targeted doxorubicin–core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, 14, 4105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]






| Combination of PDT and Chemotherapy | Type of Carriers | Advantages | Disadvantages |
|---|---|---|---|
| Without external carriers | Photosensitizers as carriers |
|
|
| Photosensitizer-drug materials | |||
| With external carriers | Transition metal based nano-platforms |
|
|
| Silica | |||
| Graphene | |||
| Liposomes | |||
| Dendrimers | |||
| Polymers | |||
| Metal–organic frameworks | |||
| Biological nanocarriers | |||
| Nano emulsions |
| Reference | Photosensitizer (Carrier) | Drug |
|---|---|---|
| [27] | citric acid/CuS@Fe3O4 | Doxorubicin |
| [28] | [(η6-p-cymene)Ru(2,3-bis(2-pyridyl)-benzoquinoxaline)(pyridine)]2+ | Ru (II) segments |
| [29] | Porphyrin | Oxaliplatin-adamantane |
| [30] | Zinc phthalocyanine | Coumarin |
| [31] | Cyclometallated Ir(III) complex | Camptothecin |
| [32] | Cu2−xSe | Doxorubicin |
| [33] | NaYF4:Yb/Tm-TiO2 | Doxorubicin |
| [34] | Silver nanoparticles | Doxorubicin |
| [35] | ZnO nanorods | Daunorubicin |
| [36] | MnO2-Pt@Au25 | Platinum (IV) prodrugs |
| [37] | Zinc phthalocyanine | Ganetespib |
| [38] | Polyelectrolytes-NaYF4:Yb/Tm | Doxorubicin |
| [39] | AgFeO2 | Quercetin |
| [40] | MnFe2O4 | Curcumin |
| [40] | Cr2Fe6O12 | Curcumin |
| [41] | Ti3C2 MXene | Doxorubicin |
| [42] | Ti3C2 MXene | Metformin |
| [43] | MoS2 | Doxorubicin |
| [44] | Boron-dipyrromethene | Lenvatinib |
| [45] | porphyrin-containing Janus macromolecular brush | Doxorubicin |
| [46] | mPEG-Hydrazone-Br2-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene | Doxorubicin |
| [47] | Ir(III) | Paclitaxel |
| [48] | Fe3O4@MnO2-Chlorin-e6 | Traditional Chinese medicine |
| Reference | Photosensitizer | Drug | Carrier |
|---|---|---|---|
| [75] | Photochlor | Prodrug banoxantrone | UiO-66-H/N3 (MOF) |
| [76] | Merocyanine 540 | Doxorubicin | YbPO4:Er, Dy |
| [77] | Mitoxantrone | Mitoxantrone | PEGylated Hollow gold nanoparticles |
| [78] | Hematoporphyrin | Docetaxel | Gd-up conversion nanoparticles core/mesoporous silica shell |
| [79] | Chlorin core star shaped block copolymer | Camptothecin-11 | Micelles |
| [80] | Rose Bengal | Platinum IV | NaGdF4:Yb/Nd@NaGdF4:Yb/Er@NaGdF4 |
| [81] | Rose Bengal | AB3, a histone deacetylase inhibitor | NaYF4:Yb/Tm/Er |
| [82] | Merocyanine 540 | Doxorubicin | NaYF4:Yb/Er |
| [83] | Chlorin-e6 | Camptothecin | Up-conversion nanoparticles |
| [84] | Pyropheophorbide | Doxorubicin | Up-conversion nanoparticles |
| [85] | Chlorin-e6 | c,c,t-[Diamine-dichlorodisuccinato-platinum(IV)] | [Mg(1−x)Alx(OH)2][An−x/n]·zH2O |
| [86] | Zinc(II) phthalocyanine | Doxorubicin | Mesoporous silica nanoparticle |
| [87] | Chlorin-e6 | Doxorubicin | Polyethylene glycol |
| [88] | Chlorin-e6 | Doxorubicin | Mesoporous silica nanoparticle |
| [89] | Hematoporphyrin | Doxorubicin | Hollow Mesoporous Silica |
| [90] | Aluminum chloride phthalocyanine | Cisplatin | Mesoporous silica nanoparticle |
| [91] | PEGylated tetraphenylporphyrin zinc | Doxorubicin | Mesoporous silica nanoparticle |
| [92] | Chlorin-e6 | Doxorubicin | Fe3O4@mSiO2(DOX)@ Human serum albumin |
| [93] | Fullerene (C60) | Doxorubicin | Mesoporous hollow silica |
| [94] | Chlorin-e6 | Cisplatin | Mesoporous silica nanoparticle |
| [95] | 2-[1-Hexyloxyethyl]-2-devinyl pyropheophorbide | Doxorubicin | Liposome |
| [96] | Chlorin-e6 | Doxorubicin | Microbubble-lipid mixture |
| [97] | Indocyanine green-octadecylamine | Doxorubicin | Light sensitive liposome |
| [98] | IR780 | Tirapazamine | Liposome |
| [99] | Porphyrin | Doxorubicin | Dendritic poly(ethylene glycol) (PEG-G3-OH) copolymer |
| [100] | 5,10,15,20-Tetraphenylchlorin | Paclitaxel | Red blood cells membrane-camouflaged nanoparticles |
| [101] | Chlorin-e6 | Doxorubicin | Hybrid protein oxygen carriers |
| [102] | Indocyanine green | Doxorubicin | Red blood cells containing oxyhemoglobin |
| [6] | Chloroaluminum phthalocyanine | Doxorubicin | Nano emulsions |
| [103] | CaFe2O4 | Curcumin | Polyvinyl alcohol |
| [104] | Hypocrellin A | 7-ethyl-10-hydroxycamptothecin | Graphene oxide |
| [105] | MnO2 | Cis-Platine | Graphene oxide |
| [106] | 4-Hydroxy coumarin | Camptothecin | Graphene oxide |
| [107] | Methylene blue | Doxorubicin | Graphene oxide |
| [108] | Zinc(II) phthalocyanine | Doxorubicin | Methoxypolyethylene glycol (mPEG) and poly(β-benzyl-l-aspartate) |
| [109] | Hematoporphyrin | Doxorubicin | Co-polymer containing arylboronic ester (BE)-modified with amphiphilic co-polymer (mPEG-PBAM). |
| [110] | NIR dye-IR820 | Docetaxel | Methoxy-poly ethylene glycol-poly caprolactone |
| [111] | Pyrolipid | Oxaliplatin | 1,2-distearoyl-sn-glycero-3-phosphocholine, cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine polyethylene glycol 2000 |
| [112] | 4,4-difluoro-4-bora-3a,4a-diaza-sindacene | Doxorubicin | mPEG-polyaspartic acid-benzaldehyde |
| [113] | Fluorogen photosensitizer | Paclitaxel | Poly(ethylene glycol)-b-poly(5-mthyl-5-propargyl-1,3-dioxan-2-one) |
| [114] | Hematoporphyrin | Doxorubicin | PEGylated (cyclo-arginine-glycine-aspartic acid-d-phenylalanine-cysteine) peptide |
| [115] | NIR fluorophore | Paclitaxel | Poly(ethylene glycol)-b-poly(5-mthyl-5-propargyl-1,3-dioxan-2-one) |
| [116] | Hyaluronic Acid-chlorin-e6 | Tirapazamine | Self-assembling amphiphilic polyethylenimine-alkyl nitroimidazole |
| [117] | Zn | Docetaxel | Co-polymers poly(ethylene oxide)-poly(ε-caprolactone)-poly(ethylene oxide) |
| [118] | 5-aminolevulinic acid | Doxorubicin | Hydroxyethyl chitosan and aldehyde-functionalized hyaluronic acid |
| [119] | Mesotetra(p-hydroxyphenyl) porphine | Cis-platinum | Mesotetra(p-hydroxyphenyl)-Pt-PEG (covalent-organic polymers) |
| [120] | Chlorin-e6 | Doxorubicin | Hyaluronic acid-chlorin-e6 |
| [121] | C60 | Doxorubicin | C60–PEI–DOX |
| [122] | Chlorin-e6 | Doxorubicin | (ε-caprolactone-co-lactide)-b-poly (ethylene glycol)-b-poly (ε-caprolactone-colactide) |
| [123] | Merocyanine 540 | Doxorubicin | UCNP-loaded (NaYF4:Yb, Er) folate-conjugated polymeric (dextran) |
| [124] | Pyropheophorbide-a | gemcitabine | Human serum albumin |
| [125] | Zinc phthalocyanine | Doxorubicin | [methoxy-poly(ethylene glycol)-poly(2-(N,N-diethylamino)ethyl methacrylate)-poly(ε-caprolactone)]4-zinc β-tetra-(4-carboxyl benzyloxyl)phthalocyani |
| [126] | Indocyanine green | Doxorubicin | Nano-scaled red blood cells |
| [127] | Purpurin 18 | Doxorubicin | mPEG-Cyclodextrin-Polyhydroxybutyrate |
| [128] | Gold nanoclusters | Doxorubicin | (ZIF-8) metal–organic framework |
| [129] | protoporphyrin IX | Doxorubicin | (ZIF-8) metal–organic framework |
| [130] | Chlorin-e6 | Tirapazamine | (polyethylene glycol)-Azo-benzene-poly (d, l-lactide-co-glycolide) |
| [131] | Chlorin-e6 | Gambogic acid | Hyaluronic acid-nitroimidazole (HA-NI) as shells, MnO2 NPs functionalized poly (l-glutamic acid) derivatives (γ-PFGA) as cores |
| [132] | Si photosensitizer | Doxorubicin | Mesoporous silica nanoparticle |
| [133] | Chlorin-e6 | Doxorubicin | Polyoligo (ethylene glycol) methacrylate-block-poly(ε-caprolactone)-azobenzene-poly(ε-caprolactone)-block-poly oligo (ethylene glycol) |
| [134] | Pyropheophorbide | paclitaxel | Poly [oligo (ethylene glycol) methyl ether methacrylate] |
| [135] | Pheophorbide a | Tirapazamine | Self-assembled gelatin nanoparticles |
| [136] | Chlorin-e6 | Doxorubicin | Poly(phosphorylcholine) |
| [137] | Chlorin-e6 | Oridonin | Side-chain selenium-grafted polymers |
| [138] | porphyrin | Doxorubicin | Tetra-β-cyclodextrin |
| [139] | Chlorin-e6 | Gemcitabine | Polymeric micelles |
| [140] | Chlorin-e6 | Gemcitabine | Multifunctional polymeric prodrug micelles |
| [141] | Chlorin-e6 | Paclitaxel | Liposomes |
| [142] | Chlorin-e6 | Docetaxel | Hyaluronic acid |
| [143] | pyropheophorbide-a | camptothecin | mPEG with thioketal linker |
| [144] | Chlorin-e6 | Doxorubicin | Block copolymers polystyrene-b-poly (acrylic acid) and oil-soluble |
| [145] | Chlorin-e6 | Perfluorohexanoate-modified cisplatin | Poly(ethylene glycol)-lysine-block-poly(L-glutamate)-imidazole |
| [146] | protoporphyrin IX | Tegafur (prodrug of 5-fluorouracil) | Heterodimers hydrogel |
| [147] | zinc phthalocyanine | Tirapazamine | Hyaluronic acid |
| [148] | Chlorin-e6 | cisplatin | Dual-effect liposome |
| [149] | Porphyrin | Paclitaxel | Porphyrin-lipid shelled nano-emulsion |
| [150] | 5-aminolevulinic acid to produce protoporphyrin IX | Doxorubicin | Nanogel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghajanzadeh, M.; Zamani, M.; Rajabi Kouchi, F.; Eixenberger, J.; Shirini, D.; Estrada, D.; Shirini, F. Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems. Pharmaceutics 2022, 14, 322. https://doi.org/10.3390/pharmaceutics14020322
Aghajanzadeh M, Zamani M, Rajabi Kouchi F, Eixenberger J, Shirini D, Estrada D, Shirini F. Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems. Pharmaceutics. 2022; 14(2):322. https://doi.org/10.3390/pharmaceutics14020322
Chicago/Turabian StyleAghajanzadeh, Mozhgan, Mostafa Zamani, Fereshteh Rajabi Kouchi, Josh Eixenberger, Dorsa Shirini, David Estrada, and Farhad Shirini. 2022. "Synergic Antitumor Effect of Photodynamic Therapy and Chemotherapy Mediated by Nano Drug Delivery Systems" Pharmaceutics 14, no. 2: 322. https://doi.org/10.3390/pharmaceutics14020322






