Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery
Abstract
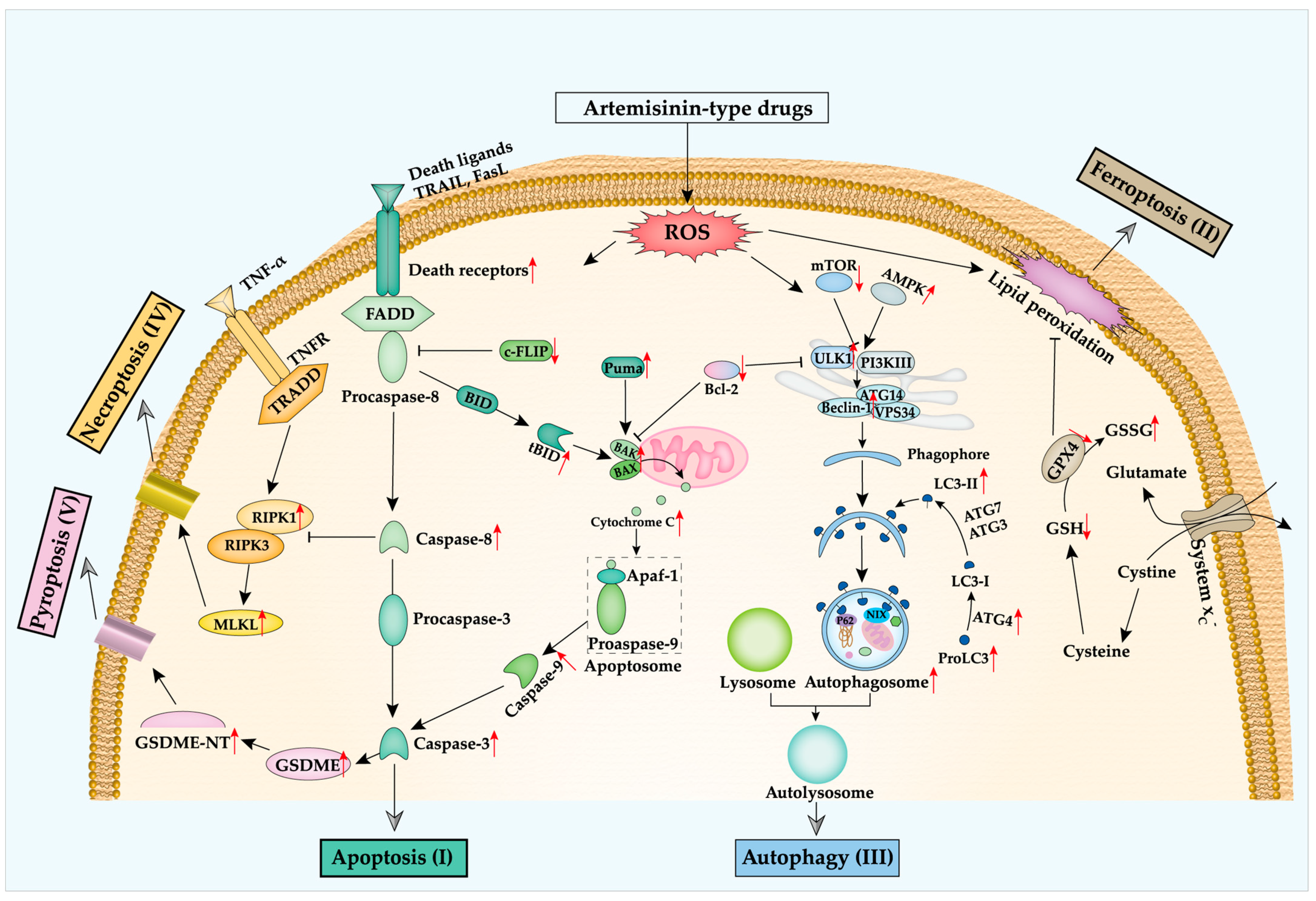
1. Introduction
2. Artemisinin and Regulated Cell Death in Cancer
2.1. Apoptosis
| Cell Lines; Cancer | Drugs | Effects | Ref. |
|---|---|---|---|
| HOS, MG-63, U-2 OS, Saos-2; Osteosarcoma | DHA | Cell viability↓; Cell apoptosis↑; G2/M phase arrest↑; Cleaved caspase-3, -8, -9↑; BAX↑; Bcl-2↓; FAS↑; Cyclin D1, B1↓; Cdc25B↓; NF-kB activity↓ | 2011 [52] |
| MCF-7, T47D, MDA-MB-231; Breast cancer | ATS | Cell death↑; ROS↑; DFO reduces ROS production and cell death; LC3 puncta↑; LC3-II↑; Cell death rescued by CQ and BafA1 | 2011 [61] |
| G-361, A375, LOX; Melanoma | DHA | Cell apoptosis↑; ROS↑; Cell viability↓; DFO reduces ROS production and cell death; Transmembrane potential↓; NOXA↑; CHOP↑; p-P53↑ | 2012 [55] |
| T47D; Breast cancer | DHA | Cell viability↓; G0/G1 phase↑; Cell apoptosis↑; tBid↑; Cytochrome C↑; Cleaved caspase-8, -9↑; Bim↑; Bcl-2↓ | 2013 [57] |
| Eca109, Ec9706; Esophageal cancer | DHA | Cell viability↓; Cell apoptosis↑; G0/G1 phase↑; Swollen mitochondria↑; Apoptotic body↑; Bcl-2, Bcl-xL↓; Bax↑; Pro-caspase-3↓; Caspase-9↑; Cyclin E↓; CDK2, CDK4↓ | 2013 [59] |
| SW1990, BxPC-3, PANC-1; Pancreatic cancer. γδ T cell | DHA | No influence on the ell viability of γδ T; DHA-treated γδ T cell reduces cancer cell viability; Increasing expression of perforin, granzyme B, CD107a, IFN-γ from γδ T cell | 2013 [63] |
| HepG2, Huh-7, LO2; Liver cancer | ART, ATS, DHA | Cell viability↓; Cell apoptosis↑; NAC and zVAD reduce cell death; Chromatin condensation↑; ROS↑; Transmembrane potential↓; Caspase-3, -8, -9 activity↑; Cytochrome C releasing↑; Bax, Bak, Bim↑; Mcl-1↓ | 2015 [60] |
| Diverse cell lines | ART and 4 derivatives | Cell death↑; Cell apoptosis↑; Transmembrane potential↓; ROS↑; Intracellular calcium↑; G2/M phase↑; Caspase-3 activity↑; Pro-caspase-3, -9↓; Caspase-9↑; Apaf-1↑; P53, Bax↑, Bcl-2↓ | 2017 [54] |
| EJ-138, HTB-9; Bladder cancer | DHA | Cell viability↓; Cell apoptosis↑; Transmembrane potential↓; ROS↑; Caspase-3 activity↑; Bax↑, Bcl-2↓; Cytochrome C↑ | 2017 [58] |
| Diverse cell lines | ATS | Cell apoptosis↑; Lipid peroxidation↑; GRP78↑; CHOP↑; PUMA↑; Tumor growth↓ | 2017 [56] |
| SK-Hep-1; Liver cancer | DHA | Cell viability↓; Cell apoptosis↑; Cleaved caspase-3, -8, -9↑; Cleaved PARP-1↑; Sp1↓; XIAP↓; p-ERK, p-P38, p-JNK↓ | 2018 [48] |
| SK-BR-3, MDA-MB-468, MCF-7; Breast cancer | ATS | Cell viability↓; Cell divisions↓; G1 phase↑; CDK1, CDK4↓; CDC25C↓; Cyclin B, Cyclin D3↓; P21↑; Cell apoptosis↑; Cleaved PARP-1↑; Caspases activation↑; Mitochondrial outer membrane permeability↑; Cytochrome C, SMAC↑; ROS↑ | 2019 [53] |
| 4T1; Mouse breast cancer | ART | Cell viability↓; Cell apoptosis↑; TGF-β↓; Tumor growth↓; Treg and MDSC expansion↓; CD4+ IFN-γ+ T cells and granzyme B+ cytotoxic T lymphocytes↑ | 2019 [62] |
2.2. Ferroptosis
| Cell Lines; Cancer | Drugs | Effects | Ref. |
|---|---|---|---|
| Diverse cell lines | ART, 10 derivatives | Artenimol induced cell death rescued by Fer-1 in CCRF-CEM cell | 2015 [81] |
| Panc-1, COLO357, AsPC-1, BxPC-3; Pancreatic cancer | ATS | ROS↑; Cell death rescued by DFO, trolox and Fer-1 | 2015 [76] |
| Head and neck squamous cell carcinoma | DHA | GPX4↓; Ras↓; P53↓; Bcl-2↓; Cell death rescued by DFO | 2016 [82] |
| DAUDI, CA-46; Burkitt’s Lymphoma | ATS | Cell death rescued by DFO, Lip-1 and Fer-1; ATF4↑; CHOP↑; CHAC1↑; Tumor growth↓ | 2019 [83] |
| U251, U373; Patient-derived glioma | DHA | Cell death↑; ROS and Malondialdehyde↑; GSH↓; GSSG↑; CHOP↑; HSPA5↑; GPX4↑ | 2019 [75] |
| PaTU8988, AsPC-1; Pancreatic cancer | ATS | Cell death rescued by Fer-1; GRP78↑ | 2019 [84] |
| HL60, KG1, THP-1; Leukemia | DHA | Cell viability↓; Dysfunction of mitochondria; Mitochondrial ROS↑; Cytoplasm ROS↑; p-AMPK↑; p-mTOR↓; Ferritin heavy chain (FTH)↓; GPX4↓; FTH over-expression prevents DHA-induced ferroptosis; Tumor growth↓ | 2019 [73] |
| U87, A172; Glioblastoma | DHA | Cell viability↓; Total ROS and lipid ROS↑; HO-1↑; GPX4↓; Mitochondrial ridges↓; Bilayer membrane density↑; Fer-1 decreases ROS production and inhibits cell death | 2020 [72] |
| MT-2, MT-4, HUT-102; Leukemia | ATS | T-cell growth↓; ROS↑; Cell death rescued by Fer-1; Tumor growth↓ | 2020 [78] |
| Diverse cell lines | ART ATS DHA AM | Cell death↑; lipid ROS↑; GSH↓; Cell death rescued by DFO or BafA1 | 2020 [79] |
| U2932, SU-DHL2, SU-DHL4, SU-DHL6, 293 T; Lymphoma | ATS | Cell viability↓; Colony formation↓; GPX4↓; FTH-1; ROS and Malondialdehyde↑; Cell death rescued by Fer-1; p-STAT3↓; Tumor growth↓ | 2021 [85] |
| Hep3B, PLC/PRF/5, Huh7, HepG2; Primary liver cancer | DHA | Cell viability↓; Lipid ROS and Malondialdehyde↑; Iron content↑; GSH/GSSG↓; GPX4↓; SLC7A11 and SLC3A2↓; CHAC↑; Tumor growth↓; p-PERK and IRE1-α↑; ATF4 and ATF6↑ | 2021 [74] |
| NCI-H1299, A549, LTEP-a-2, NCI-H23, NCI-H358; Lung cancer | ART DHA | Cell death↑; Cystine/glutamate transporter (xCT)↓; Cell death rescued by NAC | 2021 [77] |
2.3. Autophagy
2.4. Other Types of Regulated Cell Death
3. Combination Treatment of ART-Type Drugs with RCD-Targeting Biologics in Cancer
4. Delivery of Art-Type Drugs with Nanocarriers
4.1. Inorganic ART-Loaded Nanoparticles
4.1.1. Ion-Containing Inorganic ART-Loaded Nanoparticles
4.1.2. Other Inorganic ART-Loaded Nanoparticles
4.2. ART-Type Drug-Loaded Liposomes
4.3. Polymer-Based ART-Loaded Nanoparticles
4.4. Carbon-Based ART-Loaded Nanoparticles
4.5. Other Types of ART-Type Drugs-Loaded Nanocarriers
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abb. | Full Name |
| AIM2 | Interferon-inducible protein |
| AMP | Adenosine monophosphate |
| Apaf-1 | Apoptotic protease activating factor 1 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATF | Activating transcription factor |
| Atg5 | Autophagy related 5 protein |
| BafA1 | Bafilomycin A1 |
| Bax | Bcl-2-like protein 4 |
| BCE | Before common era |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| Bid | BH3 interacting-domain death agonist |
| Bim | Bcl-2-like protein 11 |
| bis-MPA | 2,2-bis(hydroxymethyl)propionic acid |
| CD107a | Lysosomal-associated membrane protein-1 |
| CD155 | Cluster of differentiation 155 |
| CD20 | B-lymphocyte antigen CD20 |
| CD4 | Cluster of differentiation 4 |
| CDC25B | Cell Division Cycle 25B |
| CDK2 | Cyclin Dependent Kinase 2 |
| CDK4 | Cyclin Dependent Kinase 4 |
| CE | Common Era |
| CHAC1 | ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1 |
| CHEMS | Cholesteryl Hemisuccinate |
| CHOL | Cholesterol |
| CHOP | DNA damage-inducible transcript 3 |
| CQ | Chloroquine |
| DFNA5 | Non-syndromic hearing impairment protein 5 |
| DFO | Deferoxamine |
| DOPE | 1,2-dioleoyl- snglycero-3-phosphoethanolamine |
| DOX | Doxorubicin |
| DPPC | 1,2-dipalmitoyl-snglycero-3-phosphocholine |
| DPTA | Diethylene Triamine Pentacetate Acid |
| DQA | Dequalinium |
| DR3 | Death receptor 3 |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| DSPC | Distearoyl Phosphatidylcholine |
| DSPE | Distearoyl Phosphatidyl Ethanolamine |
| EPC | egg phosphatidylcholine |
| FADD | Fas-associated protein with death domain |
| Fas | Fas receptor, apoptosis antigen 1 |
| FasL | Fas ligand |
| FDA | The United States Food and Drug Administration |
| Fn14 | Fibroblast growth factor-inducible 14 |
| GRP78 | Glucose regulated protein |
| GSSG | Glutathione disulfide |
| HA | Hyaluronic Acid |
| HCQ | Hydroxychloroquine sulfate |
| HMFB | Hyperthermophilic archaeon Methanothermus fervidus DNA-binding protein |
| HMGB | High mobility group box 1 protein |
| HO-1 | Heme oxygenase 1 |
| HSPA5 | Heat Shock Protein Family A (Hsp70) Member 5 |
| IC50 | The half maximal inhibitory concentration |
| IFN | Type-I interferons |
| IFNR | IFN receptor |
| IL-1β | Interleukin-1β |
| IRE1-α | Inositol-requiring enzyme 1 α |
| JNK | c-Jun N-terminal kinases |
| LPS | Lipopolysaccharides |
| Mcl-1 | Induced myeloid leukemia cell differentiation protein |
| MLKL | Mixed lineage kinase domain like pseudokinase |
| MPEG | Poly(ethylene glycol) Monomethyl Ether |
| mTOR | Mammalian target of rapamycin |
| NAC | N-acetyl-l-cysteine |
| NBR1 | Neighbor of BRCA1 gene 1 protein |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHS | N-hydroxysuccinimide |
| NHS | N-hydroxysuccinimide |
| NIX/BNIP3L | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like |
| Noxa | Phorbol-12-myristate-13-acetate-induced protein 1 |
| P53 | Tumor protein P53 |
| P62/SQSTM | Ubiquitin-binding protein p62/Sequestosome-1 |
| P90G | Phospholipon90G |
| p-AMPKα | Phospho-AMPKα |
| PBAE | poly(β- amino ester) |
| PCL | poly(ɛ-caprolactone) |
| PCL | Poly (E-caprolactone) |
| PEG | Poly (ethylene glycol) |
| PEOZ | poly(2-ethyl-2-oxazoline) |
| p-ERK | Phospho-extracellular signal-regulated kinases |
| PI3KC1 | Phosphatidylinositol 3-kinase catalytic subunit type 3 |
| PI3KII | Phosphatidylinositol 3-kinase |
| PI3P | Phosphatidylinositol 3-phosphate |
| PLA | poly(lactic acid) |
| p-MAPK | Phospho- mitogen-activated protein kinase |
| PPC | Phophatidylcholine |
| p-PERK | Phospho-ER-resident protein |
| Puma | p53 upregulated modulator of apoptosis |
| Ra1B | Ras-related protein Ral-B |
| RGD | Arginine-Glycine-Aspartic Acid |
| RIPK | Receptor-interacting serine/threonine-protein kinase 1 |
| SLC3A2 | 4F2 cell-surface antigen heavy chain |
| SLC7A11 | Cystine/glutamate transporter |
| SMAC | Second mitochondria-derived activator of caspase |
| Sp1 | Specificity protein 1 |
| SRF | Sorafenib |
| STAT3 | Signal transducer and activator of transcription 3 |
| Surf1 | Surfeit locus protein 1 |
| Tb | Terbium |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptors |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNF-α | Tumor necrosis factor α |
| TRADD | Tumor necrosis factor receptor type 1-associated DEATH domain protein |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRF2 | Telomeric repeat binding factor 2 |
| TWEAK | TNF-related weak inducer of apoptosis |
| ULK | Unc-51 Like Autophagy Activating Kinase 1 |
| USP33 | Ubiquitin carboxyl-terminal hydrolase 33 |
| VEGI | Vascular endothelial growth inhibitor |
| Vps34 | Class III PI 3-kinase |
| XIAP | X-linked inhibitor of apoptosis protein |
| zVAD | Caspase inhibitor z-VAD-fmk |
| γH2AX | H2A histone family member X |
References
- Muhammad, I.; Samoylenko, V. Antimalarial quassinoids: Past, present and future. Expert Opin. Drug Discov. 2007, 2, 1065–1084. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front. Pharmacol. 2020, 11, 529881. [Google Scholar] [CrossRef] [PubMed]
- Raphals, L.A. Early Chinese Medical Literature: The Mawangdui Medical Manuscripts (review). China Rev. Int. 2000, 7, 463–466. [Google Scholar] [CrossRef]
- Maude, R.J.; Woodrow, C.J.; White, L.J. Artemisinin Antimalarials: Preserving the “Magic Bullet”. Drug Dev. Res. 2010, 71, 12–19. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin—A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem.-Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef]
- Czechowski, T.; Weathers, P.J.; Brodelius, P.E.; Brown, G.D.; Graham, I.A. Editorial: Artemisinin—From Traditional Chinese Medicine to Artemisinin Combination Therapies; Four Decades of Research on the Biochemistry, Physiology, and Breeding of Artemisia annua. Front. Plant Sci. 2020, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y. Artemisinin (Qing Hao Su). In Thirty Great Inventions of China; Springer: Singapore, 2020; pp. 815–845. [Google Scholar]
- Li, Y.; Yu, P.L.; Chen, Y.X.; Li, L.Q.; Gai, Y.Z.; Wang, D.S.; Zheng, Y.P. Studies on analogs of artemisinine. I. The synthesis of ethers, carboxylic esters and carbonates of dihydroartemisinine (author’s transl). Acta Pharm. Sin. 1981, 16, 429–439. [Google Scholar]
- Zhang, R.B.; Xu, S.L.; Li, Y. Separation of artemisinine and its derivatives by reversed phase high performance liquid chromatography (author’s transl). Acta Pharm. Sin. 1981, 16, 460–465. [Google Scholar]
- Choudhary, A.; Sinha, M.; Devi, A.; Jindal, S.; Goyal, K. A Review on Antimalarial 1,2,4-Trioxane Derivatives. J. Drug Deliv. Ther. 2020, 10, 240–253. [Google Scholar] [CrossRef]
- Rudrapal, M.; Chetia, D. Endoperoxide antimalarials: Development, structural diversity and pharmacodynamic aspects with reference to 1,2,4-trioxane-based structural scaffold. Drug Des. Devel. Ther. 2016, 10, 3575–3590. [Google Scholar] [CrossRef]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. Exploration of artemisinin derivatives and synthetic peroxides in antimalarial drug discovery research. Eur. J. Med. Chem. 2021, 213, 113193. [Google Scholar] [PubMed]
- Grazzia, N.; Boaventura, S.; Garcia, V.L.; Gadelha, F.R.; Miguel, D.C. Dihydroartemisinin, an active metabolite of artemisinin, interferes with Leishmania braziliensis mitochondrial bioenergetics and survival. Parasitol. Res. 2021, 120, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Development of antimalarial drugs and their application in China: A historical review. Infect. Dis. Poverty 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Vil’, V.A.; Yaremenko, I.A.; Ilovaisky, A.I.; Terent’ev, A.O. Synthetic Strategies for Peroxide Ring Construction in Artemisinin. Molecules 2017, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. The problem of drug resistance in malaria. Parasitology 1985, 90, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Ouji, M.; Augereau, J.-M.; Paloque, L.; Benoit-Vical, F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 2018, 25, 24. [Google Scholar] [CrossRef]
- Sun, W.C.; Han, J.X.; Yang, W.Y.; Deng, D.A.; Yue, X.F. Antitumor activities of 4 derivatives of artemisic acid and artemisinin B in vitro. Acta Pharmacol. Sin. 1992, 13, 541–543. [Google Scholar]
- Woerdenbag, H.J.; Moskal, T.A.; Pras, N.; Malingré, T.M.; El-Feraly, F.S.; Kampinga, H.H.; Konings, A.W.T. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod. 1993, 56, 849–856. [Google Scholar] [CrossRef]
- Moore, J.C.; Lai, H.; Li, J.R.; Ren, R.L.; McDougall, J.A.; Singh, N.P.; Chou, C.K. Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett. 1995, 98, 83–87. [Google Scholar] [CrossRef]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kai, K.; Yamaji, K.; Ide, T.; Noshiro, H.; Kawaguchi, A.; Aishima, S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology 2019, 75, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Bhaw-Luximon, A.; Jhurry, D. Artemisinin and its derivatives in cancer therapy: Status of progress, mechanism of action, and future perspectives. Cancer Chemother. Pharmacol. 2017, 79, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Conradt, B. Genetic control of programmed cell death during animal development. Annu. Rev. Genet. 2009, 43, 493–523. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar]
- Susan, E. Apoptosis: A Reveiw of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 496–516. [Google Scholar]
- Chaudhry, G.E.S.; Jan, R.; Zafar, M.N.; Mohammad, H.; Muhammad, T.S.T. Vitex Rotundifolia fractions induced apoptosis in human breast cancer T-47d cell line via activation of extrinsic and intrinsic pathway. Asian Pac. J. Cancer Prev. 2019, 20, 3555–3562. [Google Scholar] [CrossRef]
- Fakai, M.I.; Abd Malek, S.N.; Karsani, S.A. Induction of apoptosis by chalepin through phosphatidylserine externalisations and DNA fragmentation in breast cancer cells (MCF7). Life Sci. 2019, 220, 186–193. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, L.; Wang, C.; Yang, Y.; Zhou, W.; Li, F.; Tan, Z. Assessment of the cytotoxicity of ionic liquids on Spodoptera frugiperda 9 (Sf-9) cell lines via in vitro assays. J. Hazard. Mater. 2018, 348, 1–9. [Google Scholar] [CrossRef]
- Wang, D.; Fung, J.N.T.; Tuo, Y.; Hu, L.; Chen, C. TWEAK/Fn14 promotes apoptosis of human endometrial cancer cells via caspase pathway. Cancer Lett. 2010, 294, 91–100. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, M.; Hu, X.; Han, L.; Yang, K.; Ba, H.; Zhang, Z.; Yin, B.; Yang, X.P.; Li, Z.; et al. STAT1 mediates transmembrane TNF-alpha-induced formation of death-inducing signaling complex and apoptotic signaling via TNFR1. Cell Death Differ. 2017, 24, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Lv, C.L.; Shi, J.G.; Zhang, C.X. MiR-543-3p promotes locomotor function recovery after spinal cord injury by inhibiting the expression of tumor necrosis factor superfamily member 15 in rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2701–2709. [Google Scholar] [PubMed]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef]
- Suo, F.; Zhou, X.; Setroikromo, R.; Quax, W.J. Receptor Specificity Engineering of TNF Superfamily Ligands. Pharmaceutics 2022, 14, 181. [Google Scholar] [CrossRef]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef]
- Deng, D.; Shah, K. TRAIL of Hope Meeting Resistance in Cancer. Trends Cancer 2020, 6, 989–1001. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, S.; Xia, Y. TWEAK/Fn14 Activation Participates in Skin Inflammation. Mediat. Inflamm. 2017, 2017, 6746870. [Google Scholar] [CrossRef]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2014; Volume 544, pp. 99–128. ISBN 9780124171589. [Google Scholar]
- Safa, A.; Day, T.; Wu, C.-H. Cellular FLICE-Like Inhibitory Protein (C-FLIP): A Novel Target for Cancer Therapy. Curr. Cancer Drug Targets 2008, 8, 37–46. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011, 18, 538–548. [Google Scholar] [CrossRef]
- Willms, A.; Schittek, H.; Rahn, S.; Sosna, J.; Mert, U.; Adam, D.; Trauzold, A. Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells. PLoS ONE 2019, 14, e0214847. [Google Scholar] [CrossRef] [PubMed]
- Boivin, W.A.; Cooper, D.M.; Hiebert, P.R.; Granville, D.J. Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab. Investig. 2009, 89, 1195–1220. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Rücker, G.; Falkenberg, M.; Manns, D.; Olbrich, A.; Fabry, U.; Osieka, R. Detection of apoptosis in KG-1a leukemic cells treated with investigational drugs. Arzneim. Forsch. 1996, 46, 196–200. [Google Scholar]
- Handrick, R.; Ontikatze, T.; Bauer, K.D.; Freier, F.; Rübel, A.; Dürig, J.; Belka, C.; Jendrossek, V. Dihydroartemisinin induces apoptosis by a bak-dependent intrinsic pathway. Mol. Cancer Ther. 2010, 9, 2497–2510. [Google Scholar] [CrossRef]
- Im, E.; Yeo, C.; Lee, H.J.; Lee, E.O. Dihydroartemisinin induced caspase-dependent apoptosis through inhibiting the specificity protein 1 pathway in hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018, 192, 286–292. [Google Scholar] [CrossRef]
- Zhou, X.; Zijlstra, S.N.; Soto-Gamez, A.; Setroikromo, R.; Quax, W.J. Artemisinin Derivatives Stimulate DR5-Specific TRAIL-Induced Apoptosis by Regulating Wildtype P53. Cancers 2020, 12, 2514. [Google Scholar] [CrossRef]
- Ilamathi, M.; Sivaramakrishnan, V. Artesunate acts as fuel to fire in sensitizing HepG2 cells towards TRAIL mediated apoptosis via STAT3 inhibition and DR4 augmentation. Biomed. Pharmacother. 2017, 88, 515–520. [Google Scholar] [CrossRef]
- Wang, K.S.; Li, J.; Wang, Z.; Mi, C.; Ma, J.; Piao, L.X.; Xu, G.H.; Li, X.; Jin, X. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol. Immunotoxicol. 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.C.; Pei, L.B.; Shi, L.L.; Yan, J.L.; Ma, X.H. Anti-tumor effects of dihydroartemisinin on human osteosarcoma. Mol. Cell. Biochem. 2011, 351, 99–108. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Fernando, W.; Hoskin, D.W. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp. Mol. Pathol. 2019, 107, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, J.; Li, Y.; Zhuang, J.; Zhang, Q.; Sun, X.; Sun, D. Synthesis and evaluation of cytotoxic activities of artemisinin derivatives. Chem. Biol. Drug Des. 2017, 90, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Cabello, C.M.; Lamore, S.D.; Bair, W.B.; Qiao, S.; Azimian, S.; Lesson, J.L.; Wondrak, G.T. The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest. New Drugs 2012, 30, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, D.H.; Lee, Y.S.; Jo, M.J.; Jeong, Y.A.; Kwon, W.T.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Molecular crosstalk between ferroptosis and apoptosis: Emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget 2017, 8, 115164–115178. [Google Scholar] [CrossRef]
- Mao, H.; Gu, H.; Qu, X.; Sun, J.; Song, B.; Gao, W.; Liu, J.; Shao, Q. Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. Int. J. Mol. Med. 2013, 31, 213–218. [Google Scholar] [CrossRef]
- Poupel, F.; Aghaei, M.; Movahedian, A.; Jafari, S.; Shahrestanaki, M. Dihydroartemisinin induces apoptosis in human bladder cancer cell lines through reactive oxygen species, mitochondrial membrane potential, and cytochrome C pathway. Int. J. Prev. Med. 2017, 8, 78. [Google Scholar]
- Du, X.X.; Li, Y.J.; Wu, C.L.; Zhou, J.H.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; Huang, P.; Yuan, H.H.; et al. Initiation of apoptosis, cell cycle arrest and autophagy of esophageal cancer cells by dihydroartemisinin. Biomed. Pharmacother. 2013, 67, 417–424. [Google Scholar] [CrossRef]
- Qin, G.; Zhao, C.B.; Zhang, L.; Liu, H.; Quan, Y.; Chai, L.; Wu, S.; Wang, X.; Chen, T. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis 2015, 20, 1072–1086. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Stein, H.A.; Turschner, S.; Toegel, I.; Mora, R.; Jennewein, N.; Efferth, T.; Eils, R.; Brady, N.R. Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J. Biol. Chem. 2011, 286, 6587–6601. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.-H.; Gao, L.-W.; Li, X.-Y.; Jin, Q.-X.; Wang, Y.-Y.; Xu, Y.-Y.; Jin, F.; Lu, S.-L.; Wei, M.-J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef]
- Zhou, Z.-H.; Chen, F.-X.; Xu, W.-R.; Qian, H.; Sun, L.-Q.; Lü, X.-T.; Chen, L.; Zhang, J.; Ji, H.-C.; Fei, S.-J. Enhancement effect of dihydroartemisinin on human γδ T cell proliferation and killing pancreatic cancer cells. Int. Immunopharmacol. 2013, 17, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Liu, F.; Wang, Q.; Song, M.; Yu, Q.; Tang, K.; Teng, T.; Wu, D.; Wang, X.; et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 1675613. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Kannan, D.; Yadav, N.; Ahmad, S.; Namdev, P.; Bhattacharjee, S.; Lochab, B.; Singh, S. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. EBioMedicine 2019, 45, 261–277. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Zhao, X. Mechanisms and Molecular Targets of Artemisinin in Cancer Treatment. Cancer Investig. 2021, 39, 675–684. [Google Scholar] [CrossRef]
- Yi, R.; Wang, H.; Deng, C.; Wang, X.; Yao, L.; Niu, W.; Fei, M.; Zhaba, W. Dihydroartemisinin initiates ferroptosis in glioblastoma through GPX4 inhibition. Biosci. Rep. 2020, 40, BSR20193314. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mi, Y.; Zhang, X.; Ma, Q.; Song, Y.; Zhang, L.; Wang, D.; Xing, J.; Hou, B.; Li, H.; et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 402. [Google Scholar] [CrossRef] [PubMed]
- Eling, N.; Reuter, L.; Hazin, J.; Hamacher-Brady, A.; Brady, N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2015, 2, 517–532. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, H.; Yao, H.; Lu, L.; He, G.; Wu, M.; Zheng, C.; Li, Y.; Chen, S.; Li, L.; et al. Artemisinin derivatives inhibit Non-small cell lung cancer cells through induction of ROS-dependent apoptosis/ferroptosis. J. Cancer 2021, 12, 4075–4085. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. Eur. J. Pharmacol. 2020, 872, 172953. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Benthani, F.A.; Wu, J.; Liang, D.; Bian, Z.-X.; Jiang, X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020, 27, 242–254. [Google Scholar] [CrossRef]
- Zou, Y.; Schreiber, S.L. Progress in Understanding Ferroptosis and Challenges in Its Targeting for Therapeutic Benefit. Cell Chem. Biol. 2020, 27, 463–471. [Google Scholar] [CrossRef]
- Ooko, E.; Saeed, M.E.M.; Kadioglu, O.; Sarvi, S.; Colak, M.; Elmasaoudi, K.; Janah, R.; Greten, H.J.; Efferth, T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine 2015, 22, 1045–1054. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Z.; Chen, L.; Zhou, Y.; Zou, P.; Feng, C.; Wang, L.; Liang, G. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016, 381, 165–175. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, G.Z.; Yin, J.L.; Bian, Z.X. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s Lymphoma. Biochem. Biophys. Res. Commun. 2019, 519, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Z.; Wang, M.; Cao, X.; Qi, J.; Wang, D.; Gong, A.; Zhu, H. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des. Devel. Ther. 2019, 13, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F.; Wu, P.; Gong, S.; Gao, J.; Tao, H.; Shen, Q.; Wang, S.; Zhou, Z.; Jia, Y. Artesunate induces apoptosis, autophagy and ferroptosis in diffuse large B cell lymphoma cells by impairing STAT3 signaling. Cell. Signal. 2021, 88, 110167. [Google Scholar] [CrossRef] [PubMed]
- Kenney, D.L.; Benarroch, E.E. The autophagy-lysosomal pathway. Neurology 2015, 85, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, I.; Lim, A.L.; Laderoute, K.; Denko, N.C. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008, 15, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tan, J.; Zhang, Q. Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol. Int. 2015, 39, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Jin, X.F.; Zhou, X.H.; Dong, X.H.; Yu, W.T.; Gao, W.J. The role of Astragaloside IV against cerebral ischemia/reperfusion injury: Suppression of apoptosis via promotion of P62-LC3-autophagy. Molecules 2019, 24, 1838. [Google Scholar] [CrossRef]
- Randall-Demllo, S.; Chieppa, M.; Eri, R. Intestinal Epithelium and Autophagy: Partners in Gut Homeostasis. Front. Immunol. 2013, 4, 301. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Zou, J.; Ma, Q.; Sun, R.; Cai, J.; Liao, H.; Xu, L.; Xia, J.; Huang, G.; Yao, L.; Cai, Y.; et al. Dihydroartemisinin inhibits HepG2.2.15 proliferation by inducing cellular senescence and autophagy. BMB Rep. 2019, 52, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Wang, F.; Wu, H.; Zhang, Y.; Liu, J.; Cai, Y.; Huang, S.; He, N.; Hu, Z.; et al. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem. Biol. Interact. 2020, 331, 109273. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Kong, R.; Ma, Z.-B.; Han, B.; Wang, Y.-W.; Pan, S.-H.; Li, Y.-H.; Sun, B. The activation of c-Jun NH₂-terminal kinase is required for dihydroartemisinin-induced autophagy in pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 8. [Google Scholar] [CrossRef]
- Jiang, F.; Zhou, J.Y.; Zhang, D.; Liu, M.H.; Chen, Y.G. Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunate-induced apoptosis. Int. J. Mol. Med. 2018, 42, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, T.; Song, Z.; Peng, L.; Gao, M.; Hermine, O.; Rousseaux, S.; Khochbin, S.; Mi, J.-Q.; Wang, J. Induction of autophagy and autophagy-dependent apoptosis in diffuse large B-cell lymphoma by a new antimalarial artemisinin derivative, SM1044. Cancer Med. 2018, 7, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Bu, S.; Sun, J.; Guo, Y.; Lai, D. Artemisinin derivatives inhibit epithelial ovarian cancer cells via autophagy-mediated cell cycle arrest. Acta Biochim. Biophys. Sin. 2018, 50, 1227–1235. [Google Scholar] [CrossRef]
- Thongchot, S.; Vidoni, C.; Ferraresi, A.; Loilome, W.; Yongvanit, P.; Namwat, N.; Isidoro, C. Dihydroartemisinin induces apoptosis and autophagy-dependent cell death in cholangiocarcinoma through a DAPK1-BECLIN1 pathway. Mol. Carcinog. 2018, 57, 1735–1750. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Zhang, J.-L.; Wu, X.-H.; Zhou, H.-J. Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio 2012, 2, 103–112. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.-S.; Zhang, J.-L.; Lou, X.-E.; Zhou, H.-J. Dihydroartemisinin induces autophagy by suppressing NF-κB activation. Cancer Lett. 2014, 343, 239–248. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Li, X.; Bai, J.; Li, J.; Li, S.; Wang, Z.; Zhou, M. Dihydroartemisinin induces autophagy-dependent death in human tongue squamous cell carcinoma cells through DNA double-strand break-mediated oxidative stress. Oncotarget 2017, 8, 45981–45993. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Fan, M.; Shen, C.; Dai, W.; Bao, Y.; Liu, J.-H.; Yu, B.-Y. Novel dihydroartemisinin derivative DHA-37 induces autophagic cell death through upregulation of HMGB1 in A549 cells. Cell Death Dis. 2018, 9, 1048. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Ren, L.; Li, J.; Li, S.; Cui, Q.; Li, S. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phyther. Res. 2019, 33, 1413–1425. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Shi, X.; Li, S.; Tang, P.M.K.; Li, Z.; Li, H.; Wei, C. Antimalarial Dihydroartemisinin triggers autophagy within HeLa cells of human cervical cancer through Bcl-2 phosphorylation at Ser70. Phytomedicine 2019, 52, 147–156. [Google Scholar] [CrossRef]
- Ma, Q.; Liao, H.; Xu, L.; Li, Q.; Zou, J.; Sun, R.; Xiao, D.; Liu, C.; Pu, W.; Cheng, J.; et al. Autophagy-dependent cell cycle arrest in esophageal cancer cells exposed to dihydroartemisinin. Chin. Med. 2020, 15, 37. [Google Scholar] [CrossRef]
- Shi, X.; Li, S.; Wang, L.; Li, H.; Li, Z.; Wang, W.; Bai, J.; Sun, Y.; Li, J.; Li, X. RalB degradation by dihydroartemisinin induces autophagy and IFI16/caspase-1 inflammasome depression in the human laryngeal squamous cell carcinoma. Chin. Med. 2020, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, L.-Y.; Lai, S.; He, Y. Dihydroartemisinin inhibits the migration of esophageal cancer cells by inducing autophagy. Oncol. Lett. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Dawood, M.; Böckers, M.; Klauck, S.M.; Fottner, C.; Weber, M.M.; Efferth, T. Multiple modes of cell death in neuroendocrine tumors induced by artesunate. Phytomedicine 2020, 79, 153332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, L.; Xiang, J.-D.; Jin, C.-S.; Li, M.-Q.; He, Y.-Y. Artesunate-induced ATG5-related autophagy enhances the cytotoxicity of NK92 cells on endometrial cancer cells via interactions between CD155 and CD226/TIGIT. Int. Immunopharmacol. 2021, 97, 107705. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Laurien, L.; Nagata, M.; Schünke, H.; Delanghe, T.; Wiederstein, J.L.; Kumari, S.; Schwarzer, R.; Corona, T.; Krüger, M.; Bertrand, M.J.M.; et al. Autophosphorylation at serine 166 regulates RIP kinase 1-mediated cell death and inflammation. Nat. Commun. 2020, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, L.C.; Weinlich, R. Necroptosis, the Other Main Caspase-Independent Cell Death. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1301, pp. 123–138. [Google Scholar]
- Button, R.W.; Lin, F.; Ercolano, E.; Vincent, J.H.; Hu, B.; Hanemann, C.O.; Luo, S. Artesunate induces necrotic cell death in schwannoma cells. Cell Death Dis. 2014, 5, e1466. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Min, K.-J.; Kwon, T.K. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Mol. Cell. Biochem. 2017, 435, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Tan, R.Z.; Jia, J.; Wu, S.L.; Wen, C.L.; Lin, X.; Wang, H.; Shi, Z.J.; Li, B.; Kang, Y.; et al. Artesunate relieves acute kidney injury through inhibiting macrophagic Mincle-mediated necroptosis and inflammation to tubular epithelial cell. J. Cell. Mol. Med. 2021, 25, 8775–8788. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Li, A.; Huang, W.; Chen, S.; Han, F.; Wang, L. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem. Biol. Interact. 2021, 340, 109434. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Y.; Qi, L.; Li, L.; Song, D.; Gan, J.; Li, Y.; Ling, X.; Song, C. Dihydroartemisinin mediating PKM2-caspase-8/3-GSDME axis for pyroptosis in esophageal squamous cell carcinoma. Chem. Biol. Interact. 2021, 350, 109704. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Wang, L.; Qin, X.; Liang, J.; Ge, P. Induction of Pyroptosis: A Promising Strategy for Cancer Treatment. Front. Oncol. 2021, 11, 635774. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-”host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, S.; Li, J.; Li, Y. Efficacy and Safety of Camrelizumab Monotherapy and Combination Therapy for Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 695512. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Soto-Gamez, A.; Wang, Y.; Zhou, X.; Seras, L.; Quax, W.; Demaria, M. Enhanced extrinsic apoptosis of therapy-induced senescent cancer cells using a death receptor 5 (DR5) selective agonist. Cancer Lett. 2022, 525, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mitsiades, C.S.; Treon, S.P.; Mitsiades, N.; Shima, Y.; Richardson, P.; Schlossman, R.; Hideshima, T.; Anderson, K.C. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: Therapeutic applications. Blood 2001, 98, 795–804. [Google Scholar] [CrossRef]
- Snajdauf, M.; Havlova, K.; Vachtenheim, J.; Ozaniak, A.; Lischke, R.; Bartunkova, J.; Smrz, D.; Strizova, Z. The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials. Front. Mol. Biosci. 2021, 8, 628332. [Google Scholar] [CrossRef]
- Van Dijk, M.; Halpin-McCormick, A.; Sessler, T.; Samali, A.; Szegezdi, E. Resistance to TRAIL in non-transformed cells is due to multiple redundant pathways. Cell Death Dis. 2013, 4, e702. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005, 12, 228–237. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Shen, X.L.; An, J.; Sun, H.; Wang, L.; Hu, Y.J.; Sun, Q.; Fu, L.C.; Sheikh, M.S.; et al. Dihydroartemisinin upregulates death receptor 5 expression and cooperates with TRAIL to induce apoptosis in human prostate cancer cells. Cancer Biol. Ther. 2010, 9, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Jia, G.; Cheng, Z.; Wang, Y.; Mu, M.; Wang, S.; Pan, S.; Gao, Y.; Jiang, H.; Dong, D.; et al. Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5. PLoS ONE 2012, 7, e37222. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, J. Iron Metabolism, Ferroptosis, and the Links With Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef]
- Högemann-Savellano, D.; Bost, E.; Blondet, C.; Sato, F.; Abe, T.; Josephson, L.; Weissleder, R.; Gaudet, J.; Sgroi, D.; Peters, P.J.; et al. The Transferrin Receptor: A Potential Molecular Imaging Marker for Human Cancer. Neoplasia 2003, 5, 495–506. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and holotransferrin. Cancer Lett. 1995, 91, 41–46. [Google Scholar] [CrossRef]
- Sadava, D.; Phillips, T.; Lin, C.; Kane, S.E. Transferrin overcomes drug resistance to artemisinin in human small-cell lung carcinoma cells. Cancer Lett. 2002, 179, 151–156. [Google Scholar] [CrossRef]
- Efferth, T.; Benakis, A.; Romero, M.R.; Tomicic, M.; Rauh, R.; Steinbach, D.; Häfer, R.; Stamminger, T.; Oesch, F.; Kaina, B.; et al. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic. Biol. Med. 2004, 37, 998–1009. [Google Scholar] [CrossRef]
- Deng, X.R.; Liu, Z.X.; Liu, F.; Pan, L.; Yu, H.P.; Jiang, J.P.; Zhang, J.J.; Liu, L.; Yu, J. Holotransferrin enhances selective anticancer activity of artemisinin against human hepatocellular carcinoma cells. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2013, 33, 862–865. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, S.; Zhao, X.; Zhao, C.; Zhao, H.; Huo, L. Mechanisms of Dihydroartemisinin and Dihydroartemisinin/Holotransferrin Cytotoxicity in T-Cell Lymphoma Cells. PLoS ONE 2015, 10, e0137331. [Google Scholar]
- Lai, H.; Sasaki, T.; Singh, N.P.; Messay, A. Effects of artemisinin-tagged holotransferrin on cancer cells. Life Sci. 2005, 76, 1267–1279. [Google Scholar] [CrossRef]
- Lai, H.; Nakase, I.; Lacoste, E.; Singh, N.P.; Sasaki, T. Artemisinin-transferrin conjugate retards growth of breast tumors in the rat. Anticancer Res. 2009, 29, 3807–3810. [Google Scholar]
- Nakase, I.; Gallis, B.; Takatani-Nakase, T.; Oh, S.; Lacoste, E.; Singh, N.P.; Goodlett, D.R.; Tanaka, S.; Futaki, S.; Lai, H.; et al. Transferrin receptor-dependent cytotoxicity of artemisinin-transferrin conjugates on prostate cancer cells and induction of apoptosis. Cancer Lett. 2009, 274, 290–298. [Google Scholar] [CrossRef]
- Zhou, X.; Soto-Gamez, A.; Nijdam, F.; Setroikromo, R.; Quax, W.J. Dihydroartemisinin-Transferrin Adducts Enhance TRAIL-Induced Apoptosis in Triple-Negative Breast Cancer in a P53-Independent and ROS-Dependent Manner. Front. Oncol. 2021, 11, 789336. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Gdynia, G.; Roth, W.; Bonavida, B.; Efferth, T. Combination treatment of malignant B cells using the anti-CD20 antibody rituximab and the anti-malarial artesunate. Int. J. Oncol. 2009, 35, 149–158. [Google Scholar] [PubMed][Green Version]
- Zeng, X.; Li, Y.; Fan, J.; Zhao, H.; Xian, Z.; Sun, Y.; Wang, Z.; Wang, S.; Zhang, G.; Ju, D. Recombinant human arginase induced caspase-dependent apoptosis and autophagy in non-Hodgkin’s lymphoma cells. Cell Death Dis. 2013, 4, e840. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, Z.; Li, L.; He, Y.; Fan, J.; Liu, Z.; Zhao, S.; Ju, D. The role of autophagy in the cytotoxicity induced by recombinant human arginase in laryngeal squamous cell carcinoma. Appl. Microbiol. Biotechnol. 2015, 99, 8487–8494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, X.; Li, Y.; Zeng, X.; Fan, J.; Sun, Y.; Xian, Z.; Zhang, G.; Wang, S.; Hu, H.; et al. Involvement of autophagy in recombinant human arginase-induced cell apoptosis and growth inhibition of malignant melanoma cells. Appl. Microbiol. Biotechnol. 2014, 98, 2485–2494. [Google Scholar] [CrossRef]
- Medhi, B.; Patyar, S.; Rao, R.S.; Byrav DS, P.; Prakash, A. Pharmacokinetic and Toxicological Profile of Artemisinin Compounds: An Update. Pharmacology 2009, 84, 323–332. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Zhang, M.; Guo, Z.; Wang, H.; He, M.; Xu, P.; Zhou, J.; Liu, Z.; Chen, Q. Mn(ii) mediated degradation of artemisinin based on Fe3O4@MnSiO3 -FA nanospheres for cancer therapy in vivo. Nanoscale 2015, 7, 12542–12551. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Xia, G.; Zhou, S.; Liu, Z.; Zhang, N.Q.; Wang, H.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, J.; Chen, R.; Shi, R.; Zhao, G.; Xia, G.; Li, R.; Liu, Z.; Tian, J.; Wang, H.H.; et al. Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 2016, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Zhu, X.; Zhang, X.; Chen, Q.; Chen, J.; Hou, L.; Zhang, Z. Visible-light-sensitive titanium dioxide nanoplatform for tumor-responsive Fe2+ liberating and artemisinin delivery. Oncotarget 2017, 8, 58738–58753. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Ma, L.; Mao, F.; Jiang, A.; Liu, D.; Wang, L.; Jia, Q.; Zhou, J. Artemisinin-Loaded Mesoporous Nanoplatform for pH-Responsive Radical Generation Synergistic Tumor Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 6155–6167. [Google Scholar] [CrossRef] [PubMed]
- Pan, U.N.; Sanpui, P.; Paul, A.; Chattopadhyay, A. Synergistic Anticancer Potential of Artemisinin When Loaded with 8-Hydroxyquinoline-Surface Complexed-Zinc Ferrite Magnetofluorescent Nanoparticles and Albumin Composite. ACS Appl. Bio Mater. 2018, 1, 1229–1235. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wang, Z.; Jin, Y. Fe3O4@SiO2 mesoporous spheres as Fe(ii) donors loaded with artemisinin and a photosensitizer to alleviate tumor hypoxia in PDT for enhanced anticancer therapy. New J. Chem. 2019, 43, 8761–8773. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, X.; Huang, L.; Yan, J.; Yu, B.Y.; Tian, J. Artemisinin-Based Smart Nanomedicines with Self-Supply of Ferrous Ion to Enhance Oxidative Stress for Specific and Efficient Cancer Treatment. ACS Appl. Mater. Interfaces 2019, 11, 29490–29497. [Google Scholar] [CrossRef]
- Guo, S.; Yao, X.; Jiang, Q.; Wang, K.; Zhang, Y.; Peng, H.; Tang, J.; Yang, W. Dihydroartemisinin-Loaded Magnetic Nanoparticles for Enhanced Chemodynamic Therapy. Front. Pharmacol. 2020, 11, 226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Zhu, X.; Zhang, Z.; Huang, H.; Hou, L. Artemisinin co-delivery system based on manganese oxide for precise diagnosis and treatment of breast cancer. Nanotechnology 2021, 32, 325101. [Google Scholar] [CrossRef]
- Dadgar, N.; Alavi, S.E.; Esfahani, M.K.M.; Akbarzadeh, A. Study of toxicity effect of pegylated nanoliposomal artemisinin on breast cancer cell line. Indian J. Clin. Biochem. 2013, 28, 410–412. [Google Scholar] [CrossRef]
- Righeschi, C.; Coronnello, M.; Mastrantoni, A.; Isacchi, B.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Strategy to provide a useful solution to effective delivery of dihydroartemisinin: Development, characterization and in vitro studies of liposomal formulations. Colloids Surf. B Biointerfaces 2014, 116, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhao, Y.; Sun, M.G.; Shi, J.F.; Ju, R.J.; Zhang, C.X.; Li, X.T.; Zhao, W.Y.; Mu, L.M.; Zeng, F.; et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 2014, 35, 5591–5604. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Faezizadeh, Z.; Mesbah-Namin, S.A.R.; Saravani, R. Experimental treatment of breast cancer-bearing BALB/c mice by artemisinin and transferrin-loaded magnetic nanoliposomes. Pharmacogn. Mag. 2015, 11, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Zhan, X.; Wang, L.; Ho, R.J.Y.; Sasaki, T. pH-responsive artemisinin dimer in lipid nanoparticles are effective against human breast cancer in a xenograft model. J. Pharm. Sci. 2015, 104, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, Y. Lysosomes activating chain reactions against cancer cells with a pH-switched prodrug/procatalyst co-delivery nanosystem. J. Mater. Chem. B 2017, 5, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Zhang, J.Y.; Luo, Q.; Xu, J.R.; Yan, Y.; Mu, L.M.; Bai, J.; Lu, W.L. Nanostructured dihydroartemisinin plus epirubicin liposomes enhance treatment efficacy of breast cancer by inducing autophagy and apoptosis. Nanomaterials 2018, 8, 804. [Google Scholar] [CrossRef]
- Ju, R.J.; Cheng, L.; Peng, X.M.; Wang, T.; Li, C.Q.; Song, X.L.; Liu, S.; Chao, J.P.; Li, X.T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cells, Nanomed. Biotechnol. 2018, 46, 616–628. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; You, C.; Sun, K.; An, P.; Sun, C.; Wang, M.; Zhu, X.; Sun, B. Iron oxide nanocarrier-mediated combination therapy of cisplatin and artemisinin for combating drug resistance through highly increased toxic reactive oxygen species generation. ACS Appl. Bio Mater. 2018, 1, 270–280. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Shi, X.; Li, Z.; Sun, Y. Effects of magnetic dihydroartemisinin nano-liposome in inhibiting the proliferation of head and neck squamous cell carcinomas. Phytomedicine 2019, 56, 215–228. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, X.; Lv, Y.; Zhao, Y. Low density lipoprotein receptor (LDLR)-targeted lipid nanoparticles for the delivery of sorafenib and Dihydroartemisinin in liver cancers. Life Sci. 2019, 239, 117013. [Google Scholar] [CrossRef]
- Liu, J.J.; Tang, W.; Fu, M.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Development of R 8 modified epirubicin–dihydroartemisinin liposomes for treatment of non-small-cell lung cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.A.; Lu, M.; Luo, Y.; Hu, Y.; Zhang, Y.; Xu, Z.; Gong, S.; Wu, Y.; Ma, X.N.; Yu, B.Y.; et al. A cancer-specific activatable theranostic nanodrug for enhanced therapeutic efficacy via amplification of oxidative stress. Theranostics 2020, 10, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid Interface Sci. 2021, 586, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Ma, J.; Zhang, H.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. LyP-1 Modification To Enhance Delivery of Artemisinin or Fluorescent Probe Loaded Polymeric Micelles to Highly Metastatic Tumor and Its Lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef]
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–936. [Google Scholar] [CrossRef]
- Nosrati, H.; Barzegari, P.; Danafar, H.; Kheiri Manjili, H. Biotin-functionalized copolymeric PEG-PCL micelles for in vivo tumour-targeted delivery of artemisinin. Artif. Cells Nanomed. Biotechnol. 2019, 47, 104–114. [Google Scholar] [CrossRef]
- Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Du, F.; Huang, J.; Lu, W.; Yu, J.; Liu, S.; Muir, B. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Colloids Surf. B Biointerfaces 2014, 115, 164–169. [Google Scholar] [CrossRef]
- Sun, Q.; Teong, B.; Chen, I.F.; Chang, S.J.; Gao, J.; Kuo, S.M. Enhanced apoptotic effects of dihydroartemisinin-aggregated gelatin and hyaluronan nanoparticles on human lung cancer cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 455–462. [Google Scholar] [CrossRef]
- Ma, W.; Xu, A.; Ying, J.; Li, B.; Jin, Y. Biodegradable core-shell copolymer-phospholipid nanoparticles for combination chemotherapy: An in vitro study. J. Biomed. Nanotechnol. 2015, 11, 1193–1200. [Google Scholar] [CrossRef]
- Phung, C.D.; Le, T.G.; Nguyen, V.H.; Vu, T.T.; Nguyen, H.Q.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. PEGylated-Paclitaxel and Dihydroartemisinin Nanoparticles for Simultaneously Delivering Paclitaxel and Dihydroartemisinin to Colorectal Cancer. Pharm. Res. 2020, 37, 129. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Y.; Zhai, S.; Chen, Q.; Xing, D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials 2015, 62, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dai, L.; Li, C.; Liu, J.; Wang, L.; Lei, J. Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol-dihydroartemisinin conjugate. Sci. Rep. 2016, 6, 29461. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Huang, H.; Yuan, S.; Wang, L.; Wang, S.; Chen, Y.; Feng, N.; Veroniaina, H.; Wu, Z.; Wu, Z.; et al. ROS-Mediated Apoptosis and Anticancer Effect Achieved by Artesunate and Auxiliary Fe(II) Released from Ferriferous Oxide-Containing Recombinant Apoferritin. Adv. Healthc. Mater. 2019, 8, 1900911. [Google Scholar] [CrossRef]
- He, Z.; Su, H.; Shen, Y.; Shi, W.; Liu, X.; Liu, Y.; Zhang, F.; Zhang, Y.; Sun, Y.; Ge, D. Poly(norepinephrine)-coated FeOOH nanoparticles as carriers of artemisinin for cancer photothermal-chemical combination therapy. RSC Adv. 2019, 9, 9968–9982. [Google Scholar] [CrossRef]
- Dong, L.; Wang, C.; Zhen, W.; Jia, X.; An, S.; Xu, Z.; Zhang, W.; Jiang, X. Biodegradable iron-coordinated hollow polydopamine nanospheres for dihydroartemisinin delivery and selectively enhanced therapy in tumor cells. J. Mater. Chem. B 2019, 7, 6172–6180. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.L.; Xie, R.; De, G.J.; Yi, H.; Zang, C.; Yang, M.Y.; Liu, L.; Ma, H.; Cai, W.Y.; Zhao, Q.H.; et al. PH-responsive artesunate polymer prodrugs with enhanced ablation effect on rodent xenograft colon cancer. Int. J. Nanomed. 2020, 15, 1771–1786. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Kokotidou, C.; Mitraki, A.; Pelecanou, M.; Sagnou, M. Advanced bis-MPA hyperbranched dendritic nanocarriers of artemisinin with anticancer potential. J. Nanoparticle Res. 2021, 23, 135. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, L.; Jiao, X.; Ji, Y.; Zhu, X.; Zhang, Z. Transferrin-mediated fullerenes nanoparticles as Fe2+-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366. [Google Scholar] [CrossRef]
- Emami, J.; Yousefian, H.; Sadeghi, H. Targeted Nanostructured Lipid Carrier for Brain Delivery of Artemisinin: Design, Preparation, Characterization, Optimization and Cell Toxicity. J. Pharm. Pharm. Sci. 2018, 21, 225s–241s. [Google Scholar] [CrossRef]
- Asgharkhani, E.; Najmafshar, A.; Chiani, M. Artemisinin (ART) drug delivery using mixed non-ionic surfactants and evaluation of their efficiency in different cancer cell lines. Int. J. Drug Deliv. Technol. 2014, 4, 67–71. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; du Plessis, L.; du Preez, J.L.; Haynes, R.K.; du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei-Parsa, M.J.; Najafabadi, M.R.H.; Haeri, A.; Zahmatkeshan, M.; Ebrahimi, S.A.; Pazoki-Toroudi, H.; Adel, M. Preparation, characterization, and evaluation of the anticancer activity of artemether-loaded nano-niosomes against breast cancer. Breast Cancer 2020, 27, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gallis, B.; Taya, M.; Wang, S.; Ho, R.J.Y.; Sasaki, T. pH-Responsive Artemisinin Derivatives and Lipid Nanoparticle Formulations Inhibit Growth of Breast Cancer Cells In Vitro and Induce Down-Regulation of HER Family Members. PLoS ONE 2013, 8, e59086. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Q.; Qi, Z.; Deng, T.; Liu, F. Recent Progresses in Cancer Nanotherapeutics Design Using Artemisinins as Free Radical Precursors. Front. Chem. 2020, 8, 472. [Google Scholar] [CrossRef]
- Duan, Y.; Qin, W.; Suo, F.; Zhai, X.; Guan, Y.; Wang, X.; Zheng, Y.; Liu, H. Design, synthesis and in vitro evaluation of stilbene derivatives as novel LSD1 inhibitors for AML therapy. Bioorg. Med. Chem. 2018, 26, 6000–6014. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. Design of drug delivery systems containing artemisinin and its derivatives. Molecules 2017, 22, 323. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, C.J.; Steiert, M.; Talmadge, J.E.; Derfus, A.M.; Barry, S.E. Targeted nanoparticles for detecting and treating cancer. Drug Dev. Res. 2006, 67, 70–93. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and biocompatibility of carbon nanoparticles. J. Nanosci. Nanotechnol. 2006, 6, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cheng, Y.; Tan, J.; Li, J.; Cheng, H.; Hu, H.; Du, C.; Zhao, S.; Yan, Y.; Liu, M. Carbon Nanomaterials With Hollow Structures: A Mini-Review. Front. Chem. 2021, 9, 668336. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Attama, A.A.; Umeyor, C.E. The use of solid lipid nanoparticles for sustained drug release. Ther. Deliv. 2015, 6, 669–684. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Hanafy, N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]

| Cell Lines; Cancer | Drugs | Effects | Ref. |
|---|---|---|---|
| K562; Leukemia | DHA | Autophagosome formation↑; LC3-I and LC3-II↑; ROS↑; TfR↓; Cell viability↓ | 2012 [100] |
| Eca109, Ec9706; Esophageal cancer | DHA | Autophagosome formation↑; LC3-I and LC3-II↑ | 2013 [59] |
| Diverse cell lines | DHA | Autophagosome and autolysosome formation↑; LC3-I and LC3-II↑; P62↓; p-IκBα; ROS scavenger 4-Hydroxy-TEMPO (TEMPO) reduces autophagic vacuoles | 2014 [101] |
| BxPC-3, PANC-1; Pancreatic cancer | DHA | Cell growth↓; LC3-1↓; LC3-II↑; 3MA enhances DHA-induced apoptosis; p-JNK↑; Beclin 1↑; ROS↑; JNK inhibitor and beclin-1 siRNA suppress DHA-induced autophagy | 2014 [95] |
| Cal-27; Tongue squamous cell carcinoma | DHA | Cell viability↓; Colony formation↓; Autolysosome formation↑; LC3-II↑; DNA damage↑; Nuclear p-STAT3↓; Beclin-1↑; Tumor growth↓ | 2017 [102] |
| SKOV3; Ovarian cancer | ATS DHA | Cell viability↓; Beclin-1↑; LC3-II↑; Autophagosome formation↑; Cell viability rescued by CQ and BafA1 | 2018 [98] |
| Cholangiocarcinoma | DHA | Cell viability↓; Colony formation↓; LC3-I and LC3-II↑; P62↓; PI3KC1↓; AKT and mTOR↓; BCL-1↓; Vps34↑; Beclin-1↑; Spautin-1 inhibits DHA-induced autophagy and cell death | 2018 [99] |
| Diverse cell lines | DHA-37 | Cell viability↓; Cell viability rescued by autophagy inhibitors CQ, 3-MA or LY294002; LC3-II↑; P62↓; Autolysosome formation↑; HMGB1↑; p-MAPK and P38↑; Tumor growth↓ | 2018 [103] |
| HCT116; Colon cancer | ATS | Cell viability↓; Autolysosome formation↑; Atg5↑; Beclin-1↑; LC3-II↑; Autophagy inhibitor HCQ promotes ATS-induced apoptosis; Tumor growth↓ | 2018 [96] |
| SU-DHL-4, SU-DHL-10, OCI-LY3; Diffuse large B cell lymphoma | SM1044 | Autolysosome formation↑; LC3-II↑; Autophagy inhibitors CQ and BafA1 inhibit DHA-induced apoptosis; p-AMPK↑; ULK1↑; Ceramide↑; Caramide inhibitor S1P and l-cycloserine, the Ca2+/calmodulin-dependent kinase kinases inhibitor STO-609 inhibit AMPK activation; Tumor growth↓ | 2018 [97] |
| HepG2215; Hepatocellular carcinoma | DHA | Cell viability↓; Colony formation↓; DNA damage↑; Autolysosome formation↑; P62↓; LC3-II↑; ROS↑; cell mobility↓; | 2019 [104] |
| HeLa; Cervical cancer | DHA | Cell viability↓; Tumor growth↓; LC3 puncta↑; LC3-II↑; Autolysosome formation↑; ROS↑; γH2AX↑; DNA damage↑; p-mTOR | 2019 [105] |
| Eca109; Esophagus squamous cell carcinoma | DHA | Cell viability↓; Tumor growth↓; ROS↑; LC3 puncta↑; P62↓; LC3-II↑; TRF2↓; NAC reduces LC3 puncta | 2020 [106] |
| Diverse cell lines | DHA | Cell viability↓; Colony formation↓; Tumor growth↓; LC3-II↑; Beclin-1↑; P62↓; Autolysosome formation↑; IFI16↓; Ra1B↓; USP33↓ | 2020 [107] |
| TE-1, Eca109; Esophageal cancer | DHA | Cell migration↓; LC3 puncta↑; LC3↑; P62/SQSTM↓; 3MA or overexpression of Akt restores DHA-suppressed migration; p-AKT and p-mTOR↓; E-cadherin↑; N-cadherin↓; Vimentin↓ | 2020 [108] |
| EJ, T24; Bladder cancer | ATS | Cell viability↓; Cell migration↓; Colony formation↓; Autolysosome formation↑; p-AMPK and p-ULK1↑; p-mTOR↓; LC3-II/I ratio↑; 3MA inhibits ATS-induced apoptosis; AMPK activator enhances ATS-induced autophagy and apoptosis; AMPK inhibitor, 3MA, and NAC suppresses ATS-induced apoptosis; ROS↑ | 2020 [94] |
| BON-1, QGP-1; Pancreatic neuroendocrine cancer | ATS | Cell viability↓; Cell death rescued by 3MA; LC3-II↑; DHA induces apoptosis, ferroptosis, and autophagy | 2020 [109] |
| Ishikawa, AN3CA; Endometrial carcinoma | ATS | Cell viability↓; Cell migration↓; CD155↑; P62↓; LC3-II/I ratio↑; ATG5↑; ATS-treated cancer cell triggers NK92 cytotoxicity | 2021 [110] |
| U2932, SU-DHL2, SU-DHL4, SU-DHL6, 293 T; Diffuse large B cell lymphoma | ATS | Cell viability↓; Colony formation↓; Apoptosis↑; P62↓; LC3-II/I ratio↑; Acidic vesicular organelles formation↑; CQ reduces ATS-induced apoptosis; p-STAT3↑; Knockdown of STAT3 enhances ATS-induced autophagy, apoptosis, and ferroptosis; Tumor growth↓ | 2021 [85] |
| Cell Lines; Cancer | Drugs | Effects | Ref. |
|---|---|---|---|
| Necroptosis | |||
| Diverse cell lines | ATS | Cell viability↓; p-MLKL↑; RIPK1↑; Caspase inhibitor z-VAD-fmk (zVAD), Nec and siRIPK1 rescue ATS-induced cell death | 2014 [115] |
| Diverse cell lines | ATS | Cell viability↓; ROS↑; Mitochondrial ROS↑; zVAD, Nec, siRIPK1, and ROS scavengers rescue ATS-induced cell death; | 2017 [116] |
| MT-2, MT-4, HUT-102; Leukemia | ATS | T-cell growth↓; ROS↑; Nec rescues ATS-induced cell death; Tumor growth↓ | 2020 [78] |
| Pyroptosis | |||
| MCF-7, MDA-MB-231; Breast cancer | DHA | Cell viability↓; Colony formation↓; LDH↑; AIM2↑; Cleaved caspase 3↑; GSDME/DFNA5↑; HMFB1↑; IL-1β↑; shAIM2 and shDFNA5 restore cell survival and colony formation; Tumor growth↓ | 2021 [118] |
| Eca109, Ec9706; Esophageal squamous cell carcinoma | DHA | Cell viability↓; LDH↑; IL-1β↑; GSDME-NT↑; Cleaved caspase 3↑; Caspase inhibitor Ac-DEVD-CHO reduces GSDME-NT, LDH, IL-1β, and rescue cell viability; Tumor growth↓ | 2021 [119] |
| Carrier Materials | Cargo | Cell Lines; Cancer | Main Outcomes | Ref. |
|---|---|---|---|---|
| Inorganic-based NPs | ||||
| MnSiO3, Fe3O4 | ART | A549; Lung cancer | Mn2+ release↑; Antitumor activity in vivo↑ | 2015 [153] |
| Fe (III) carboxylate | DHA | HeLa; Cervical cancer. A549; Lung cancer | Co-release of DHA and Fe3+; Complete tumor cure with no observable side effects on normal tissues | 2016 [154] |
| Dual metal-organic-frameworks | ART | HeLa; Cervical cancer | High tumor inhibition rate (~2-fold of free ART); No obvious effect on the major organs of mice | 2016 [155] |
| HA-TiO2 | ART | MCF-7; Breast cancer | Generation of ROS under visual light irradiation; Higher concentration of ART in tumor tissue | 2017 [156] |
| Mesoporous NiO, Tb-DPTA | ART | HeLa; Cervical cancer | Ni2+ release↑; Antitumor activity in vitro and in vivo↑ | 2018 [157] |
| ZnFe2O4 | ART | Diverse cell lines | Lower cell viability than free ART | 2018 [158] |
| SiO2, Fe3O4 | ART | HepG-2; Liver cancer | Easy release of Fe2+ by weak acidic etching; Enhanced production of ROS with NIR light irradiation | 2019 [159] |
| Mesoporous silica | ART, TF | MCF-7; Breast cancer. CT26; Colon cancer | Co-delivery of iron to cancer cells; Release of ART in the presence of cathepsin B; ROS↑; Glutathione↓; Anti-cancer efficacy in vitro and in vivo↑ | 2019 [160] |
| FeCl3 · 6H2O, Na3Cit · 2H2O, NaOAc | DHA | MCF-7, MDA-MB-231, MDA-MB-453; Breast cancer | Fe2+ release↑; High toxicity to intractable breast cancer cells | 2020 [161] |
| Hollow mesoporous manganese trioxide | ART, Mn | MCF-7; Breast cancer | Deep penetration of solid tumors | 2021 [162] |
| Liposomes | ||||
| PPC, PEG2000 | ART | MCF-7; Breast cancer | Half IC50 compared to free ART | 2013 [163] |
| P90G, CHOL | DHA | MCF-7; Breast cancer | Better cellular uptake efficiency | 2014 [164] |
| DQA-PEG2000-DSPE | AM, DOX | C6; Brain cancer | Transport of drug across BBB, elimination of brain CSCs; Destruction of vasculogenic mimicry channels | 2014 [165] |
| DPPC, DSPC, CHOL | ART, TF | MCF-7, MDA-MB-231; Breast cancer | 10- and 5.5-fold higher levels of ART and TF production than free drugs; Tumor volume in mice↓ | 2015 [166] |
| DPPC, mPEG2000 | ART Dimer | MDA-MB-231; Breast cancer | Better anti-tumor efficacy than Paclitaxel | 2015 [167] |
| Hollow mesoporous silica, Fe3O4 | ART | ZR75-30; Ductal carcinoma | Lysosomal environment-responsively released ART result in decreased cell viability | 2017 [168] |
| EPC, CHOL, PEG2000-DSPE | DHA, Epirubicin | MDA-MB-435S, MDA-MB-231, MCF-7; Breast cancer | Drug circulation↑; Targeting delivery to the tumor; Anticancer efficacy↑ than free DHA or Epitubicin | 2018 [169] |
| DSPE-PEG2000-NHS | DHA, Daunorubicin | MDA-MB-435S; Breast cancer | More accumulation in tumor than free DHA; Better antitumor efficacy with no obvious toxicity in mice | 2018 [170] |
| CHOL, cRGD-PEG-DSPE, phospholipids, Fe3O4 | ART, Cisplatin | A549/R; NSCLC | The 15.17-fold lower IC50 value of free cisplatin against A549/R cells, ROS↑; Cell apoptosis rates↑ | 2018 [171] |
| FeCl3 · 6H2O, FeSO4 · 7H2O, sodium oleate, sodium hydroxide, Acetonitrile. | DHA | HNSCC; Head and neck squamous cell carcinoma | Significant targeting effect in a magnetic field; Better inhibition of HNSCC in mice than free DHA | 2019 [172] |
| Cholesteryl oleate, glyceryl trioleate, CHOL, DOPE | DHA, SRF | HepG2; Liver cancer | BAX and Bcl-2↑; Exhibited a 3-fold higher SubG1% of cells than free DHA or SRF | 2019 [173] |
| EPC, CHOL, DSPE-PEG2000, DSPE-PEG2000-R8 | DHA, Epirubicin | A549; NSCLC: | Increased drug accumulation; Enhanced specificity and anti-tumor efficacy in vivo | 2019 [174] |
| DSPE-PEG2000, DOPE, CHEMS | DHA, TF | HepG2; Liver cancer | High oxidative state at the tumor site; Eradication of HepG2 tumor in mice | 2020 [175] |
| DSPE-PEG2000-HE-R6 | ART | 4 T1; Breast cancer | Longer retention time in tumors and higher efficiency in tumor suppression | 2021 [176] |
| Micelles | ||||
| PEG-PCL | ART | MDA-MB-435S; Breast cancer | Specific delivery of ART to tumor site; Higher antitumor efficacy than other ART formulations in vivo with low toxicity | 2012 [177] |
| PCL-PEG-PCL | ART | MCF-7, 4T1; Breast cancer | Prolong in vivo residence time in rats | 2018 [178] |
| Biotin-PEG-PCL | ART | MCF-7; Breast cancer | Tumor inhibition; No toxic effects on HFF2 fibroblast cells | 2019 [179] |
| Polymer-based NPs | ||||
| mPEG | ATS | L1210; Leukemia. MCF7; Breast cancer | Controllable release of ATS in the presence of esterase | 2014 [180] |
| Formulation I: Gelatin; Formulation II: Hyaluronan | DHA | A549; NSCLC | Formation of DHA nanosized aggregates in an electrostatic field; Higher anticancer proliferation activities than DHA alone in A549 cells. | 2014 [181] |
| Poly(lactic-co-glycolic acid) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine | DHA, DOX | HeLa; Cervical cancer. HepG2; Liver cancer | Increased doxorubicin accumulation in cell nuclei; cytotoxicity↑ | 2015 [182] |
| PEG | DHA, Paclitaxel | HT-29; Colon cancer | Higher accumulation in the tumor site; Tumor growth in vivo↓; Systemic toxicity↓ | 2015 [183] |
| Graphene oxide | DHA, TF | EMT6; Breast cancer | Significant enhancement of delivery specificity and tumor cytotoxicity; Complete tumor cure in mice | 2015 [184] |
| PEG | DHA, TF | LLC; Lung cancer | High solubility (~102-fold of free DHA); Relatively high drug loading; Circulating half-life↑; One-fifth the size of the tumor in free DHA | 2016 [185] |
| H-apoferritin | AS | Hela; Cervical cancer | pH-responsive release of AS; Cytotoxic ROS↑; Cytotoxicity↑; Biocompatibility↑; No additional side effects | 2019 [186] |
| PNE, FeOOH | ART | 4T1; Mouse breast cancer | Extremely low toxicity to normal tissue; Tumor elimination after 7-day treatment; No tumor recurrence in 30 days after treatment. | 2019 [187] |
| Iron coordinated hollow polydopamine nanospheres | DHA | HeLa; Cervical cancer | 3.05-fold higher anti-tumor efficacy than free DHA | 2019 [188] |
| PEOz-PLA-PBAE | ATS dimer | CT-26; Colon cancer | Enhanced cellular uptake of the drug depot by the cancer cells; Enhanced anti-tumor efficacy in vivo | 2020 [189] |
| Bis-MPA, PEG | ART | MCF-7, MDA-231; Breast cancer | Completely non-toxic towards healthy fibroblasts | 2021 [190] |
| Carbon-based NPs | ||||
| HA-C60 | AS | MCF-7; Breast cancer | Increased intracellular accumulation of AS in tumor; Remarkably enhanced antitumor efficacy | 2015 [191] |
| NLCs | ||||
| Cholesterol, oleic acid, stearylamine | ART | U87MG; Malignant gliomas | High entrapment efficiency; Controlled drug release for brain administration | 2018 [192] |
| Niosomes | ||||
| Span 60, Tween 60, PEG-600 | ART | MCF-7; Breast cancer | 4-fold higher cytotoxic activity than free ART | 2014 [193] |
| Span 60, CHOL | Artemisone | A-375; Melanoma | Highly selective cytotoxicity towards melanoma cells, not to normal skin cells | 2015 [194] |
| Span, Tween, CHOL | AM, Paclitaxel | 4T1; Mouse breast cancer | Superior tumor necrosis and smaller tumor volume than free AM | 2020 [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Suo, F.; Haslinger, K.; Quax, W.J. Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery. Pharmaceutics 2022, 14, 395. https://doi.org/10.3390/pharmaceutics14020395
Zhou X, Suo F, Haslinger K, Quax WJ. Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery. Pharmaceutics. 2022; 14(2):395. https://doi.org/10.3390/pharmaceutics14020395
Chicago/Turabian StyleZhou, Xinyu, Fengzhi Suo, Kristina Haslinger, and Wim J. Quax. 2022. "Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery" Pharmaceutics 14, no. 2: 395. https://doi.org/10.3390/pharmaceutics14020395
APA StyleZhou, X., Suo, F., Haslinger, K., & Quax, W. J. (2022). Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery. Pharmaceutics, 14(2), 395. https://doi.org/10.3390/pharmaceutics14020395








