Bone Morphogenetic Protein-, Antimicrobial Agent-, and Analgesic-Incorporated Nanofibrous Scaffolds for the Therapy of Alveolar Clefts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacture of Growth-Factor- and Drug-Loaded Scaffolds
2.2. Scanning Electron Microscopy
2.3. Identification of the Sheath–Core Structure
2.4. Differential Scanning Calorimetry (DSC)
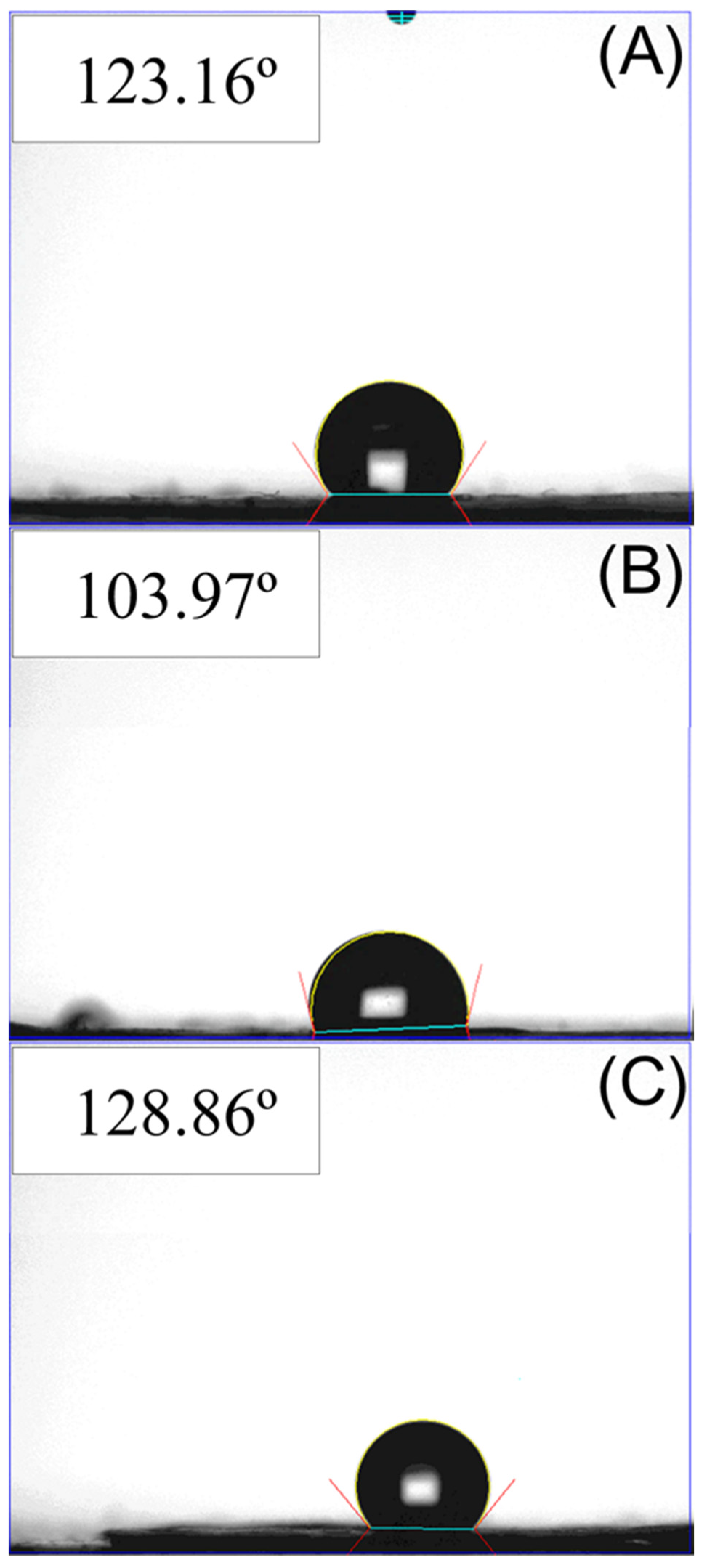
2.5. Wetting Angle Measurement
2.6. In Vitro Drug and Biomolecule Release
2.7. In vivo Animal Test
2.8. Postoperative Activity
2.9. Micro-CT Examination and Bone Growth Evaluation
2.10. Statistical Analysis
3. Results
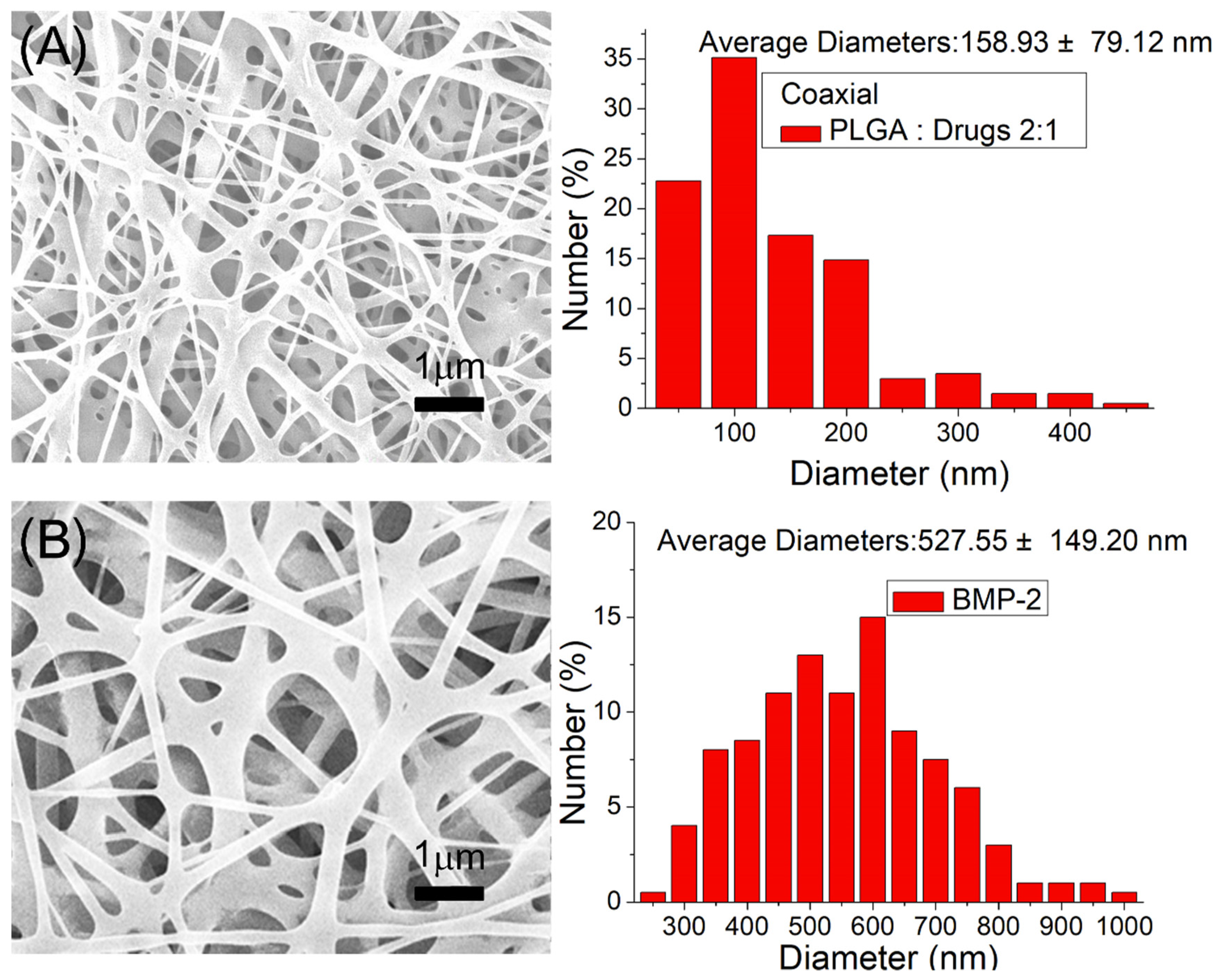
3.1. Characterization of Printed Scaffolds and Coelectrospun Nanofibers
3.2. Drug and Biomolecule Release Patterns
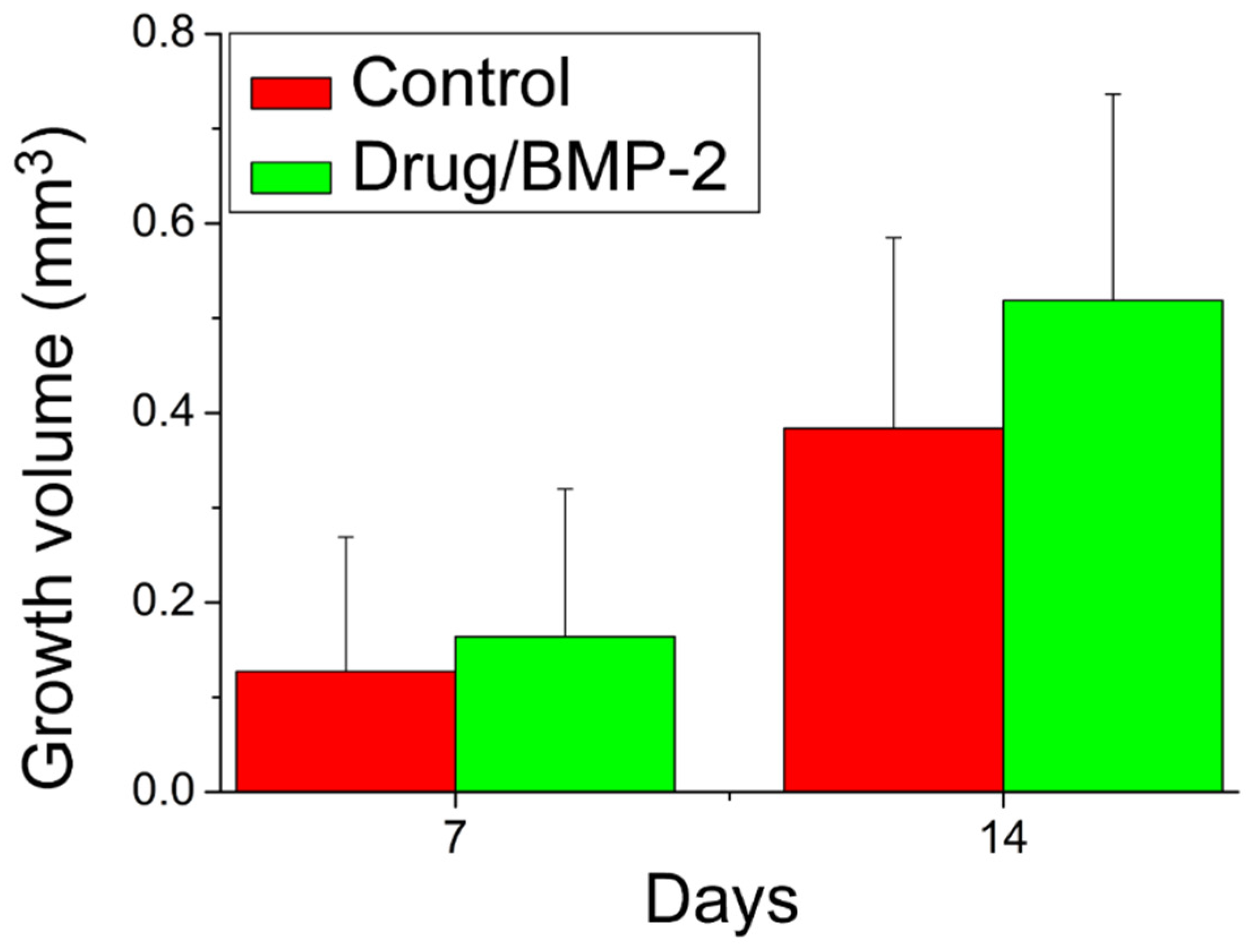
3.3. Animal Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Denadai, R.; Chou, P.Y.; Pai, B.C.; Chen, C.; Lin, C.C.H.; Huang, C.S.; Chen, Y.R.; Lo, L.J. Skeletofacial reconstruction for cleft-related deformities: Four decades of evolving cleft care. Ann. Plast Surg. 2020, 85, 3–11. [Google Scholar] [CrossRef]
- Chou, P.Y.; Denadai, R.; Hallac, R.R.; Dumrongwongsiri, S.; Hsieh, W.C.; Pai, B.C.; Lo, L.J. Comparative volume analysis of alveolar defects by 3D simulation. J. Clin. Med. 2019, 8, 1401. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.R.; Lin, Y.C.; Pai, B.C.J.; Tseng, H.J.; Lo, L.J.; Chou, P.Y. Progressive Comparison of Density Assessment of Alveolar Bone Graft in Patients with Unilateral and Bi-lateral Cleft. J. Clin. Med. 2021, 10, 5143. [Google Scholar] [CrossRef]
- Brudnicki, A.; Regulski, P.A.; Sawicka, E.; Fudalej, P.S. Alveolar Volume Following Different Timings of Secondary Bone Grafting in Patients with Unilateral Cleft Lip and Palate. A Pilot Study. J. Clin. Med. 2021, 10, 3524. [Google Scholar] [CrossRef]
- Ghassmi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.H. Current methods for the treatment of alveolar cleft. Arch. Plast. Surg. 2017, 44, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Harris, M.A.; Rossini, G.; Dunstan, C.R.; Dallas, S.L.; Feng, J.Q.; Mundy, G.R.; Harris, S.E. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 1997, 60, 283–290. [Google Scholar] [CrossRef]
- Fisher, M.; Yee, K.; Alba, B.; Tanna, N.; Bastidas, N.; Bradley, J.P. Applications of bone morphogenetic protein-2: Alternative therapies in craniofacial reconstruction. J. Craniofac. Surg. 2019, 30, 1952–1959. [Google Scholar] [CrossRef]
- Jahanbin, A.; Zarch, H.H.; Irani, S.; Eslami, N.; Kermani, H. Recombinant human bone morphogenic protein-2 combined with autogenous bone graft for reconstruction of alveolar cleft. J. Craniofac. Surg. 2019, 30, e209–e213. [Google Scholar] [CrossRef]
- Canan, L.W., Jr.; da Silva Freitas, R.; Alonso, N.; Tanikawa, D.Y.S.; Rocha, D.L.; Coelho, J.C.U. Human bone morphogenetic protein-2 use for maxillary reconstruction in cleft lip and palate patients. J. Craniofac. Surg. 2012, 23, 1627–1633. [Google Scholar] [CrossRef]
- Liu, B.; Yin, N.B.; Xiao, R.; Li, B.H.; Li, H.D.; Chen, S.X.; Li, S.L.; Wang, Y.Q. Evaluating the efficacy of recombinant human bone morphogenic protein-2 in the treatment of alveolar clefts with autologous bone grafting using computer-aided engineering techniques. Br. J. Oral. Maxillofac. Surg. 2021, 59, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Reichman, D.E.; Greenberg, J.A. Reducing surgical site infections: A review. Rev. Obstet. Gynecol. 2009, 2, 212–221. [Google Scholar] [PubMed]
- Spagnolo, A.M.; Ottria, G.; Amicizia, D.; Perdelli, F.; Cristina, M.L. Operating theatre quality and prevention of surgical site infections. J. Prev. Med. Hyg. 2013, 54, 131–137. [Google Scholar] [PubMed]
- Pogatzki-Zahn, E.M.; Segelcke, D.; Schug, S.A. Postoperative pain—From mechanisms to treatment. Pain Rep. 2017, 2, e588. [Google Scholar] [CrossRef]
- Fregoso, G.; Wang, A.; Tseng, K.; Wang, J. Transition from acute to chronic pain: Evaluating risk for chronic postsurgical pain. Pain Physician 2019, 22, 479–488. [Google Scholar] [PubMed]
- Lee, J.W.; Cho, D.-W. 3D Printing technology over a drug delivery for tissue engineering. Curr. Pharm. Des. 2015, 21, 1606–1617. [Google Scholar] [CrossRef]
- Tamay, D.G.; Usal, T.D.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, T.E. A review of ketorolac as a prehospital analgesic. J. Paramed. Prac. 2017, 9, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Gillies, M.; Ranakusuma, A.; Hoffmann, T.; Thorning, S.; McGuire, T.; Glasziou, P.; Mar, C.D. Common harms from amoxicillin: A systematic review and meta-analysis of randomized placebo-controlled trials for any indication. CMAJ 2015, 187, E21–E31. [Google Scholar] [CrossRef] [Green Version]
- Hines, D.J.; Kaplan, D. Poly (lactic-co-glycolic acid) controlled release systems: Experimental and modeling insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The design of poly(lactide-co-glycolide) nanocarriers for medical applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Xiao, Y.; Liu, P.; Wnag, S.; Zhao, Y.; Shen, M.; Shi, X. Electrospun PEGylated PLGA nanofibers for drug encapsulation and release. Mater. Sci. Eng. C 2018, 91, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan, S.; Kumar, K.J.; Parasuraman, S. Simple and sensitive method for the analysis of ketorolac in human plasma using high-performance liquid chromatography. J. Young Pharm. 2013, 5, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraka, M.M.; Elsadek, M.E.; Ibrahim, A.M. HPLC method for the simultaneous determination of secnidazole, omeprazole and amoxicillin mixture in pure forms and pharmaceutical formulations. Asian J. Pharm. Analy. Med. Chem. 2014, 2, 197–207. [Google Scholar]
- Liu, K.S.; Kao, C.W.; Tseng, Y.Y.; Chen, S.K.; Lin, Y.T.; Lu, C.J.; Liu, S.J. Assessment of antimicrobial agents, analgesics, and epidermal growth factors-embedded anti-adhesive poly(lactic-co-glycolic acid) nanofibrous membranes: In vitro and in vivo studies. Int. J. Nanomed. 2021, 16, 4471–4480. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.M.J.; Maheshbabu, C.; Kumar, N.; Sachdeva, R.K. Effect of carbopol 940 on drug release profile and floating characteristics of floating drug delivery system of amoxicillin trihydrate using HPMC different grades. J. Appl. Biopharm. Pharmacokinet. 2014, 2, 29–39. [Google Scholar]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Gaertner, D.J.; Hallman, T.M.; Hankenson, F.C.; Batchelder, M.A. Chapter 10-Anesthesia and analgesia for laboratory rodents. In Anesthesia and Analgesia in Laboratory Animals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 239–297. [Google Scholar]
- Pilmis, B.; Lourtet-Hascoet, J.; Barraud, O.; Piau, C.; Isnard, C.; Hery-Arnaud, G.; Amara, M.; Merens, A.; Farfour, E.; Thomas, E.; et al. Be careful about MICs to amoxicillin for patients with Streptococci -related infective endocarditis. Int. J. Antimicrob. Agents 2019, 53, 850–854. [Google Scholar] [CrossRef]
- Velasco, M.A.; Lancheros, Y.; Garzon-Alvarado, A. Geometric and mechanical properties evaluation of scaffolds for bone tissue applications designing by a reaction-diffusion models and manufactured with a material jetting system. J. Comput. Des. Eng. 2016, 3, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Helder, M.N.; Knippenberg, M.; Klein-Nulend, J.; Wuisman, P.I. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007, 13, 1799–1808. [Google Scholar] [CrossRef]
- Kroeze, R.J.; Helder, M.N.; Govaert, L.E.; Smit, T.H. Biodegradable polymers in bone tissue engineering. Materials 2009, 2, 833–856. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, P.-Y.; Lee, D.; Weng, C.-C.; Wu, R.-C.; Liao, C.-T.; Liu, S.-J. Bone Morphogenetic Protein-, Antimicrobial Agent-, and Analgesic-Incorporated Nanofibrous Scaffolds for the Therapy of Alveolar Clefts. Pharmaceutics 2022, 14, 374. https://doi.org/10.3390/pharmaceutics14020374
Chou P-Y, Lee D, Weng C-C, Wu R-C, Liao C-T, Liu S-J. Bone Morphogenetic Protein-, Antimicrobial Agent-, and Analgesic-Incorporated Nanofibrous Scaffolds for the Therapy of Alveolar Clefts. Pharmaceutics. 2022; 14(2):374. https://doi.org/10.3390/pharmaceutics14020374
Chicago/Turabian StyleChou, Pang-Yun, Demei Lee, Chi-Chang Weng, Ren-Chin Wu, Chien-Tun Liao, and Shih-Jung Liu. 2022. "Bone Morphogenetic Protein-, Antimicrobial Agent-, and Analgesic-Incorporated Nanofibrous Scaffolds for the Therapy of Alveolar Clefts" Pharmaceutics 14, no. 2: 374. https://doi.org/10.3390/pharmaceutics14020374
APA StyleChou, P.-Y., Lee, D., Weng, C.-C., Wu, R.-C., Liao, C.-T., & Liu, S.-J. (2022). Bone Morphogenetic Protein-, Antimicrobial Agent-, and Analgesic-Incorporated Nanofibrous Scaffolds for the Therapy of Alveolar Clefts. Pharmaceutics, 14(2), 374. https://doi.org/10.3390/pharmaceutics14020374



_周(Chou).jpg)



