Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoparticles
2.1.1. Silica (SiO2) Nanoparticles
2.1.2. Lactate-Loaded Silica Nanoparticles
2.1.3. Titania (TiO2) Nanoparticles
2.1.4. Lactate-Loaded Titania Nanoparticles
2.2. Characterization of Nanoparticles
2.3. Glioma Cell Cultures
2.4. Ultrastructural Analysis of Nanoparticles by TEM
2.5. Evaluation of Nanoparticle Uptake by Glioma Cells
2.6. Evaluation of Cellular Metabolic Activity
2.7. Evaluation of Cytotoxicity
2.8. Evaluation of Cell Supernatant Acidification
2.9. Evaluation of Cellular Metabolic Activity under Hypoxia
2.10. Evaluation of Cytotoxicity on Cocultured Normoxic and Hypoxic Glioma Cells
2.11. Evaluation of the Effects of Nanoparticles in an In Vivo Orthotopic Malignant Glioma Model
2.12. Statistical Analysis
3. Results
3.1. Nanoparticle Characterization
3.2. Cumulative Lactate Release from Nanoparticles
3.3. Nanoparticle Treatment Induces Intracellular Accumulation of Nanometer-Sized Electron-Dense Particles
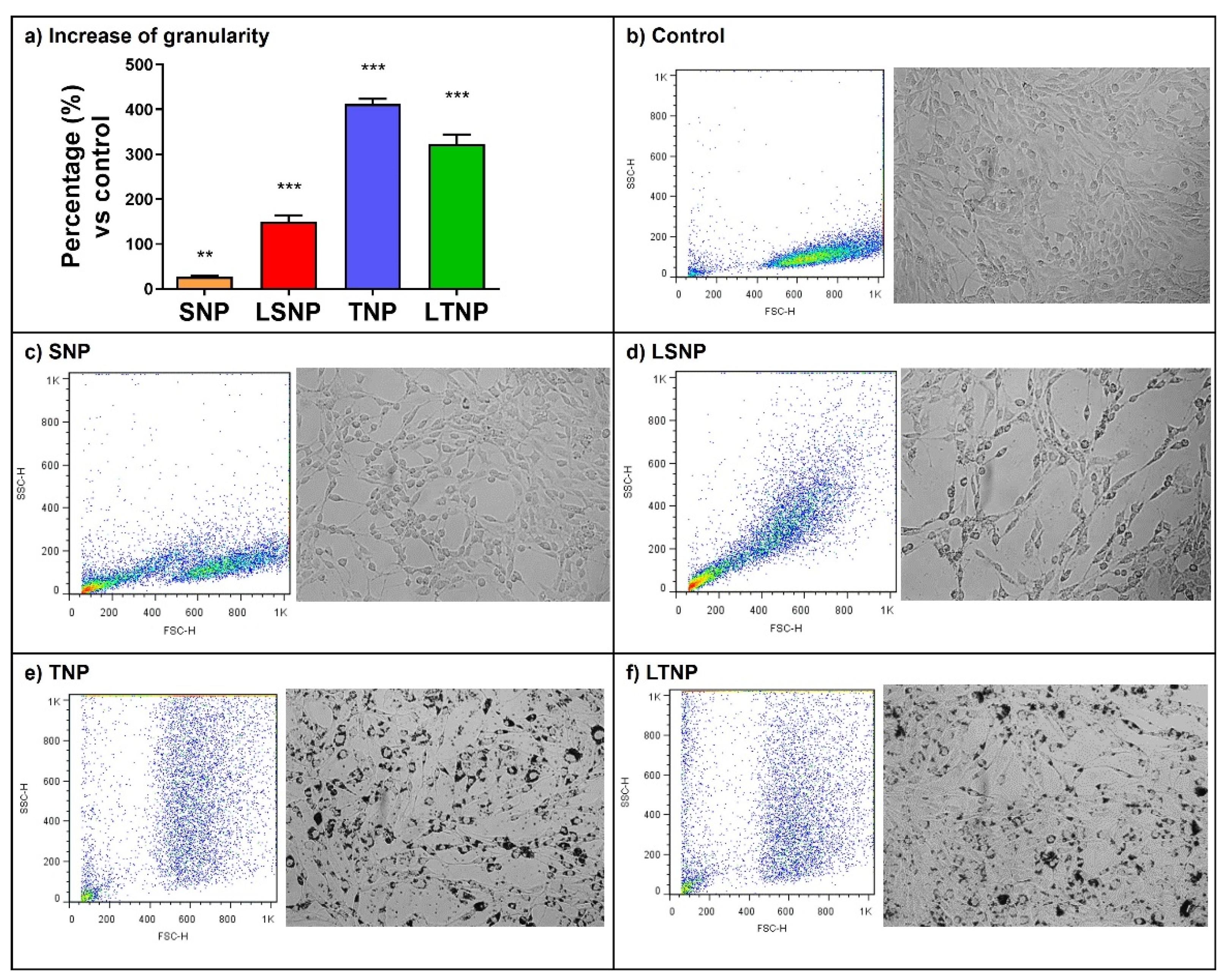
3.4. Nanoparticle Treatment Increases Cell Granularity of C6 Glioma Cells
3.5. The Cell Metabolic Activity Decreases in a Dose-Dependent Manner after Treatment
3.6. LSNP Are Highly Cytotoxic and Accompanied by Supernatant Acidification
3.7. Decrease in Cell Metabolic Activity Induced by Nanoparticles Is Inhibited during Hypoxia
3.8. LSNP-Induced Cytotoxicity Is Inhibited by the Interaction between Normoxic and Hypoxic Tumor Cells
3.9. LSNP Treatment Increases Median Survival in Model of Orthotopic Malignant Glioma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; pp. 197–241. [Google Scholar]
- Musah-Eroje, A.; Watson, S. Adaptive Changes of Glioblastoma Cells Following Exposure to Hypoxic (1% Oxygen) Tumour Microenvironment. Int. J. Mol. Sci. 2019, 20, 2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duraj, T. Beyond the Warburg Effect: Oxidative and Glycolytic Phenotypes Coexist Within the Metabolic Heterogeneity of Glioblastoma. Cells 2021, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Guyon, J.; Romero-Garmendia, I.; Bikfalvi, A.; Daubon, T. FSMP-14. Metabolic symbiosis in glioblastoma: Lactate dehydrogenases take the lead. Neuro Oncol. Adv. 2021, 3, i19. [Google Scholar] [CrossRef]
- Nikoobakht, M.; Shamshiripour, P.; Azimi Nekoo, Z.; Fallah Haghmohammadi, S. Elevated Lactate and Total Protein Levels in Stereotactic Brain Biopsy Specimen; Potential Biomarkers of Malignancy and Poor Prognosis. Arch. Iran. Med. 2019, 22, 125–131. [Google Scholar]
- Nakajima, E.C.; Van Houten, B. Metabolic symbiosis in cancer: Refocusing the Warburg lens. Mol. Carcinog. 2013, 52, 329–337. [Google Scholar] [CrossRef]
- Benjamin, D.; Robay, D.; Hindupur, S.K.; Pohlmann, J.; Colombi, M.; El-Shemerly, M.Y.; Maira, S.-M.; Moroni, C.; Lane, H.A.; Hall, M.N. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018, 25, 3047–3058. [Google Scholar] [CrossRef] [Green Version]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Strategies of temozolomide in future glioblastoma treatment. OncoTargets Ther. 2017, 10, 265–270. [Google Scholar] [CrossRef] [Green Version]
- AbouAitah, K.; Lojkowski, W. Delivery of Natural Agents by Means of Mesoporous Silica Nanospheres as a Promising Anticancer Strategy. Pharmaceutics 2021, 13, 143. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Lin, C.; Wang, L.; Guo, H.; Wang, X. Hypoxia and hypoxia-inducible factors in glioblastoma multiforme progression and therapeutic implications. Exp. Cell Res. 2012, 318, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, A.M.; Wielockx, B. PHD2: From hypoxia regulation to disease progression. Hypoxia 2016, 4, 53–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Garcia, S.; Moreno-Altamirano, M.; Prado-Garcia, H.; Sánchez-García, F. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, T.; Ortiz, E.; Meza, D.; Basaldella, E.; Bokhimi, X.; Magaña, C.; Sepúlveda, A.; Rodríguez, F.; Ruiz, J. Controlled release of phenytoin for epilepsy treatment from titania and silica based materials. Mater. Chem. Phys. 2011, 126, 922–929. [Google Scholar] [CrossRef]
- Lopez, T.; Ortiz-Islas, E.; Guevara, P.; Gómez, E. Catalytic nanomedicine technology: Copper complexes loaded on titania nanomaterials as cytotoxic agents of cancer cell. Int. J. Nanomed. 2013, 8, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Borshchevskaya, L.; Gordeeva, T.; Kalinina, A.; Sineoky, S. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Orozco-Ibarra, M.; Muñoz-Sánchez, J.; Zavala-Medina, M.; Pineda, B.; Magaña-Maldonado, R.; Vazquez-Contreras, E.; Maldonado, P.; Pedraza-Chaverri, J.; Chanez-Cardenas, M. Aged garlic extract and S-allylcysteine prevent apoptotic cell death in a chemical hypoxia model. Biol. Res. 2016, 49, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Percino, J.; Pacheco, J.A.; Soriano-Moro, G.; Cerón, M.; Castro, M.E.; Chapela, V.M.; Bonilla-Cruz, J.; Lara-Ceniceros, T.E.; Flores-Guerrero, M.; Saldivar-Guerra, E. Synthesis, characterization and theoretical calculations of model compounds of silanols catalyzed by TEMPO to elucidate the presence of Si–O–Si and Si–O–N bonds. RSC Adv. 2015, 5, 79829–79844. [Google Scholar] [CrossRef]
- Bagheri, S.; Kamyar, S.; Abd Hamid, S.B. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. J. Chem. 2012, 2013. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Li, Y.; Miao, J.; Wang, W.; Nundlall, K.; Chen, S. Reactive oxygen species-sensitive thioketal-linked mesoporous silica nanoparticles as drug carrier for effective antibacterial activity. Mater. Des. 2020, 195, 109021. [Google Scholar] [CrossRef]
- Ortiz-Islas, E.; Sosa-Arróniz, A.; Manríquez-Ramírez, M.E.; Rodríguez-Pérez, C.E.; Tzompantzi, F.; Padilla, J.M. Mesoporous silica nanoparticles functionalized with folic acid for targeted release Cis-Pt to glioblastoma cells. Rev. Adv. Mater. Sci 2021, 60, 25–37. [Google Scholar] [CrossRef]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar] [PubMed]
- Salazar, A.; Pérez-de la Cruz, V.; Muñoz-Sandoval, E.; Chavarria, V.; García Morales, M.D.; Espinosa-Bonilla, A.; Sotelo, J.; Jiménez-Anguiano, A.; Pineda, B. Potential Use of Nitrogen-Doped Carbon Nanotube Sponges as Payload Carriers Against Malignant Glioma. Nanomaterials 2021, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Ge, M.; Zhou, G.; Sun, W.; Liu, D.; Liang, X.-J.; Zhang, J. A Distinct Endocytic Mechanism of Functionalized-Silica Nanoparticles in Breast Cancer Stem Cells. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucker, R.M.; Massaro, E.J.; Sanders, K.M.; Degn, L.L.; Boyes, W.K. Detection of TiO2 nanoparticles in cells by flow cytometry. Cytom. Part A 2010, 77A, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Romano-Feinholz, S.; Salazar-Ramiro, A.; Munoz-Sandoval, E.; Magana-Maldonado, R.; Hernandez Pedro, N.; Rangel Lopez, E.; Gonzalez Aguilar, A.; Sanchez Garcia, A.; Sotelo, J.; Perez de la Cruz, V.; et al. Cytotoxicity induced by carbon nanotubes in experimental malignant glioma. Int. J. Nanomed. 2017, 12, 6005–6026. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.; Alkawareek, M.; Dreaden, E.; Brown, D.; Alkilany, A.; Farokhzad, O.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46. [Google Scholar] [CrossRef]
- Surin, A.; Sharipov, R.; Krasil’nikova, I.; Boyarkin, D.; Lisina, O.; Gorbacheva, L.; Avetisyan, A.; Pinelis, V. Disruption of Functional Activity of Mitochondria during MTT Assay of Viability of Cultured Neurons. Biochemistry 2017, 82, 737–749. [Google Scholar] [CrossRef]
- Madesh, M.; Balasubramanian, K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J. Biochem. Biophys. 1998, 35, 184–188. [Google Scholar] [PubMed]
- Leite, T.C.; Coelho Rg Fau-Da Silva, D.; Da Silva, D.; Fau-Coelho, W.S.; Coelho Ws Fau-Marinho-Carvalho, M.M.; Marinho-Carvalho Mm Fau-Sola-Penna, M.; Sola-Penna, M. Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett. 2011, 585, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stambaugh, R.; Post, D. Substrate and product inhibition of rabbit muscle lactic dehydrogenase heart (H4) and muscle (M4) isozymes. J. Biol. Chem. 1966, 241, 1462–1467. [Google Scholar] [CrossRef]
- Tsai, K.-L.; Wang, S.-M.; Chen, C.-C.; Fong, T.-H.; Wu, M.-L. Mechanism of oxidative stress-induced intracellular acidosis in rat cerebellar astrocytes and C6 glioma cells. J. Physiol. 2004, 502, 161–174. [Google Scholar] [CrossRef]
- Ringel, F.; Chang, R.C.C.; Staub, F.; Baethmann, A.; Plesnila, N. Contribution of Anion Transporters to the Acidosis-Induced Swelling and Intracellular Acidification of Glial Cells. J. Neurochem. 2000, 75, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Bergersen, L.H. Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow Metab. 2015, 35, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Dimmer, K.S.; Friedrich, B.; Lang, F.; Deitmer, J.W.; Broer, S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000, 350, 219–227. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Marsi, T.; Ricci, R.; Toniato, T.; Vasconcellos, L.; Elias, C.; Silva, A.; Furtado, A.; Magalhães, L.; Silva-Filho, E.; Marciano, F.; et al. Electrospun Nanofibrous Poly (Lactic Acid)/Titanium Dioxide Nanocomposite Membranes for Cutaneous Scar Minimization. Front. Bioeng. Biotechnol. 2019, 7, 421. [Google Scholar] [CrossRef]
- Yuan, Y.; Hilliard, G.; Ferguson, T.; Millhorn, D.E. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003, 278, 15911–15916. [Google Scholar] [CrossRef] [Green Version]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef] [PubMed]
- Betriu, N.; Semino, C. Development of a 3D Co-Culture System as a Cancer Model Using a Self-Assembling Peptide Scaffold. Gels 2018, 4, 65. [Google Scholar] [CrossRef] [Green Version]
- Koh, B.; Jeon, H.; Kim, D.; Kang, D.; Kim, K. Effect of fibroblast co-culture on the proliferation, viability and drug response of colon cancer cells. Oncol. Lett. 2018, 17, 2409–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Yan, T.; Zhu, H.; Liang, X.; Leiss, L.; Sakariassen, P.; Skaftnesmo, K.; Huang, B.; Costea, D.; Enger, P.; et al. A co-culture model with brain tumor-specific bioluminescence demonstrates astrocyte-induced drug resistance in glioblastoma. J. Transl. Med. 2014, 12, 278. [Google Scholar] [CrossRef]
- Cruz-Bermúdez, A.; Laza-Briviesca, R.; Vicente-Blanco, R.J.; García-Grande, A.; Coronado, M.J.; Laine-Menéndez, S.; Alfaro, C.; Sanchez, J.C.; Franco, F.; Calvo, V.; et al. Cancer-associated fibroblasts modify lung cancer metabolism involving ROS and TGF-β signaling. Free Radic. Biol. Med. 2019, 130, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Chen, S.; Chen, X.; Lin, W.; Li, W.; Ma, J.; Wu, T.; Cui, X.; Ji, H.; Li, Y.; et al. Cancer-Associated fibroblasts enhance pancreatic cancer cell invasion by remodeling the metabolic conversion mechanism. Oncol. Rep. 2017, 37, 1971–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druzhkova, I.; Shirmanova, M.; Lukina, M.; Dudenkova, V.; Mishina, N.; Zagaynova, E. The metabolic interaction of cancer cells and fibroblasts—Coupling between NAD(P)H and FAD, intracellular pH and hydrogen peroxide. Cell Cycle 2016, 15, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- DeSouza, R.M.; Shaweis, H.; Han, C.; Sivasubramiam, V.; Brazil, L.; Beaney, R.; Sadler, G.; Al-Sarraj, S.; Hampton, T.; Logan, J.; et al. Has the survival of patients with glioblastoma changed over the years? Br. J. Cancer 2016, 114, 146–150. [Google Scholar] [CrossRef]
- Bisdas, S.; Schafer, R.; Kolb, R.; Bender, B.; Klose, U. Lactate as clinical tumour biomarker: Optimization of lactate detection and quantification in MR spectroscopic imaging of glioblastomas. Eur. J. Radiol. 2020, 130, 109171. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Wilson, E.B.; Short, S.; Melcher, A.A.; Biggs, M.; Diakos, C.I.; Howell, V.M. Glycolysis and Fatty Acid Oxidation Inhibition Improves Survival in Glioblastoma. Front. Oncol. 2021, 11, 633210. [Google Scholar] [CrossRef]
- Colen, C.B.; Shen, Y.; Ghoddoussi, F.; Yu, P.; Francis, T.B.; Koch, B.J.; Monterey, M.D.; Galloway, M.P.; Sloan, A.E.; Mathupala, S.P. Metabolic Targeting of Lactate Efflux by Malignant Glioma Inhibits Invasiveness and Induces Necrosis: An In Vivo Study. Neoplasia 2011, 13, 620–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicks, R.T.; Azadi, J.; Mangraviti, A.; Zhang, I.; Hwang, L.; Joshi, A.; Bow, H.; Hutt-Cabezas, M.; Martin, K.L.; Rudek, M.A.; et al. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro Oncol. 2015, 17, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Zhu, Y.; Gao, C.; Ling, C.; Qin, J.; Wang, Q.; Huang, Y.; Lu, W.; Wang, J. Menthol-modified BSA nanoparticles for glioma targeting therapy using an energy restriction strategy. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavarria, V.; Ortiz-Islas, E.; Salazar, A.; Pérez-de la Cruz, V.; Espinosa-Bonilla, A.; Figueroa, R.; Ortíz-Plata, A.; Sotelo, J.; Sánchez-García, F.J.; Pineda, B. Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics 2022, 14, 327. https://doi.org/10.3390/pharmaceutics14020327
Chavarria V, Ortiz-Islas E, Salazar A, Pérez-de la Cruz V, Espinosa-Bonilla A, Figueroa R, Ortíz-Plata A, Sotelo J, Sánchez-García FJ, Pineda B. Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics. 2022; 14(2):327. https://doi.org/10.3390/pharmaceutics14020327
Chicago/Turabian StyleChavarria, Víctor, Emma Ortiz-Islas, Alelí Salazar, Verónica Pérez-de la Cruz, Alejandra Espinosa-Bonilla, Rubén Figueroa, Alma Ortíz-Plata, Julio Sotelo, Francisco Javier Sánchez-García, and Benjamín Pineda. 2022. "Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor" Pharmaceutics 14, no. 2: 327. https://doi.org/10.3390/pharmaceutics14020327
APA StyleChavarria, V., Ortiz-Islas, E., Salazar, A., Pérez-de la Cruz, V., Espinosa-Bonilla, A., Figueroa, R., Ortíz-Plata, A., Sotelo, J., Sánchez-García, F. J., & Pineda, B. (2022). Lactate-Loaded Nanoparticles Induce Glioma Cytotoxicity and Increase the Survival of Rats Bearing Malignant Glioma Brain Tumor. Pharmaceutics, 14(2), 327. https://doi.org/10.3390/pharmaceutics14020327






