Numerical Investigation on the Anti-Angiogenic Therapy-Induced Normalization in Solid Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Geometry
2.2. Schematic View of AAIN Function
2.3. Governing Equations
2.3.1. Fluid Flow Mathematical Model
2.3.2. Solute Transport Mathematical Model
2.4. Numerical Solution Details
2.4.1. Mesh Independent Solution
2.4.2. Boundary Conditions
2.4.3. Numerical Modeling Procedure
2.5. Baseline Value of Parameters
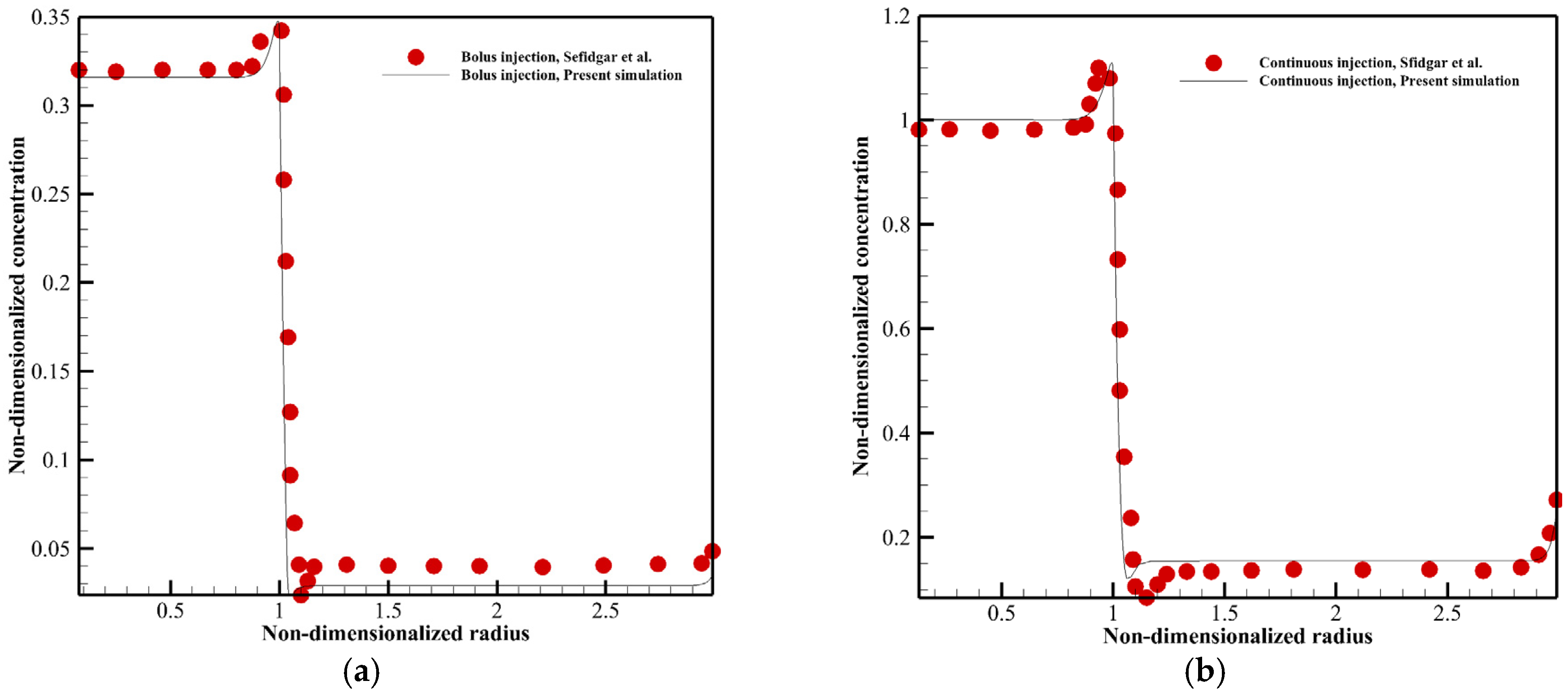
3. Validation of Numerical Model
4. Results and Discussion
4.1. Fluid Flow Analysis
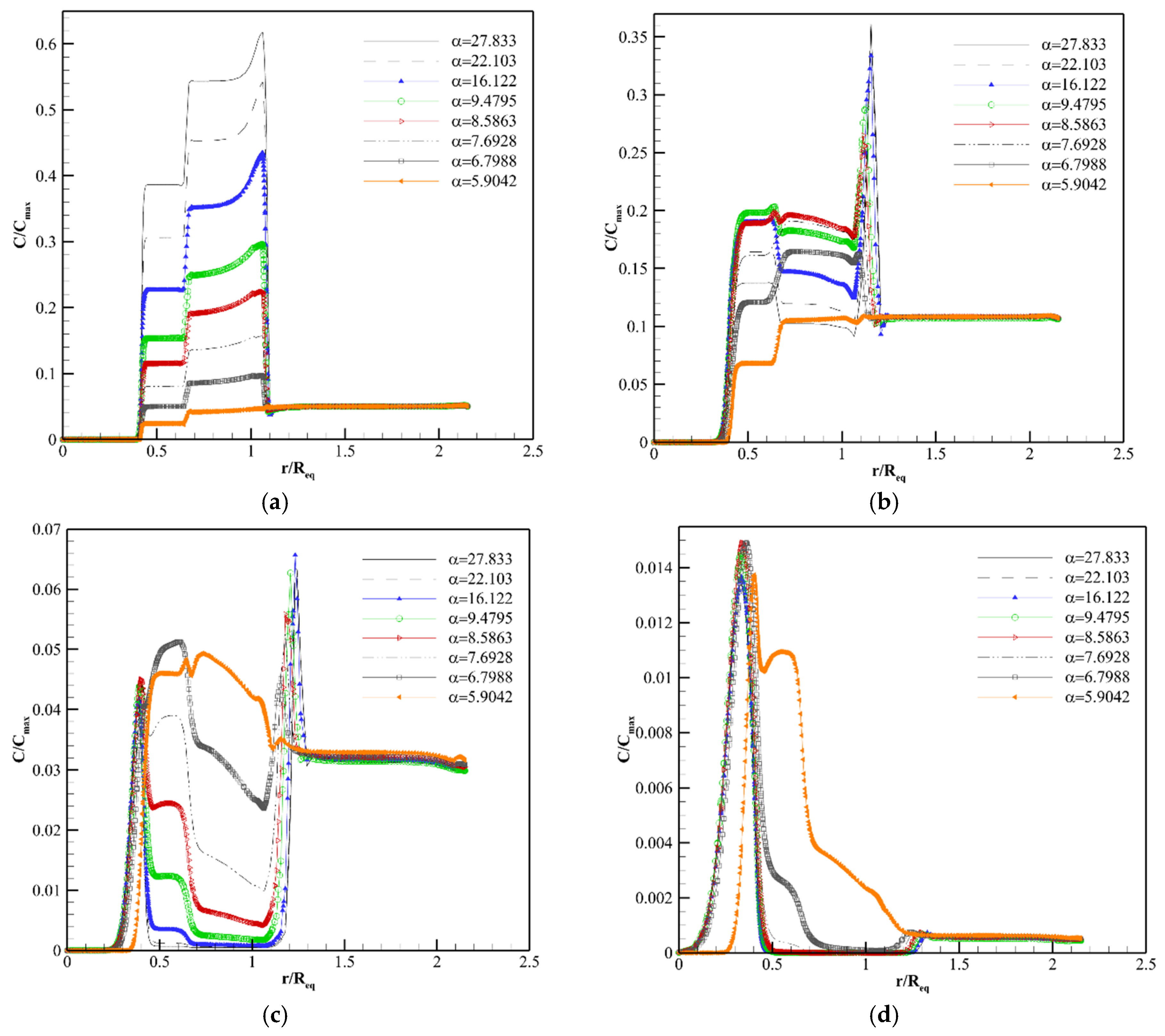
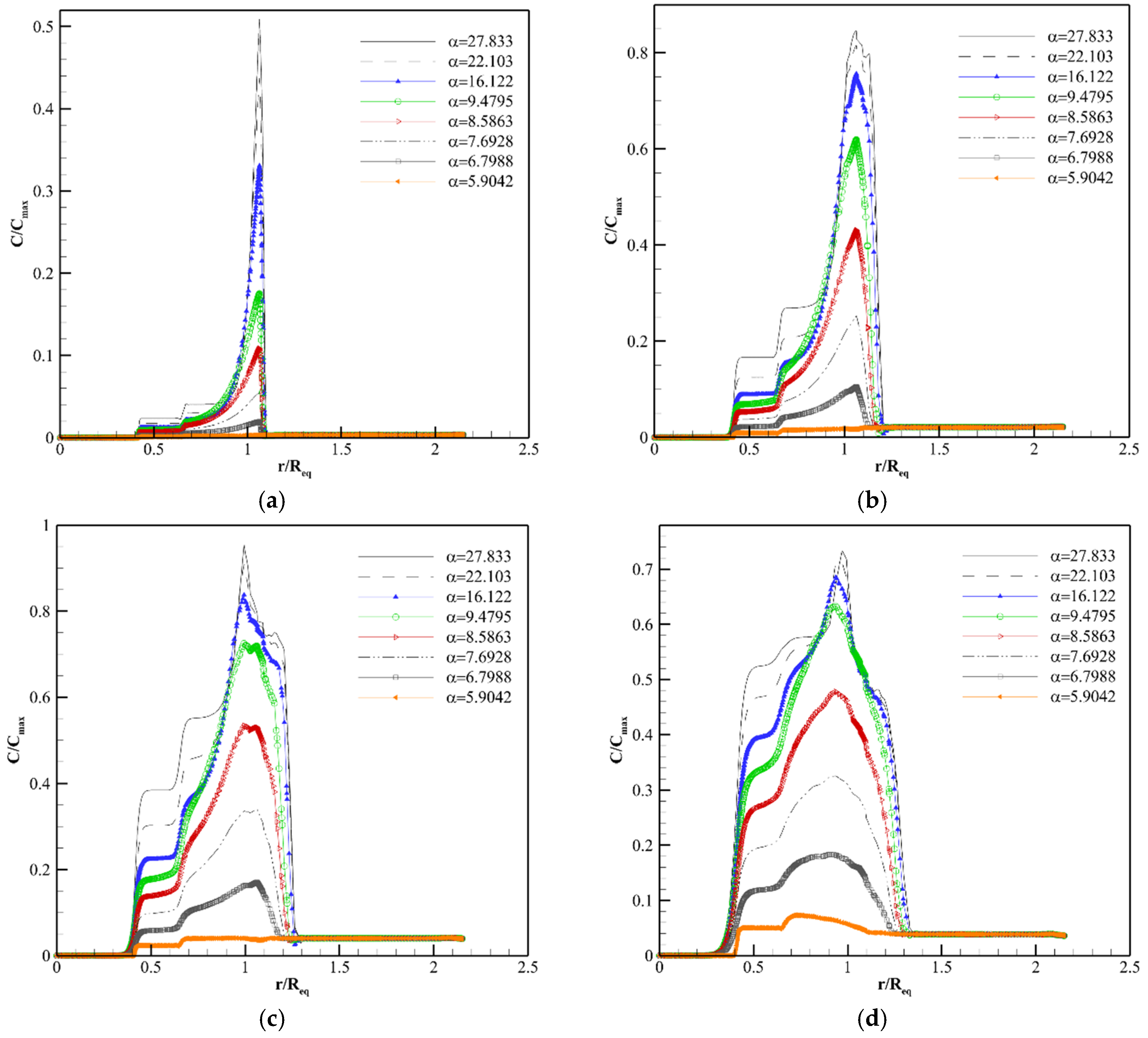
4.2. Solute Transport Analysis
4.3. Limitations and Future Works
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, A.R.A.; Quaranta, V. Integrative mathematical oncology. Nat. Cancer 2008, 8, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Byrne, H.M. Dissecting cancer through mathematics: From the cell to the animal model. Nat. Cancer 2010, 10, 221–230. [Google Scholar] [CrossRef]
- Altrock, P.M.; Liu, L.L.; Michor, F. The mathematics of cancer: Integrating quantitative models. Nat. Cancer 2015, 15, 730–745. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Nivlouei, S.J.; Soltani, M.; Carvalho, J.; Travasso, R.; Salimpour, M.R.; Shirani, E. Multiscale modeling of tumor growth and angiogenesis: Evaluation of tumor-targeted therapy. PLoS Comput. Biol. 2021, 17, e1009081. [Google Scholar] [CrossRef]
- Nikmaneshi, M.R.; Firoozabadi, B.; Mozafari, A.; Munn, L.L. A multi-scale model for determining the effects of pathophysiology and metabolic disorders on tumor growth. Sci. Rep. 2020, 10, 3025. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-Y.; Chang, W.-I.; Horng, T.-L.; Lin, W.-L. Numerical modeling of nanodrug distribution in tumors with heterogeneous vasculature. PLoS ONE 2017, 12, e0189802. [Google Scholar] [CrossRef] [Green Version]
- D’Esposito, A.; Sweeney, P.W.; Ali, M.; Saleh, M.; Ramasawmy, R.; Roberts, T.A.; Agliardi, G.; Desjardins, A.; Lythgoe, M.F.; Pedley, R.B.; et al. Computational fluid dynamics with imaging of cleared tissue and of in vivo perfusion predicts drug uptake and treatment responses in tumours. Nat. Biomed. Eng. 2018, 2, 773–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashkooli, F.M.; Soltani, M.; Rezaeian, M.; Taatizadeh, E.; Hamedi, M.-H. Image-based spatio-temporal model of drug delivery in a heterogeneous vasculature of a solid tumor—Computational approach. Microvasc. Res. 2019, 123, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W. Effects of Focused-Ultrasound-and-Microbubble-Induced Blood-Brain Barrier Disruption on Drug Transport under Liposome-Mediated Delivery in Brain Tumour: A Pilot Numerical Simulation Study. Pharmaceutics 2020, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Kashkooli, F.M.; Soltani, M.; Hamedi, M.-H. Drug delivery to solid tumors with heterogeneous microvascular networks: Novel insights from image-based numerical modeling. Eur. J. Pharm. Sci. 2020, 151, 105399. [Google Scholar] [CrossRef] [PubMed]
- Kashkooli, F.M.; Soltani, M.; Momeni, M.M. Computational modeling of drug delivery to solid tumors: A pilot study based on a real image. J. Drug Deliv. Sci. Technol. 2021, 62, 102347. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M. Computational modeling of thermal combination therapies by magneto-ultrasonic heating to enhance drug delivery to solid tumors. Sci. Rep. 2021, 11, 19539. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.; Wijeratne, P.A.; Vavourakis, V. From Tumour Perfusion to Drug Delivery and Clinical Translation of in Silico Cancer Models. Methods 2021, 185, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M. Numerical Modeling of Drug Delivery to Solid Tumor Microvasculature. PhD Thesis, University of Waterloo, Waterloo, ON, Canada, 2013. [Google Scholar]
- Bodzioch, M.; Bajger, P.; Forys, U. Angiogenesis and Chemotherapy Resistance: Optimizing Chemotherapy Scheduling Using Mathematical Modeling. J. Cancer Res. Clin. Oncol. 2021, 147, 2281–2299. [Google Scholar] [CrossRef] [PubMed]
- Jain, R. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Baxter, L.; Jain, R. Transport of Fluid and Macromolecules in Tumors I. Role of Interstitial Pressure and Convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Baxter, L.; Jain, R. Transport of Fluid and Macromolecules in Tumors II. Role of Heterogeneous Perfusion and Lymphatics. Microvasc. Res. 1990, 40, 246–263. [Google Scholar] [CrossRef]
- Baxter, L.T.; Jain, R.K. Transport of Fluid and Macromolecules in Tumors III Role of Binding and Metabolism. Microvasc. Res. 1991, 41, 5–23. [Google Scholar] [CrossRef]
- Soltani, M.; Chen, P. Numerical Modeling of Fluid Flow in Solid Tumors. PLoS ONE 2011, 6, e20344. [Google Scholar] [CrossRef]
- Soltani, M.; Chen, P. Effect of Tumor Shape and Size on Drug Delivery to Solid Tumors. J. Biol. Eng. 2012, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Sefidgar, M.; Soltani, M.; Raahemifar, K.; Bazmara, H.; Nayinian, S.M.M.; Bazargan, M. Effect of Tumor Shape, Size, and Tissue Transport Properties on Drug Delivery to Solid Tumors. J. Biol. Eng. 2014, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Steuperaert, M.; Labate, G.F.; Debbaut, C.; Wever, O.D.; Vanhove, C.; Ceelen, W.; Segers, P. Mathematical Modeling of Intraperitoneal Drug Delivery: Simulation of Drug Distribution in a Single Tumor Nodule. Drug Deliv. 2017, 24, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steuperaert, M.; Debbaut, C.; Carlier, C.; Wever, O.D.; Descamps, B.; Vanhove, C.; Segers, P. A 3D CFD Model of the Interstitial Fluid Pressure and Drug Distribution in Heterogeneous Tumor Nodules during Intraperitoneal Chemotherapy. Drug Deliv. 2019, 26, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Sefidgar, M.; Soltani, M.; Raahemifar, K.; Sadeghi, M.; Bazmara, H.; Bazargan, M.; Mousavi Naeenian, M. Numerical Modeling of Drug Delivery in a Dynamic Solid Tumor Microvasculature. Microvasc. Res. 2015, 99, 43–56. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M. Evaluation of Solid Tumor Response to Sequential Treatment Cycles via a New Computational Hybrid Approach. Sci. Rep. 2021, 11, 21475. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. The Next Frontier of Molecular Medicine: Delivery of Therapeutics. Nat. Med. 1998, 4, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Rajora, A.K.; Ravishankar, D.; Zhang, H.; Rosenholm, J.M. Recent Advances and Impact of Chemotherapeutic and Antiangiogenic Nanoformulations for Combination Cancer Therapy. Pharmaceutics 2020, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Amit, L.; Ben-Aharon, I.; Vidal, L.; Leibovici, L.; Stemmer, S.M. The Impact of Bevacizumab (Avastin) on Survival in Metastatic Solid Tumors- A Meta-Analysis and Systematic Review. PLoS ONE 2013, 8, e51780. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a Humanized Anti-VEGF Monoclonal Antibody for Cancer Therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Pian, L.; Chen, Z.; Jing, C.; Ruiguang, Z.; Jinghua, R.; Yuhui, H.; Gang, W. Combinational Therapy of Interferon-α and Chemotherapy Normalizes Tumor Vasculature by Regulating Pericytes Including The Novel Marker RGS5 in Melanoma. J. Immunother. 2011, 34, 320–326. [Google Scholar]
- Grossman, R.; Brastianos, H.; Blakeley, J.O.; Mangraviti, A.; Lal, B.; Zadnik, P.; Tyler, B. Combination of Anti-VEGF Therapy and Temozolomide in Two Experimental Human Glioma Models. J. Neuro. Oncol. 2014, 116, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, Y.; Ogawara, K.-I.; Fushimi, A.; Abe, S.; Ishikawa, K.; Araki, T.; Higaki, K. Deeper Penetration into Tumor Tissues and Enhanced in vivo Antitumor Activity of Liposomal Paclitaxel by Pretreatment with Angiogenesis Inhibitor SU5416. Mol. Pharm. 2012, 9, 3486–3494. [Google Scholar] [CrossRef] [PubMed]
- Gremonprez, F.; Descamps, B.; Izmer, A.; Vanhove, C.; Vanhaecke, F.; Wever, O.D.; Ceelen, W. Pretreatment with VEGF(R)-Inhibitors Reduces Interstitial fluid pressure, Increases Intraperitoneal Chemotherapy, Drug Penetration, and Impedes Tumor Growth in a Mouse Colorectal Carcinomatosis Model. Oncotarget 2015, 6, 29889–29900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escorcia, F.E.; Henke, E.; McDevitt, M.R.; Villa, C.H.; Smith-Jones, P.; Blasberg, R.G.; Scheinberg, D.A. Selective Killing of Tumor Neovasculature Paradoxically Improves Chemotherapy Delivery to Tumors. Cancer Res. 2010, 70, 9277–9286. [Google Scholar] [CrossRef] [Green Version]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a Hallmark of Solid Tumors-Clinical Perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, L.; Li, Y.; Liu, J.; Liu, Y. Nano Drug Delivery System Reconstruct Tumour Vasculature for The Tumour Vascular Normalisation. J. Drug Target. 2021, 30, 119–130. [Google Scholar] [CrossRef]
- Liang, P.; Ballou, B.; Lv, X.; Si, W.; Bruchez, M.P.; Huang, W.; Dong, X. Monotherapy and Combination Therapy Using Anti-Angiogenic Nanoagents to Fight Cancer. Adv. Mater. 2021, 33, 2005155. [Google Scholar] [CrossRef]
- Jain, R.K.; Tong, R.T.; Munn, L.L. Effect of Vascular Normalization by Antiangiogenic Therapy on Interstitial Hypertension, Peritumor Edema, and Lymphatic Metastasis: Insights from a Mthematical Model. Cancer Res. 2007, 67, 2729–2735. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Aghanajafi, C.; Soltani, M. Numerical Modelling of Drug Delivery in an Isolated Solid Tumor under the Influence of Vascular Normalization. In Recent Developments in Mathematical, Statistical and Computational Sciences; Kilgour, D.M., Kunze, H., Makarov, R., Melnik, R., Wang, X., Eds.; Springer Proceedings in Mathematics and Statistics; Springer: Cham, Switzerland, 2021; Volume 343. [Google Scholar]
- Zhan, W. Convection Enhanced Delivery of Anti-Angiogenic and Cytotoxic Agents in Combination Therapy against Brain Tumour. Eur. J. Pharm. Sci. 2020, 141, 105094. [Google Scholar] [CrossRef]
- Yonucu, S.; Yilmaz, D.; Phipps, C.; Unlu, M.B.; Kohandel, M. Quantifying the Effects of Antiangiogenic and Chemotherapy Drug Combinations on Drug Delivery and Treatment Efficacy. PLoS Comput. Biol. 2017, 13, e1005724. [Google Scholar] [CrossRef] [PubMed]
- Meaney, C.; Rhebergen, S.; Kohandel, M. In Silico Analysis of Hypoxia Activated Prodrugs in Combination with Anti Angiogenic Therapy through Nanocell Delivery. PLoS Comput. Biol. 2020, 16, e1007926. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, P.W.; d’Esposito, A.; Walker-Samuel, S.; Shipley, R.J. Modelling the Transport of Fluid through Heterogeneous, Whole Tumours in Silico. PLoS Comput. Biol. 2019, 15, e1006751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Long, Q.; Xu, S.; Padhani, A.R. Study of Tumor Blood Perfusion and its Variation Due to Vascular Normalization by Anti-Angiogenic Therapy Based on 3D Angiogenic Microvasculature. J. Biomech. 2009, 42, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Moath, A.; Xiao, Y.X. The Influence of Tumour Vasculature on Fluid Flow in Solid Tumours: A Mathematical Modelling Study. Biophys. Rep. 2021, 7, 35–54. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Jain, R.K. Combining Two Strategies to Improve Perfusion and Drug Delivery in Solid Tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 18632–18637. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Cao, J.; Zhang, P.; Liu, Y.; Cheng, H. Coupled Hybrid Continuum-Discrete Model of Tumor Angiogenesis and Growth. PLoS ONE 2016, 11, e0163173. [Google Scholar]
- Swabb, E.A.; Wei, J.; Gullino, P.M. Diffusion and Convection in Normal and Neoplastic Tissues. Cancer Res. 1974, 34, 2814–2822. [Google Scholar]
- Patlak, C.S.; Goldstein, D.A.; Hoffman, J.F. The Flow of Solute and Solvent across a Two-Membrane System. J. Theor. Biol. 1963, 5, 426–442. [Google Scholar] [CrossRef]
- Orlanski, I. A Simple Boundary Condition for Unbounded Hyperbolic Flows. J. Comput. Phys. 1976, 21, 251–269. [Google Scholar] [CrossRef]
- Rippe, B.; Haraldsson, B. Capillary Permeability in Rat Hindquarters as Determined by Estimations of Capillary Reflection Coefficients. Acta Physiol. Scand. 1986, 127, 289–303. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of Molecules across Tumor Vasculature. Cancer Metastasis Rev. 1987, 6, 559–593. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.G.; Boucher, Y.; Tomaso, E.D.; Duda, D.G.; Munn, L.L.; Tong, R.T.; Jain, R.K. Direct Evidence that the VEGF-Specific Antibody Bevacizumab Has Antivascular Effects in Human Rectal Cancer. Nat. Med. 2004, 10, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Okuhata, Y.; Wolfe, A.; Sirvastav, S.; Oie, S.; Ferrara, N.; Brasch, R.C. Decrease in Tumor Apparent Permeability-Surface Area Product to a MRI Macromolecular Contrast Medium Following Angiogenesis Inhibition with Correlations to Cytotoxic Drug Accumulation. Microcirculation 2004, 11, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular Normalization by Vascular Endothelial Growth Factor Receptor 2 Blockade Induces Pressure Gradients across the Vasculature and Improves Drug Penetration in Tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, K.; Perl, W. Osmotic Reflection Coefficients of Canine Subcutaneous Adipose Tissue Endothelium. Microvasc. Res. 1978, 16, 224–236. [Google Scholar] [CrossRef]
- Anderson, J.L.; Malone, D.M. Mechanism of Osmotic Flow in Porous Membranes. Biophys. J. 1974, 1974. 14, 957–982. [Google Scholar] [CrossRef] [Green Version]
- Deen, W.M. Hindered Transport of Large Molecules in Liquid-Filled Pores. AIChE J. 1987, 33, 1409–1425. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of Molecules in the Tumor Interstitium: A Review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Covell, D.G.; Barbet, J.; Holton, O.D.; Balck, C.D.; Parker, R.J.; Weinstein, J.N. Pharmacokinetics of monoclonal immunoglobulin G1, F (ab′) 2, and Fab′ in mice. Cancer Res. 1986, 46, 3969–3978. [Google Scholar]
- Gerlowski, L.E.; Jain, R.K. Microvascular Permeability of Normal and Neoplastic Tissues. Microvasc. Res. 1986, 31, 288–305. [Google Scholar] [CrossRef]
- Ozturk, D.; Yonucu, S.; Yilmaz, D.; Unlu, M.B. Influence of Vascular Normalization on Interstitial Flow and Delivery of Liposomes in Tumors. Phys. Med. Biol. 2015, 60, 1477–1496. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Stemmer-Rachamimov, A.O.; Barker II, F.G.; Halpin, C.; Padera, T.P.; Tyrrell, A.; Tomaso, E.D. Hearing Improvement after Bevacizumab in Patients with Neurofibromatosis Type 2. N. Engl. J. Med. 2009, 361, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Pishko, G.L.; Astary, G.W.; Mareci, T.H.; Sarntinoranont, M. Sensitivity Analysis of an Image-Based Solid Tumor Computational Model with Heterogeneous Vasculature and Porosity. Ann. Biomed. Eng. 2011, 39, 2360–2373. [Google Scholar] [CrossRef] [Green Version]
- Taghian, A.G.; Abi-Raad, R.; Assaad, S.I.; Casty, A.; Ancukiewicz, M.; Yeh, E.; Powell, S.N. Paclitaxel Decreases the Interstitial Fluid Pressure and Improves Oxygenation in Breast Cancers in Patients Treated With Neoadjuvant Chemotherapy: Clinical Implications. J. Clin. Oncol. 2005, 23, 1951–1961. [Google Scholar] [CrossRef]
- Ferretti, S.; Allegrini, P.R.; O′Reilly, T.; Schnell, C.; Stumm, M.; Wartmann, M.; McSheehy, P.M. Patupilone Induced Vascular Disruption in Orthotopic Rodent Tumor Models Detected by Magnetic Resonance Imaging and Interstitial Fluid Pressure. Clin. Cancer Res. 2005, 11, 7773–7784. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Du, W.; Fu, F.; Yuan, L.; Wu, H.; Dai, W.; Zhang, Q. The Reduction of Tumor Interstitial Fluid Pressure by Liposomal Imatinib and its Effect on Combination Therapy with Liposomal Doxorubicin. Biomaterials 2013, 34, 2277–2288. [Google Scholar] [CrossRef]
- Jain, R. Physiological Barriers to Delivery of Monoclonal Antibodies and other Macromolecules in Tumors. Cancer Res. 1990, 50, 814–819. [Google Scholar]
- Browder, T.; Butterfield, C.E.; Kräling, B.M.; Shi, B.; Marshall, B.; O’Reilly, M.S.; Folkman, J. Antiangiogenic Scheduling of Chemotherapy Improves Efficacy against Experimental Drug-resistant Cancer. Cancer Res. 2000, 60, 1878–1886. [Google Scholar]
- Teicher, B.A. A systems approach to cancer therapy. Cancer Metastasis Rev. 1996, 15, 247–272. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor Microvasculature and Microenvironment: Targets for Anti-angiogenesis and Normalization. Microvasc. Res. 2007, 74, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Kashkooli, F.M.; Soltani, M.; Momeni, M.M.; Rahmim, A. Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework. Front. Oncol. 2021, 11, 655781. [Google Scholar] [CrossRef]
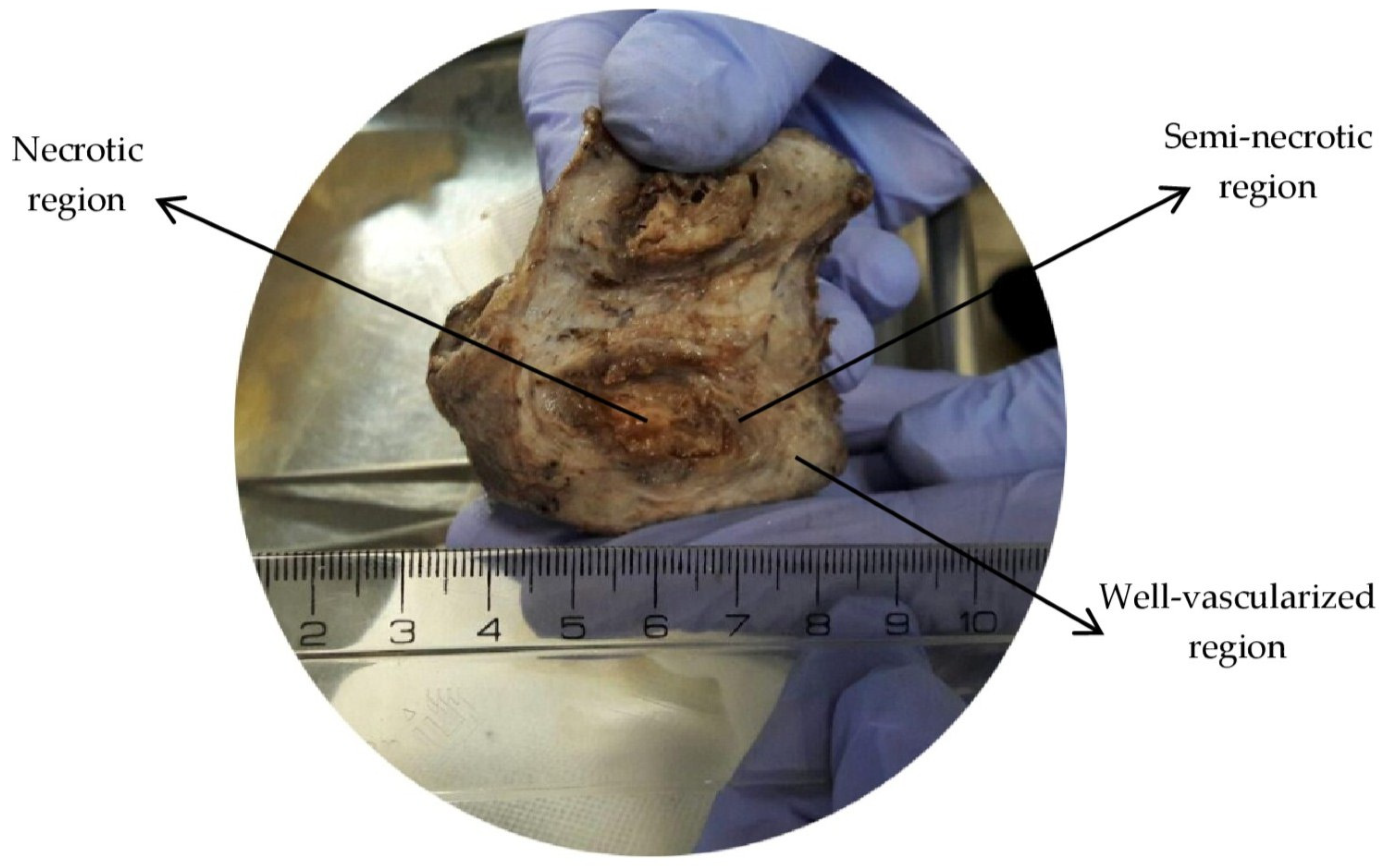
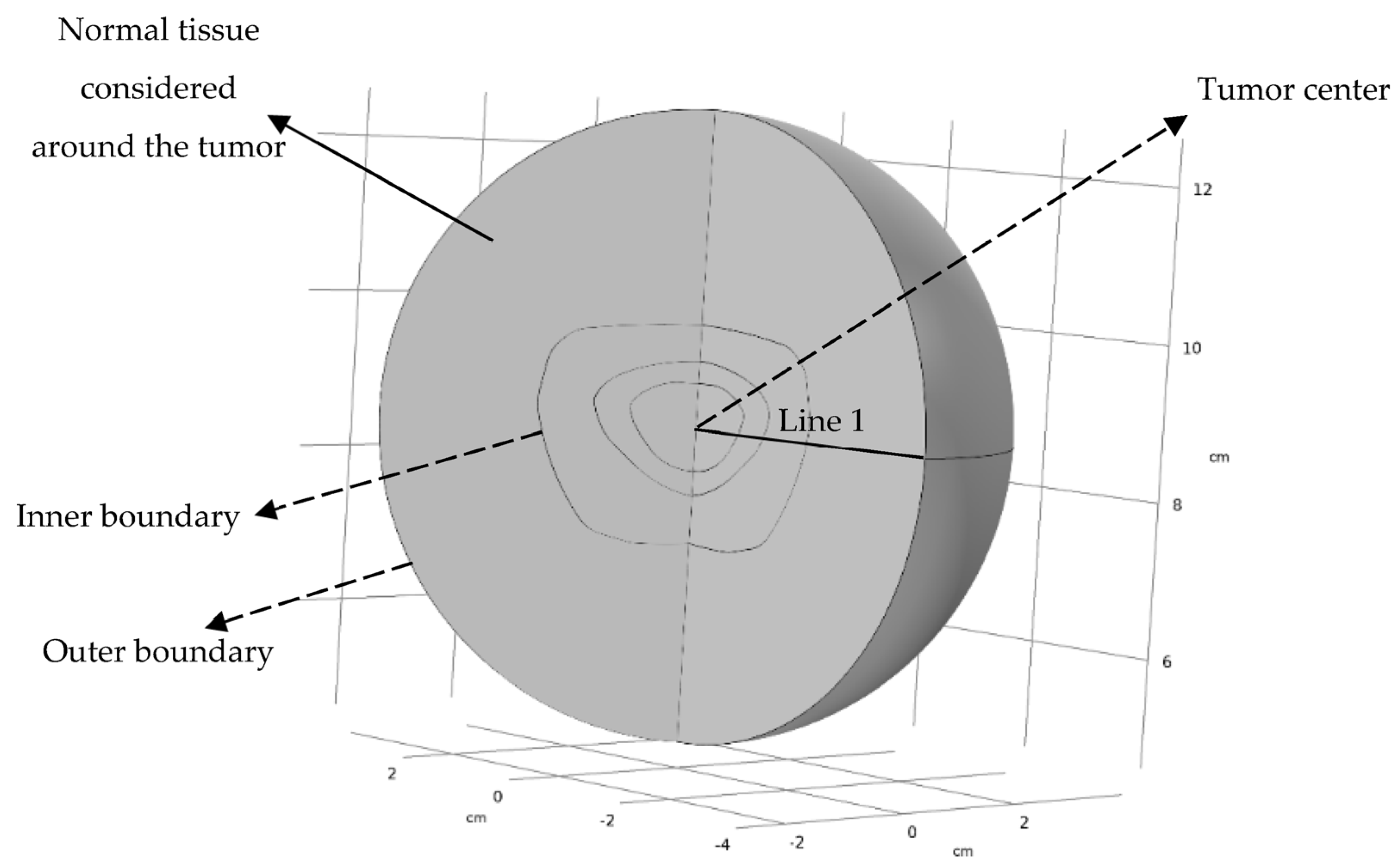

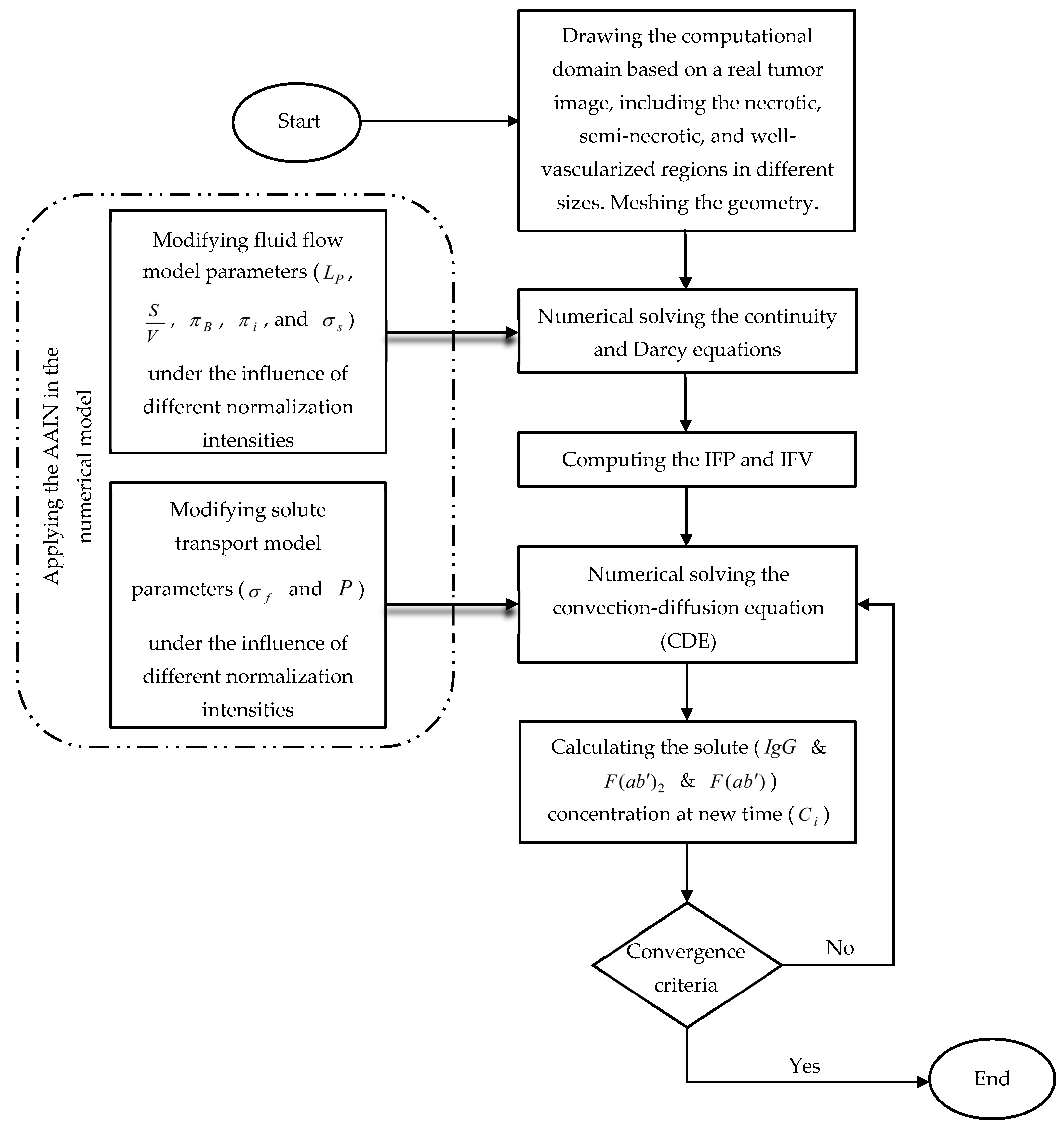
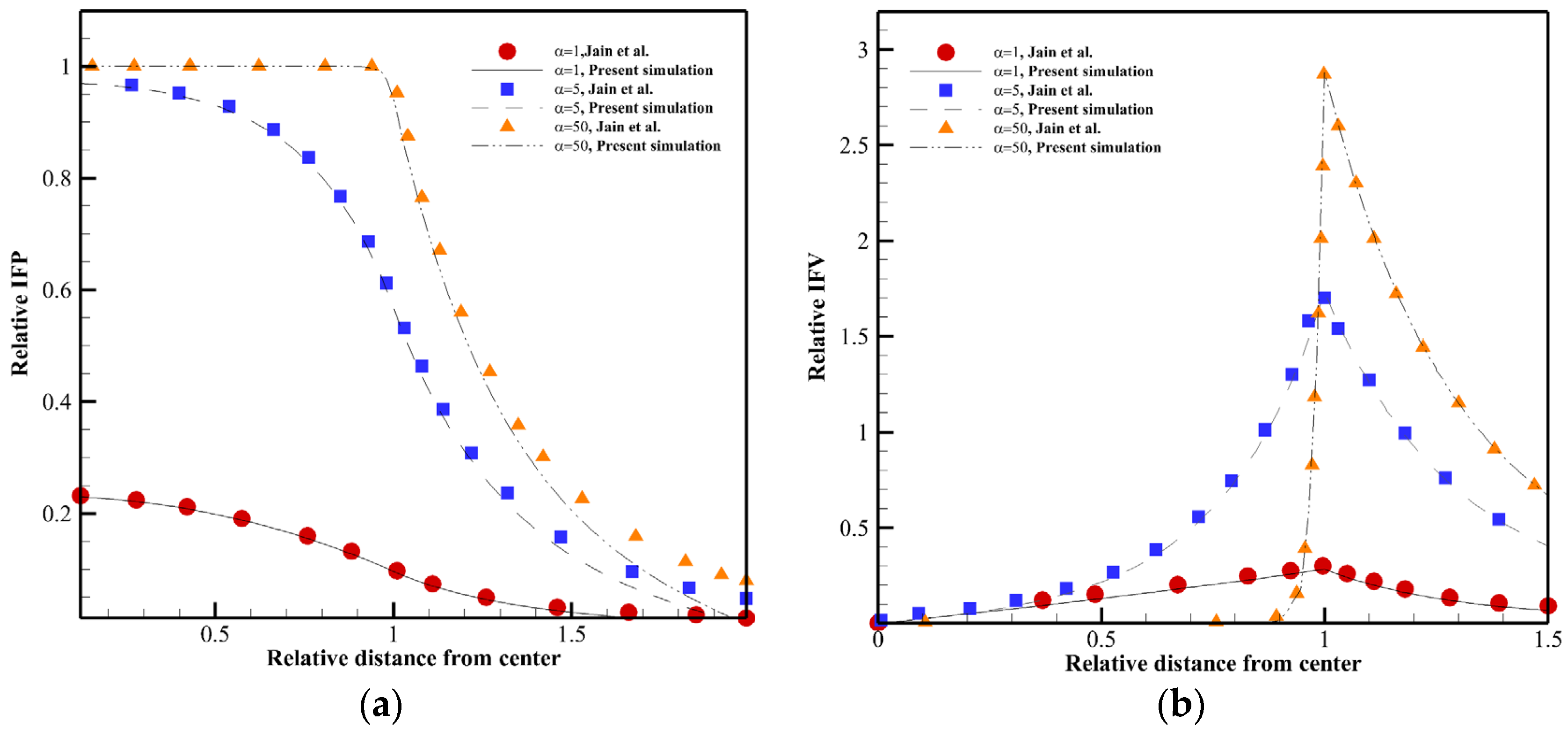









| Variable | Description | Equation | Zone(s) under the Influence of Variable |
|---|---|---|---|
| 1,3 | Source term of the fluid flow analysis | Tumor and Normal tissues | |
| 1 | Sink term of the fluid flow analysis | Normal tissue | |
| 1,2,3 | Source term of the solute transport analysis | Tumor and Normal tissues | |
| Sink term of the solute transport analysis | Normal tissue |
| Zone | Tumor Center | Inner Boundary | Outer Boundary |
|---|---|---|---|
| Fluid flow | 1 1 | ||
| Solute transport | 1 1 | 2 |
| Parameter | Description | Normal Tissue | Normalized Tissue | Tumor Tissue | Reference(s) |
|---|---|---|---|---|---|
| Hydraulic conductivity of the microvascular wall | [18,40,53] | ||||
| Hydraulic conductivity of the interstitium | [40] | ||||
| Surface area of vessel wall per unit volume of tissue | 70 | 116 a | 200 | [22,23,55,56] | |
| Vascular pressure | 15.6 | 15.6 | 15.6 | [22,23] | |
| Osmotic pressure of the plasma | 20 | 19.2 | 19.8 | [40] | |
| Osmotic pressure of the interstitial fluid | 10 | 15.1 | 17.3 | [40] | |
| Average osmotic reflection coefficient for plasma proteins | [58] and calculated based on [59,60] | ||||
| Hydrostatic pressure of the lymphatics | 0 | - | - | [66] | |
| Product of hydraulic conductivity of the lymphatic wall and surface area of lymphatic wall per unit volume of tissue | - | - | [66] | ||
| Osmotic filtration reflection coefficient | [62] and calculated based on [59,60] | ||||
| Effective diffusion coefficient | [63] | ||||
| Microvessel permeability coefficient | [23,63,65] | ||||
| b | Drug half-life in plasma | [18] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, M.; Aghanajafi, C.; Soltani, M.; Raahemifar, K. Numerical Investigation on the Anti-Angiogenic Therapy-Induced Normalization in Solid Tumors. Pharmaceutics 2022, 14, 363. https://doi.org/10.3390/pharmaceutics14020363
Mohammadi M, Aghanajafi C, Soltani M, Raahemifar K. Numerical Investigation on the Anti-Angiogenic Therapy-Induced Normalization in Solid Tumors. Pharmaceutics. 2022; 14(2):363. https://doi.org/10.3390/pharmaceutics14020363
Chicago/Turabian StyleMohammadi, Mahya, Cyrus Aghanajafi, M. Soltani, and Kaamran Raahemifar. 2022. "Numerical Investigation on the Anti-Angiogenic Therapy-Induced Normalization in Solid Tumors" Pharmaceutics 14, no. 2: 363. https://doi.org/10.3390/pharmaceutics14020363







