Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Samples
2.2.2. Breaking Strength
2.2.3. In vitro Mucoadhesion Test
2.2.4. X-ray Powder Diffractometry (XRPD)
2.2.5. Positron Annihilation Lifetime Spectroscopy (PALS)
2.2.6. Atomic Force Microscopy (AFM)
2.2.7. Scanning Electron Microscopy
2.2.8. Surface Free Energy (SFE)
2.2.9. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.2.10. Dissolution Test
3. Results and Discussion
3.1. Breaking Strength
3.2. Mucoadhesion Force
3.3. Results of XRPD
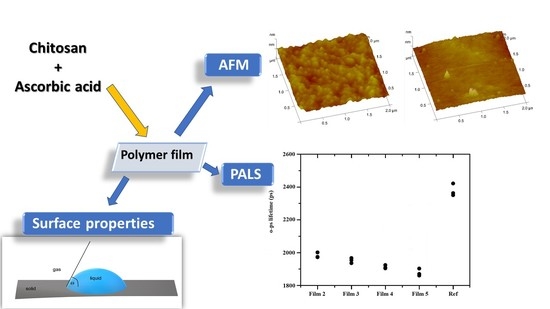
3.4. Free Volumes of the Films
3.5. Morphology of the Films
3.6. Morphology of the Films
3.7. Results of SFE
3.8. Results of FTIR
3.9. Results of Dissolution Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mojsiewicz-Pienkowska, K.; Jamrógiewicz, M.; Zebrowska, M.; Mikolaszek, B.; Sznitowska, M. Double layer adhesive silicone dressing as a potential dermal drug delivery film in scar treatment. Int. J. Pharm. 2015, 481, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mikolaszek, B.; Jamrógiewicz, M.; Mojsiewicz-Pienkowska, K.; Zebrowska, M.; Sznitowska, M.; Strankowska, J. Physical and Mechanical Evaluation of Silicone-Based Double-Layer Adhesive Patch Intended for Keloids and Scar Treatment Therapy. Polymers 2016, 8, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerec, M.; Bogataj, M.; Mugerle, B.; Gašperlin, M.; Mrhar, A. Mucoadhesion on pig vesical mucosa: Influence of polycarbophil/calcium interactions. Int. J. Pharm. 2002, 241, 135–143. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.M.; Bermejo, P.; Ruiz-Caro, R.; Veiga, M.D. Dapivirine Bioadhesive Vaginal Tablets Based on Natural Polymers for the Prevention of Sexual Transmission of HIV. Polymers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of Zwitterionic Polypeptide Nanoformulation with High Doxorubicin Loading Content for Targeted Drug Delivery. Langmuir 2019, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sheng, Y.; Li, K.; Sai, S.; Feng, J.; Li, Y.; Zhang, J.; Han, J.; Tian, B. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomaterialia 2022, 138, 193–207. [Google Scholar] [CrossRef]
- Kelemen, A.; Katona, B.; Módra, S.; Aigner, Z.; Sebe, I.; Pintye-Hódi, K.; Zelkó, R.; Regdon, G., Jr.; Kristó, K. Effects of Sucrose Palmitate on the Physico-Chemical and Mucoadhesive Properties of Buccal Films. Molecules 2020, 25, 5248. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Senel, S.; Kremer, M.J.; Kas, S.; Wertz, P.W.; Hincal, A.A.; Squier, C.A. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials 2000, 21, 2067–2071. [Google Scholar] [CrossRef]
- Rossi, S.; Sandri, G.; Ferrari, F.; Bonferoni, M.C.; Caramella, C. Buccal delivery of acyclovir from films based on chitosan and polyacrylic acid. Pharmaceut. Develop. Technol. 2003, 8, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Muzzarelli, C.; Caramella, C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur. J. Pharm. Sci. 2004, 21, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Ashri, L.Y.; Ela, A.E.S.F.A.E.; Ibrahim, M.A.; Alshora, D.H.; Naguib, M.J. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101720. [Google Scholar] [CrossRef]
- Koland, M.; Charyulu, R.N.; Vijayanarayana, K.; Prabhu, P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int. J. Pharm. Investig. 2011, 1, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Abouhussein, D.M.N.; El-Bary, A.A.; Shalaby, S.H.; El Nabarawi, M.A. Chitosan Mucoadhesive Buccal Films: Effect of Different Casting Solvents on Their Physicochemical Properties. Int. J. Pharm. Pharm. Sci. 2016, 8, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Kumria, R.; Al-Dhubiab, B.E.; Shah, J.; Nair, A.B. Formulation and Evaluation of Chitosan-Based Buccal Bioadhesive Films of Zolmitriptan. J. Pharm. Innov. 2018, 13, 133–143. [Google Scholar] [CrossRef]
- Hyppola, R.; Husson, I.; Sundholm, F. Evaluation of physical properties of plasticized ethyl cellulose films cast from ethanol solution part 1. Int. J. Pharm. 1996, 133, 161–170. [Google Scholar] [CrossRef]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control Rel. 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bodini, R.B.; Guimarães, J.d.G.L.; Monaco-Lourenço, C.A.; Aparecida de Carvalho, R. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J. Drug Deliv. Sci. Technol. 2019, 51, 403–410. [Google Scholar] [CrossRef]
- Campisi, G.; Paderni, C.; Saccone, R.; Di Fede, O.; Wolff, A.; Giannola, L.I. Human Buccal Mucosa as an Innovative Site of Drug Delivery. Curr. Pharm. Des. 2010, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Techn. 2019, 24, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunta, J.R.; Goskonda, V.R.; Brotherton, H.O.; Khan, M.A.; Reddy, I.K. Effect of Menthol and Related Terpenes on the Percutaneous Absorption of Propranolol across Excised Hairless Mouse Skin. J. Pharm. Sci. 1997, 86, 1369–1373. [Google Scholar]
- Twarog, C.; Fattah, S.; Heade, J.; Maher, S.; Fattal, E.; Brayden, D.J. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics 2019, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Marciello, M.; Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Caramella, C. Chitosan Ascorbate: A Chitosan Salt with Improved Penetration Enhancement Properties Chitosan Ascorbate as Penetration Enhancer. Pharm. Dev. Techn. 2008, 13, 513–552. [Google Scholar] [CrossRef]
- Caramella, C.; Ferrari, F.; Bonferoni, M.C.; Rossi, S.; Sandri, G. Chitosan and its derivatives as drug penetration enhancers. J. Drug Deliv. Sci. Technol. 2010, 20, 5–13. [Google Scholar] [CrossRef]
- Jovanović, M.; Tomić, N.; Cvijić, S.; Stojanović, D.; Ibrić, S.; Uskoković, P. Mucoadhesive Gelatin Buccal Films with Propranolol Hydrochloride: Evaluation of Mechanical, Mucoadhesive, and Biopharmaceutical Properties. Pharmaceutics 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, A.; Gottnek, M.; Regdon, G., Jr.; Pintye-Hódi, K. New equipment for measurement of the force of adhesion of mucoadhesive films. J. Adhes. Sci. Technol. 2015, 29, 1360–1367. [Google Scholar] [CrossRef] [Green Version]
- Pintye-Hódi, K.; Regdon, G., Jr.; Erős, I.; Süvegh, K.; Marek, T.; Kéry, I.; Zelkó, R. Metolose–PEG interaction as seen by positron annihilation spectroscopy. Int. J. Pharm. 2006, 313, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabó, B.; Zelkó, R. A pozitron annihilációs élettartam spektroszkópia és gyógyszerészeti alkalmazása. Acta Pham. Hung. 2012, 82, 23–32. [Google Scholar]
- Süvegh, K.; Vértes, A.; Hyodo, T. Positronium as a sensitive detector of changes in molecular structure. Adv. Mol. Struct. Res. 1999, 5, 313–357. [Google Scholar]
- Bajdik, J.; Fehér, M.; Pintye-Hódi, K. Effect of plasticizer on surface of free films prepared from aqueous solutions of salts of cationic polymers with different plasticizers. Appl. Surf. Sci. 2007, 253, 7303–7308. [Google Scholar] [CrossRef]
- Buckton, G. Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Adv. Drug Deliver. Rev. 1997, 26, 17–27. [Google Scholar] [CrossRef]
- Kristó, K.; Bajdik, J.; Kleinebudde, P.; Pintye-Hódi, K. Effect of lubricant on spreading of coating liquid on surface of tablets containing pancreatin. Pharm. Dev. Techn. 2010, 15, 354–359. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- Ström, G. Krüss Users’ Manual. Krüss. K121. In Contact Angle and Adsorption Measuring System; Krüss GmbH: Hamburg, Germany, 1996; pp. 50–77. [Google Scholar]
- Elzayat, E.M.; Abdel-Rahman, A.A.; Ahmed, S.M.; Alanazi, F.K.; Habib, W.A.; Sakr, A. Studying the impact of formulation and processing parameters on the release characteristics from hydroxypropyl methylcellulose matrix tablets of diclofenac. Acta Pol. Pharm. 2016, 73, 439–452. [Google Scholar]
- Douroumis, D.; Gryczke, A.; Schminke, S. Development and Evaluation of Cetirizine HCl Taste-Masked Oral Disintegrating Tablets. Aaps Pharmscitech 2010, 12, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and Antioxidant Properties of Edible Chitosan Ascorbate Films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Liping, L.; Kexin, L.; Huipu, D.; Jia, L.; Jie, Z. Study on Preparation of a Chitosan/Vitamin C Complex and Its Properties in Cosmetics. Nat. Prod. Commun. 2020, 15, 1934578X20946876. [Google Scholar] [CrossRef]












| Sample | Ascorbic Acid (w/w%) | Chitosan (w/w%) | Glycerine (w/w%) | Acetic Acid (w/w%) | Active Agent (w/w%) |
|---|---|---|---|---|---|
| Reference (Ref) | - | 2 | 1 | 2 | - |
| Film 2 | 2 | 2 | 1 | - | - |
| Film 3 | 3 | 2 | 1 | - | - |
| Film 4 | 4 | 2 | 1 | - | - |
| Film 5 | 5 | 2 | 1 | - | - |
| Cetirizine film | 2 | 2 | 1 | - | 0.5 |
| Diclofenac film | 2 | 2 | 1 | - | 0.5 |
| Sample | γtot (mN/m) | γd (mN/m) | γp (mN/m) |
|---|---|---|---|
| Ref | 43.87 | 30.55 | 13.53 |
| Film 2 | 70.32 | 35.35 | 34.98 |
| Film 3 | 66.53 | 37.53 | 29.01 |
| Film 4 | 60.04 | 31.31 | 28.73 |
| Film 5 | 56.93 | 31.13 | 25.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristó, K.; Módra, S.; Hornok, V.; Süvegh, K.; Ludasi, K.; Aigner, Z.; Kelemen, A.; Sovány, T.; Pintye-Hódi, K.; Regdon, G., Jr. Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid. Pharmaceutics 2022, 14, 345. https://doi.org/10.3390/pharmaceutics14020345
Kristó K, Módra S, Hornok V, Süvegh K, Ludasi K, Aigner Z, Kelemen A, Sovány T, Pintye-Hódi K, Regdon G Jr. Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid. Pharmaceutics. 2022; 14(2):345. https://doi.org/10.3390/pharmaceutics14020345
Chicago/Turabian StyleKristó, Katalin, Szilvia Módra, Viktória Hornok, Károly Süvegh, Krisztina Ludasi, Zoltán Aigner, András Kelemen, Tamás Sovány, Klára Pintye-Hódi, and Géza Regdon, Jr. 2022. "Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid" Pharmaceutics 14, no. 2: 345. https://doi.org/10.3390/pharmaceutics14020345
APA StyleKristó, K., Módra, S., Hornok, V., Süvegh, K., Ludasi, K., Aigner, Z., Kelemen, A., Sovány, T., Pintye-Hódi, K., & Regdon, G., Jr. (2022). Investigation of Surface Properties and Free Volumes of Chitosan-Based Buccal Mucoadhesive Drug Delivery Films Containing Ascorbic Acid. Pharmaceutics, 14(2), 345. https://doi.org/10.3390/pharmaceutics14020345