Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery
Abstract
:1. Introduction
2. Single Stimuli
2.1. Internal Stimuli
2.1.1. pH-Responsive
2.1.2. Redox Potential-Responsive
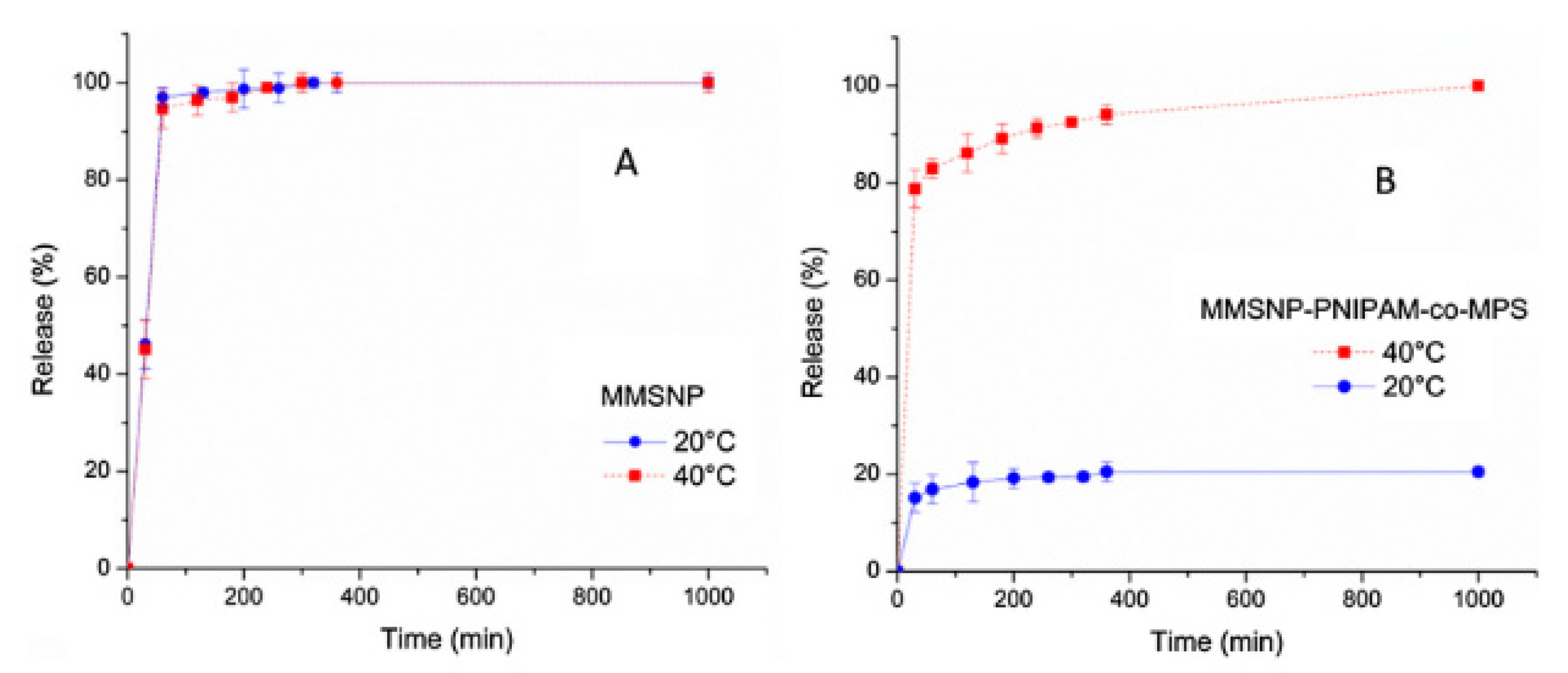
2.1.3. Thermo-Responsive
2.2. External Stimuli
2.2.1. Light-Responsive
2.2.2. Ultrasound-Responsive
2.2.3. Others
3. Combination of Various Stimuli for Polymers
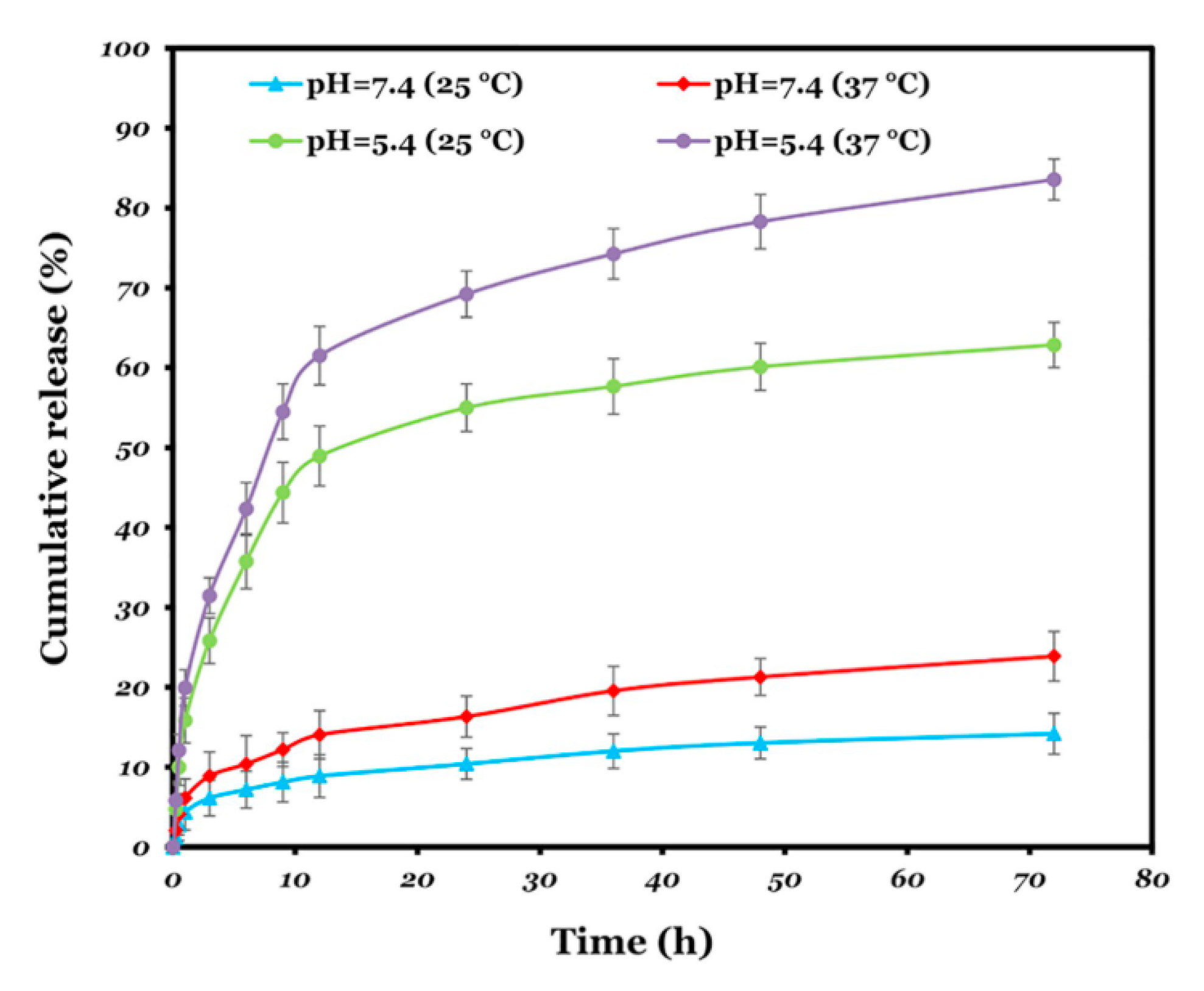
3.1. pH/Temperature-Responsive Polymers
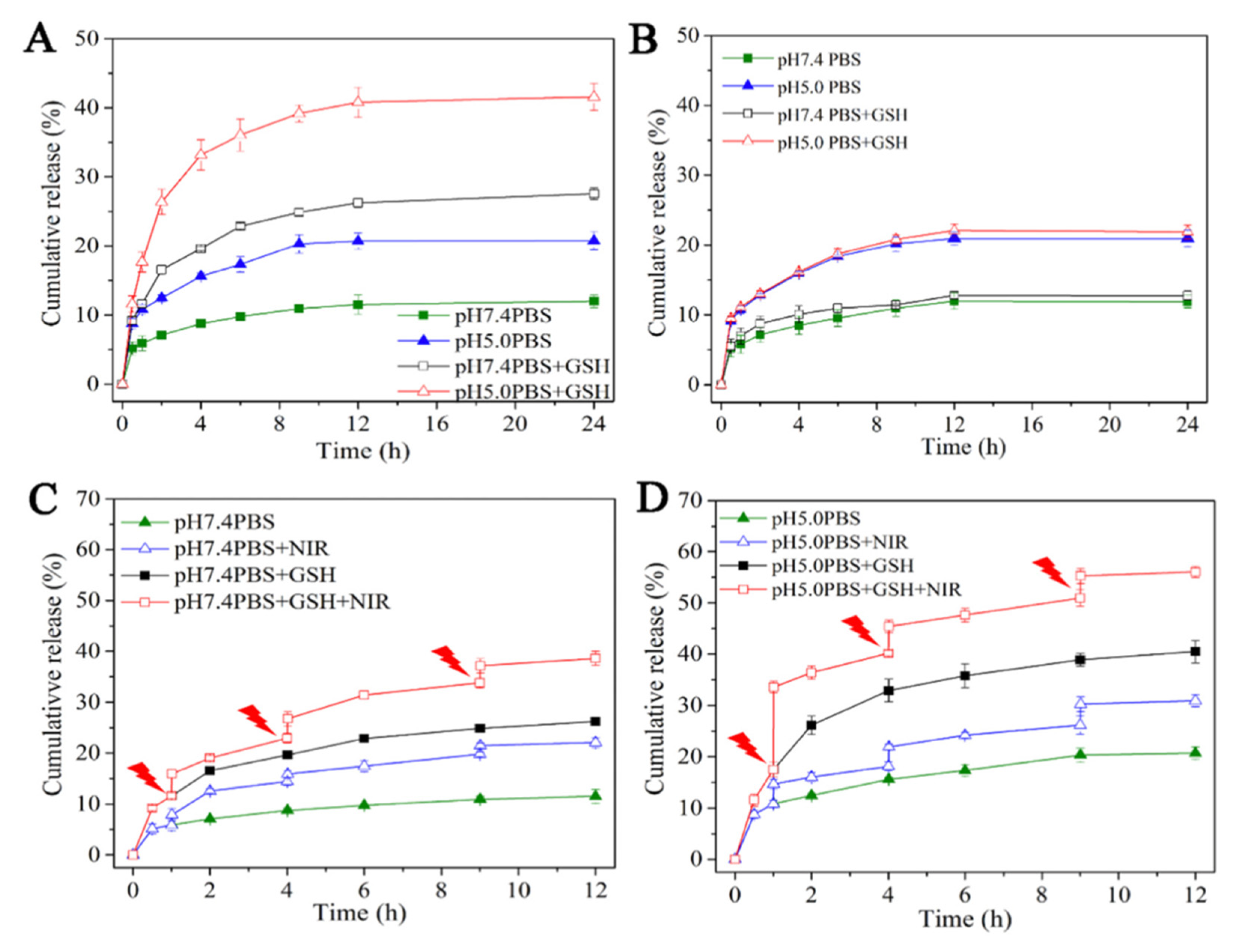
3.2. pH/Redox-Responsive Polymers
3.3. Double-pH-Responsive Polymers
3.4. Multiple-Stimuli-Responsive Polymers
4. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Daviau, T.; Brem, H. Morphological Characterization of Polyanhydride Biodegradable Implant Gliadel during in Vitro and in Vivo Erosion Using Scanning Electron Microscopy. Pharm. Res. 1996, 13, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Polymers Blending as Release Modulating Tool in Drug Delivery. Front. Mater. 2021, 8, 752813. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in multiple stimuli-responsive drug-delivery systems for cancer therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef]
- Hatai, J.; Hirschhäuser, C.; Niemeyer, J.; Schmuck, C. Multi-Stimuli-Responsive Supramolecular Polymers Based on Noncovalent and Dynamic Covalent Bonds. ACS Appl. Mater. Interfaces 2020, 12, 2107–2115. [Google Scholar] [CrossRef]
- Johnson, L.; Gray, D.M.; Niezabitowska, E.; McDonald, T.O. Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability. Nanoscale 2021, 13, 7879–7896. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Hershberger, K.K.; Gauger, A.J.; Bronstein, L.M. Utilizing Stimuli Responsive Linkages to Engineer and Enhance Polymer Nanoparticle-Based Drug Delivery Platforms. ACS Appl. Bio Mater. 2021, 4, 4720–4736. [Google Scholar] [CrossRef]
- Liu, G.; Lovell, J.F.; Zhang, L.; Zhang, Y. Stimulus-Responsive Nanomedicines for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 6380. [Google Scholar] [CrossRef] [PubMed]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym.-Plast. Technol. Mater. 2021, 1996–2024. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Cui, L.; Zhuang, X.; Ding, J.; Chen, X. Advances in Stimuli-Responsive Polypeptide Nanogels. Small Methods 2018, 3, 1700307. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as Drug-Delivery Systems: A Comprehensive Overview. Ther. Deliv. 2019, 10, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Salatin, S.; Naderinia, A.; Jelvehgari, M. Novel Pentablock Copolymers as Thermosensitive Self-Assembling Micelles for Ocular Drug Delivery. Adv. Pharm. Bull. 2017, 7, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Vicario-De-la-torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-González, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem.-Int. Ed. 2020, 36, 15342–15377. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Zhao, M.; Wu, J.; Lin, D. Construction of a Tumor Microenvironment PH-Responsive Cleavable PEGylated Hyaluronic Acid Nano-Drug Delivery System for Colorectal Cancer Treatment. Biomater. Sci. 2020, 8, 1885–1896. [Google Scholar] [CrossRef]
- Sun, C.Y.; Liu, Y.; Du, J.Z.; Cao, Z.T.; Xu, C.F.; Wang, J. Facile Generation of Tumor-PH-Labile Linkage-Bridged Block Copolymers for Chemotherapeutic Delivery. Angew. Chem.-Int. Ed. 2016, 55, 1010–1014. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Kang, Y.; Wang, M. Glutathione-Responsive Polymeric Micelles Formed by a Biodegradable Amphiphilic Triblock Copolymer for Anticancer Drug Delivery and Controlled Release. ACS Biomater. Sci. Eng. 2015, 1, 585–592. [Google Scholar] [CrossRef]
- Tseng, W.C.; Fang, T.Y.; Lin, Y.C.; Huang, S.J.; Huang, Y.H. Reversible Self-Assembly Nanovesicle of UCST Response Prepared with Multi-L-Arginyl-Poly-L-Aspartate Conjugated with Polyethylene Glycol. Biomacromolecules 2018, 19, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Conzatti, G.; Cavalie, S.; Combes, C.; Torrisani, J.; Carrere, N.; Tourrette, A. PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion. Colloids Surf. B: Biointerfaces 2017, 151, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, M.; Lin, Z.; Shi, X. Fast Near-Infrared Light Responsive Shape Memory Composites: Polydopamine Nanospheres Hybrid Polynorbornene. Polymer 2020, 206, 122898. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, D.; Hu, P.; Gao, L.; Chen, D.; Qiao, Y.; Wu, Y.; Jiang, X.; Li, G. Polydopamine-Coated Gold Nanostars for near-Infrared Cancer Photothermal Therapy by Multiple Pathways. J. Mater. Sci. 2019, 54, 12036–12048. [Google Scholar] [CrossRef]
- Li, Y.; Tong, R.; Xia, H.; Zhang, H.; Xuan, J. High Intensity Focused Ultrasound and Redox Dual Responsive Polymer Micelles. Chem. Commun. 2010, 46, 7739–7741. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.-L.; Korin, N.; Kanapathipillai, M.; Mammoto, A.; Mammoto, T.; Jiang, A.; Mannix, R.; Uzun, O.; Johnson, C.; Bhatta, D.; et al. Ultrasound-Sensitive Nanoparticle Aggregates for Targeted Drug Delivery. Biomaterials 2017, 139, 187–194. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, Y.; Zhao, G. Near-Infrared Light-, Magneto-, and pH-Responsive GO-Fe3O4/Poly(N-isopropylacrylamide)/alginate Nanocomposite Hydrogel Microcapsules for Controlled Drug Release. Langmuir 2021, 37, 5522–5530. [Google Scholar] [CrossRef]
- García-García, G.; Fernández-álvarez, F.; Cabeza, L.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Gemcitabine-loaded magnetically responsive poly(ε-caprolactone) nanoparticles against breast cancer. Polymers 2020, 12, 2790. [Google Scholar] [CrossRef]
- Rifaie-Graham, O.; Galensowske, N.F.B.; Dean, C.; Pollard, J.; Balog, S.; Gouveia, M.G.; Chami, M.; Vian, A.; Amstad, E.; Lattuada, M.; et al. Shear Stress-Responsive Polymersome Nanoreactors Inspired by the Marine Bioluminescence of Dinoflagellates. Angew. Chem.-Int. Ed. 2021, 60, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Augustijns, P.; Grimm, M.; Koziolek, M.; Lemmens, G.; Parrott, N.; Pentafragka, C.; Reppas, C.; Rubbens, J.; Van Den Abeele, J.; et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019, 134, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. From Drug Dosage Forms to Intelligent Drug-Delivery Systems: A Change of Paradigm; Alvarez-Lorenzo, A., Concheiro, A., Eds.; RSC Smart Materials No. 2 Smart Materials for Drug Delivery: Volume 1; Royal Society of Chemistry: London, UK, 2013; Volume 1. [Google Scholar]
- Ojugo, A.S.E.; Mcsheehy, P.M.J.; Mcintyre, D.J.O.; Mccoy, C.; Stubbs, M.; Leach, M.O.; Judson, I.R.; Grif, J.R. Measurement of the Extracellular PH of Solid Tumours in Mice by Magnetic Resonance Spectroscopy: A Comparison of Exogenous 19 F and 31 P Probes. NMR Biomed. 1999, 12, 495–504. [Google Scholar] [CrossRef]
- Kost, J.; Langer, R. Responsive Polymeric Delivery Systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Nasab, N.A.; Kumleh, H.H.; Beygzadeh, M.; Teimourian, S.; Kazemzad, M. Delivery of Curcumin by a PH-Responsive Chitosan Mesoporous Silica Nanoparticles for Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 78–85. [Google Scholar] [CrossRef]
- Hu, L.; Xiong, C.; Wei, G.; Yu, Y.; Li, S.; Xiong, X.; Zou, J.-J.; Tian, J. Stimuli-Responsive Charge-Reversal MOF@polymer Hybrid Nanocomposites for Enhanced Co-Delivery of Chemotherapeutics towards Combination Therapy of Multidrug-Resistant Cancer. J. Colloid Interface Sci. 2022, 608, 1882–1893. [Google Scholar] [CrossRef]
- Saw, P.E.; Yao, H.; Lin, C.; Tao, W.; Farokhzad, O.C.; Xu, X. Stimuli-Responsive Polymer-Prodrug Hybrid Nanoplatform for Multistage SiRNA Delivery and Combination Cancer Therapy. Nano Lett. 2019, 19, 5967–5974. [Google Scholar] [CrossRef]
- Elbaz, N.M.; Owen, A.; Rannard, S.; McDonald, T.O. Controlled Synthesis of Calcium Carbonate Nanoparticles and Stimuli-Responsive Multi-Layered Nanocapsules for Oral Drug Delivery. Int. J. Pharm. 2020, 574, 118866. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy Angewandte. Nanomedicine 2014, 53, 2–47. [Google Scholar] [CrossRef]
- Felber, A.E.; Dufresne, M.H.; Leroux, J.C. PH-Sensitive Vesicles, Polymeric Micelles, and Nanospheres Prepared with Polycarboxylates. Adv. Drug Deliv. Rev. 2012, 64, 979–992. [Google Scholar] [CrossRef]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles That Are Responsive to Intracellular PH Change. Angew. Chem.-Int. Ed. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Alsehli, M. Polymeric Nanocarriers as Stimuli-Responsive Systems for Targeted Tumor (Cancer) Therapy: Recent Advances in Drug Delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-Responsive Polymeric Nanocarriers for the Controlled Transport of Active Compounds: Concepts and Applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A PH-Responsive Polymer Based on Dynamic Imine Bonds as a Drug Delivery Material with Pseudo Target Release Behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery ☆. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular Drug Release Nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione Responsive Polymers and Their Application in Drug Delivery Systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Qi, J. Nano-Drug Design Based on the Physiological Properties of Glutathione. Molecules 2021, 26, 5567. [Google Scholar] [CrossRef]
- Smith, C.V.; Jones, D.P.; Guenthner, T.M.; Lash, L.H.; Lauterburg, B.H. Compartmentation of Glutathione: Implications for the Study of Toxicity and Disease. Toxicol. Appl. Pharmacol. 1996, 140, 1–12. [Google Scholar] [CrossRef]
- Stratford, I.J.; Adams, G.E.; Bremner, J.C.M.; Cole, S.; Edwards, H.S.; Robertson, N.; Wood, P.J. Manipulation and Exploitation of the Tumour Environment for Therapeutic Benefit. Int. J. Radiat. Biol. 1994, 65, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Tachibana, C.; Rahr Winther, J.; Appenzeller-Herzog, C. Intracellular Glutathione Pools Are Heterogeneously Concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive Imaging of Tumor Redox Status and Its Modification by Tissue Glutathione Levels. Cancer Res. 2002, 62, 307–312. [Google Scholar] [PubMed]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-Responsive Polymers for Drug Delivery: From Molecular Design to Applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Wright, A.J.; Fellows, G.A.; Griffiths, J.R.; Wilson, M.; Bell, B.A.; Howe, F.A. Ex-vivo HRMAS of adult brain tumours: Metabolite quantification and assignment of tumour biomarkers. Mol. Cancer 2010, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yan, X.; Yuan, T.; Liang, J.; Fan, Y.; Gu, Z.; Zhang, X. Disassemblable Micelles Based on Reduction-Degradable Amphiphilic Graft Copolymers for Intracellular Delivery of Doxorubicin. Biomaterials 2010, 31, 7124–7131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Sun, T.; Wang, J. Redox-Responsive Nanoparticles from the Single Disulfide Bond-Bridged Block Copolymer as Drug Carriers for Overcoming Multidrug Resistance in Cancer Cells. Bioconjugate Chem. 2011, 22, 1939–1945. [Google Scholar] [CrossRef]
- Breitenbach, B.B.; Steiert, E.; Konhäuser, M.; Vogt, L.M.; Wang, Y.; Parekh, S.H.; Wich, P.R. Double Stimuli-Responsive Polysaccharide Block Copolymers as Green Macrosurfactants for near-Infrared Photodynamic Therapy. Soft Matter 2019, 15, 1423–1434. [Google Scholar] [CrossRef]
- Sun, H.; Guo, B.; Li, X.; Cheng, R.; Meng, F.; Liu, H.; Meng, F.; Zhong, Z. Shell-Sheddable Micelles Based on Dextran-SS-Poly(ε-Caprolactone) Diblock Copolymer for Efficient Intracellular Release of Doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, K.; Luo, J.; Xiao, W.; Lee, J.S.; Gonik, A.M.; Kato, J.; Dong, T.A.; Lam, K.S. Biomaterials Well-defined, Reversible Disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials 2011, 32, 6633–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lokitz, B.S.; Armes, S.P.; McCormick, C.L. Synthesis of Reversible Shell Cross-Linked Micelles for Controlled Release of Bioactive Agents. Macromolecules 2006, 39, 2726–2728. [Google Scholar] [CrossRef]
- Takeoka, Y.; Aoki, T.; Sanui, K.; Ogata, N.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Watanabe, M. Electrochemical Control of Drug Release from Redox-Active Micelles. J. Control. Release 1995, 33, 79–87. [Google Scholar] [CrossRef]
- Hyperthermia in Cancer Treatment-National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/types/surgery/hyperthermia-fact-sheet (accessed on 25 March 2019).
- Kujawa, P.; Winnik, F.M. Volumetric Studies of Aqueous Polymer Solutions Using Pressure Perturbation Calorimetry: A New Look at the Temperature-Induced Phase Transition of Poly(N-Isopropylacrylamide) in Water and D2O. Macromolecules 2001, 34, 4130–4135. [Google Scholar] [CrossRef]
- Cummings, C.; Murata, H.; Koepsel, R.; Russell, A.J. Dramatically Increased PH and Temperature Stability of Chymotrypsin Using Dual Block Polymer-Based Protein Engineering. Biomacromolecules 2014, 15, 763–771. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y. Recent Advances in Multi-Temperature-Responsive Polymeric Materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Lee, J.; Ku, K.H.; Kim, M.; Shin, J.M.; Han, J.; Park, C.H.; Yi, G.R.; Jang, S.G.; Kim, B.J. Stimuli-Responsive, Shape-Transforming Nanostructured Particles. Adv. Mater. 2017, 29, 1700608. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Z.; Tang, Y. Conjugated Polymers-Based Thermal-Responsive Nanoparticles for Controlled Drug Delivery, Tracking, and Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Bio Mater. 2019, 2, 4485–4492. [Google Scholar] [CrossRef]
- Kuckling, D.; Adler, H.-J.P.; Arndt, K.-F.; Ling, L.; Habicher, W.D. Temperature and pH dependent solubility of novel poly(N-Isopropylacrylamide) copolymers. Macromol. Chem. Phys. 2000, 201, 273–280. [Google Scholar] [CrossRef]
- Principi, T.; Goh, C.C.E.; Liu, R.C.W.; Winnik, F.M. Solution Properties of Hydrophobically Modified Copolymers of N-Isopropylacrylamide and N-Glycine Acrylamide: A Study by Microcalorimetry and Fluorescence Spectroscopy. Macromolecules 2000, 33, 2958–2966. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, L.; Ying, X.; Jian, Y.; Hong, Y.; Hu, F.; Du, Y. Antitumor Drug Delivery Modulated by a Polymeric Micelle with an Upper Critical Solution Temperature. Angew. Chem.-Int. Ed. 2015, 54, 3126–3131. [Google Scholar] [CrossRef]
- Li, L.; Kiick, K.L. Resilin-Based Materials for Biomedical Applications. ACS Macro Letters. 2013, 2, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Fang, T.Y.; Kao, H.Y.; Tseng, W.C. Nanoassembly of UCST Polypeptide for NIR-Modulated Drug Release. Biochem. Eng. J. 2021, 176, 108194. [Google Scholar] [CrossRef]
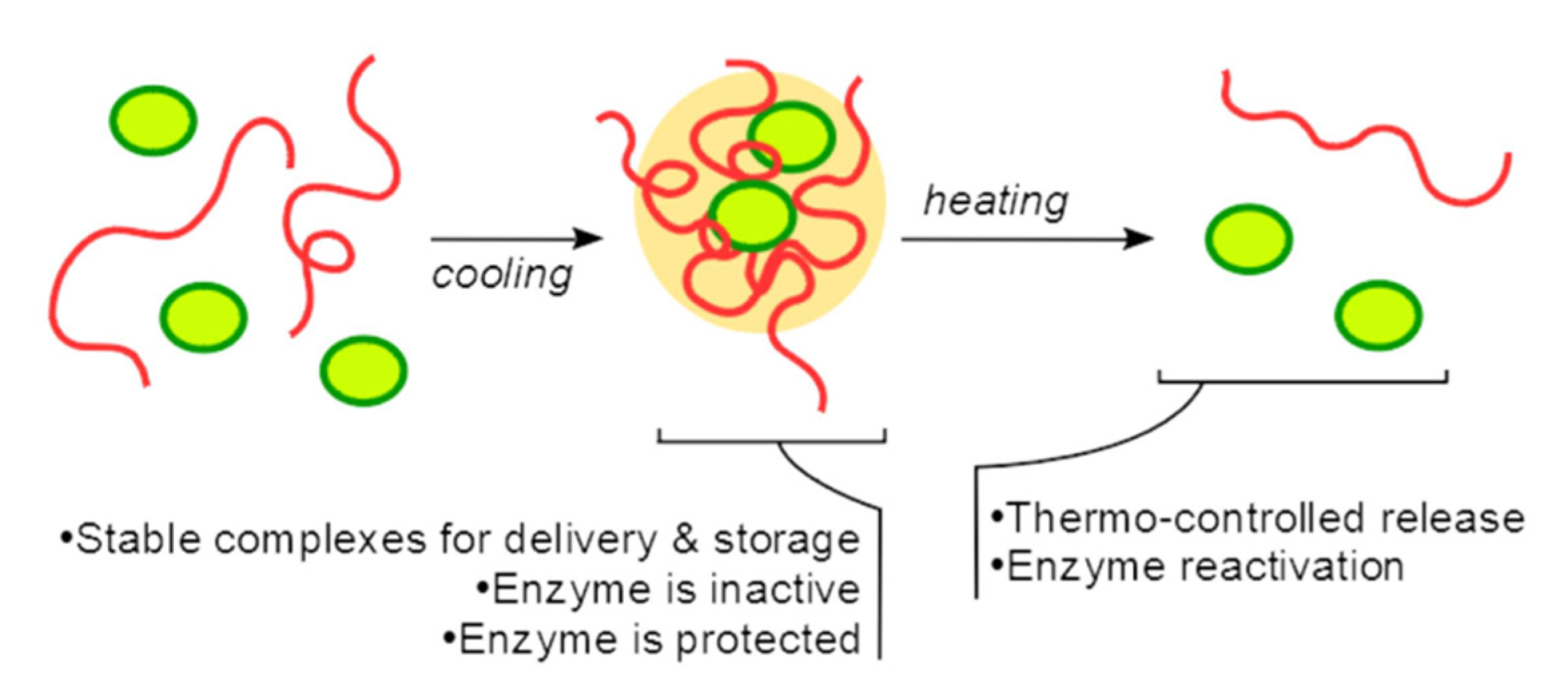
- Semenyuk, P.I.; Kurochkina, L.P.; Mäkinen, L.; Muronetz, V.I.; Hietala, S. Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide). Polymers 2021, 13, 3601. [Google Scholar] [CrossRef]
- Dugave, C.; Demange, L. Cis−Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Fang, T.; Liu, X.; Ni, Y.; Lu, C.; Xu, Z. Precise stimulation of near-infrared light responsive shape-memory polymer composites using upconversion particles with photothermal capability. Compos. Sci. Technol. 2017, 152, 190–197. [Google Scholar] [CrossRef]
- Bisby, R.H.; Mead, C.; Morgan, C.G. Wavelength-Programmed Solute Release from Photosensitive Liposomes. Biochem. Biophys. Res. Commun. 2000, 276, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Shinkai, S. The concept of molecular machinery is useful for design of stimuli-responsive gene delivery systems in the mammalian cell. J. Incl. Phenom. Macrocycl. Chem. 2007, 58, 205–219. [Google Scholar] [CrossRef]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on the Skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Sukhorukov, G.B. UV Light Stimulated Encapsulation and Release by Polyelectrolyte Microcapsules. Adv. Colloid Interface Sci. 2014, 207, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Chen, C.J.; Li, D.D.; Wang, S.S.; Ji, J. Near-Infrared Light-Sensitive Micelles for Enhanced Intracellular Drug Delivery. J. Mater. Chem. 2012, 22, 16865–16871. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.; Yang, H.H.; Liu, G.; Chen, X. Multifunctional Fe3O4@polydopamine Core-Shell Nanocomposites for Intracellular MRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Liu, M.; Yan, B.; Li, Y.S.; Lan, J.; Shi, L.; Ran, R. Polydopamine/Polystyrene Nanocomposite Double-Layer Strain Sensor Hydrogel with Mechanical, Self-Healing, Adhesive and Conductive Properties. Mater. Sci. Eng. C 2020, 109, 110567. [Google Scholar] [CrossRef]
- Xu, C.; Gao, F.; Wu, J.; Niu, S.; Li, F.; Jin, L.; Shi, Q.; Du, L. Biodegradable Nanotheranostics with Hyperthermia-Induced Bubble Ability for Ultrasound Imaging–Guided Chemo-Photothermal Therapy. Int. J. Nanomed. 2019, 14, 7141–7153. [Google Scholar] [CrossRef]
- Sun, X.; Meng, Z.; Yu, Q.; Wang, X.; Zhao, Z. Engineering PDA-Coated CM-CS Nanoparticles for Photothermo-Chemotherapy of Osteosarcoma and Bone Regeneration. Biochem. Eng. J. 2021, 175. [Google Scholar] [CrossRef]
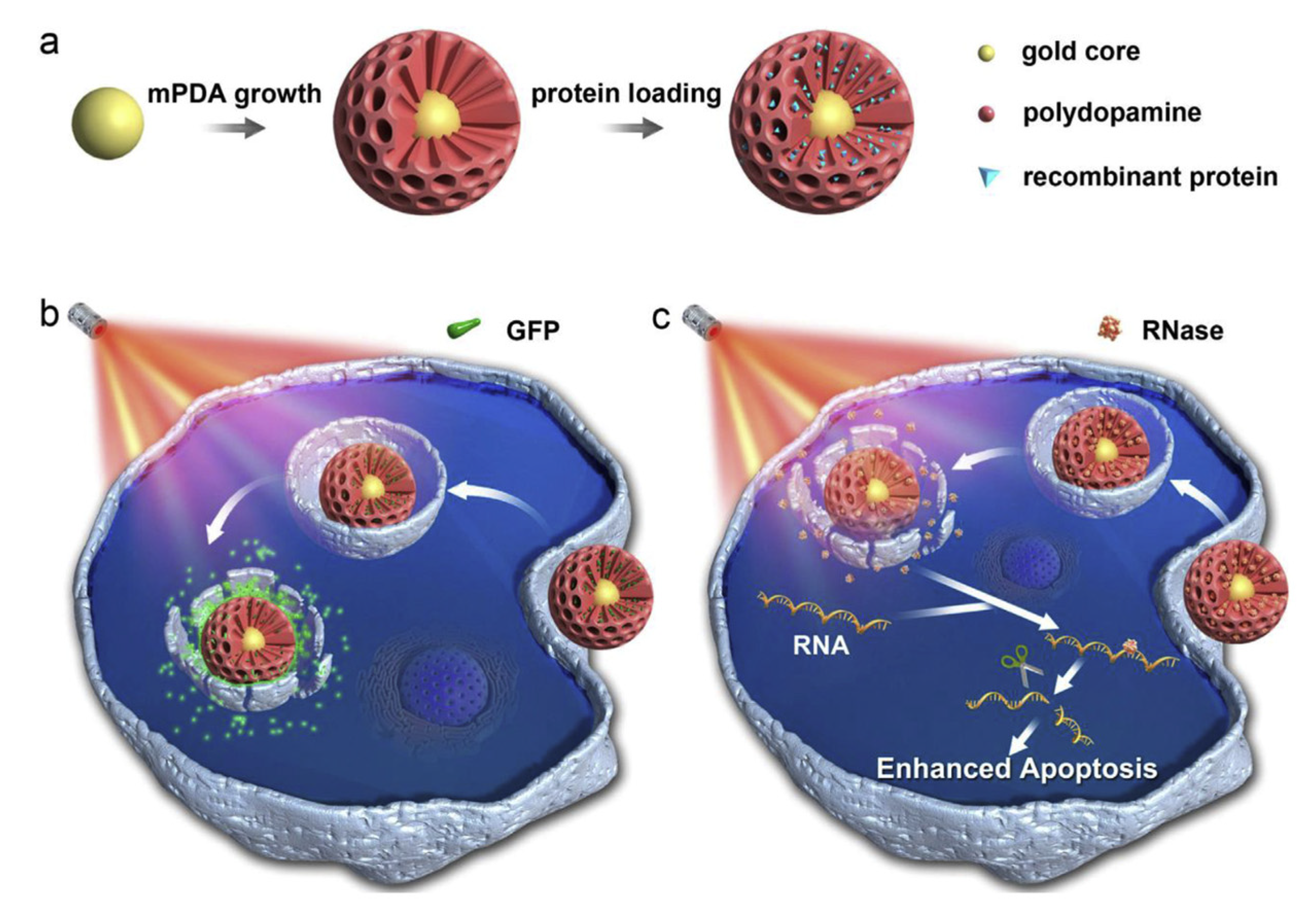
- Wu, D.; Zhou, J.; Chen, X.; Chen, Y.; Hou, S.; Qian, H.; Zhang, L.; Tang, G.; Chen, Z.; Ping, Y.; et al. Mesoporous Polydopamine with Built-in Plasmonic Core: Traceable and NIR Triggered Delivery of Functional Proteins. Biomaterials 2020, 238, 119847. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Ultrasonic-Activated Micellar Drug Delivery for Cancer Treatment. J. Pharm. Sci. 2007, 98, 795–811. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.; Sun, M.; Yang, B.; Xiao, J.; Du, J. Ultrasound-Responsive Polymersomes Capable of Endosomal Escape for Efficient Cancer Therapy. J. Control. Release 2020, 322, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Du, J.Z. Ultrasound-Responsive Homopolymer Nanoparticles. Chin. J. Polym. Sci. (Engl. Ed.) 2020, 38, 349–356. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, J.; Song, Q.; Göstl, R.; Herrmann, A. Toward Drug Release Using Polymer Mechanochemical Disulfide Scission. J. Am. Chem. Soc. 2020, 142, 14725–14732. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Fang, J.Y.; Wang, S.W.; Lee, R.S. Synthesis and characterization of triple-responsive PNiPAAm-S-S-P(αN3CL-g-alkyne) copolymers bearing cholesterol and fluorescence monitor. React. Funct. Polym. 2018, 130, 29–42. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272, 115358. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic acid-terminated poly(2-diethyl amino ethyl methacrylate) brush-gated magnetic mesoporous nanoparticles as a smart drug delivery system. Polymers 2021, 13, 59. [Google Scholar] [CrossRef]
- Asgari, M.; Soleymani, M.; Miri, T.; Barati, A. Design of thermosensitive polymer-coated magnetic mesoporous silica nanocomposites with a core-shell-shell structure as a magnetic/temperature dual-responsive drug delivery vehicle. Polym. Adv. Technol. 2021, 32, 4101–4109. [Google Scholar] [CrossRef]
- Wang, Y.; Pisapati, A.V.; Zhang, X.F.; Cheng, X. Recent Developments in Nanomaterial-Based Shear-Sensitive Drug Delivery Systems. Adv. Healthc. Mater. 2021, 10, 2002196. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Yao, S.; Wu, X.; Liu, S.; Yang, Q.; Zhang, Y.; Du, J.; Qi, S.; Li, Y. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C 2021, 126, 112164. [Google Scholar] [CrossRef]
- Gebeyehu, B.T.; Huang, S.-Y.; Lee, A.-W.; Chen, J.-K.; Lai, J.-Y.; Lee, D.-J.; Cheng, C.-C. Dual Stimuli-Responsive Nucleobase-Functionalized Polymeric Systems as Efficient Tools for Manipulating Micellar Self-Assembly Behavior. Macromolecules 2018, 51, 1189–1197. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Guo, Y.; Li, Y.; Ma, X.; Lei, Z. Synthesis of Temperature, PH, Light and Dual-Redox Quintuple-Stimuli-Responsive Shell-Crosslinked Polymeric Nanoparticles for Controlled Release. Mater. Sci. Eng. C 2018, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Poddar, P.; Maity, P.; Maiti, S.; Sahoo, S.; Dhara, S.; Dhara, D. Synthesis of a New Triple-Responsive Biocompatible Block Copolymer: Self-Assembled Nanoparticles as Potent Anticancer Drug Delivery Vehicle. React. Funct. Polym. 2020, 154, 104679. [Google Scholar] [CrossRef]
- Jiang, X.; Li, R.; Feng, C.; Lu, G.; Huang, X. Triple-Stimuli-Responsive Ferrocene-Containing Homopolymers by RAFT Polymerization. Polym. Chem. 2017, 8, 2773–2784. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Song, X.; Zhang, Y.; Cao, Y.; Xue, Y.; Wu, F.; Yu, F.; Wu, M.; Zhu, X. Multifunctional Theranostic Nanosystems Enabling Photothermal-Chemo Combination Therapy of Triple-Stimuli-Responsive Drug Release with Magnetic Resonance Imaging. Biomater. Sci. 2020, 8, 1875–1884. [Google Scholar] [CrossRef]
- Lei, W.; Sun, C.; Jiang, T.; Gao, Y.; Yang, Y.; Zhao, Q.; Wang, S. Polydopamine-Coated Mesoporous Silica Nanoparticles for Multi-Responsive Drug Delivery and Combined Chemo-Photothermal Therapy. Mater. Sci. Eng. C 2019, 105, 110103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Gao, X.; Zhao, N.; Cheng, X.; Yuan, W. Amphiphilic Copolymers with Light-PH-Temperature Triple Stimuli-Responses: Preparation, Self-Assembly and Controlled Drug Release. Mater. Lett. 2021, 284, 129008. [Google Scholar] [CrossRef]
- Ganguly, R.; Saha, P.; Banerjee, S.L.; Pich, A.; Singha, N.K. Stimuli-Responsive Block Copolymer Micelles Based on Mussel-Inspired Metal-Coordinated Supramolecular Networks. Macromol. Rapid Commun. 2021, 42, 2100312. [Google Scholar] [CrossRef]
- Muttaqien, S.E.; Nomoto, T.; Dou, X.; Takemoto, H.; Matsui, M.; Nishiyama, N. Photodynamic Therapy Using LCST Polymers Exerting PH-Responsive Isothermal Phase Transition. J. Control. Release 2020, 328, 608–616. [Google Scholar] [CrossRef]
- Nikravan, G.; Haddadi-Asl, V.; Salami-Kalajahi, M. Synthesis of Dual Temperature–and PH-Responsive Yolk-Shell Nanoparticles by Conventional Etching and New Deswelling Approaches: DOX Release Behavior. Colloids Surf. B Biointerfaces 2018, 165, 1–8. [Google Scholar] [CrossRef]
- Hiruta, Y.; Kanda, Y.; Katsuyama, N.; Kanazawa, H. Dual Temperature-and PH-Responsive Polymeric Micelle for Selective and Efficient Two-Step Doxorubicin Delivery. RSC Adv. 2017, 7, 29540–29549. [Google Scholar] [CrossRef] [Green Version]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. PH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-Targeting and PH/Redox-Dual-Stimuli-Responsive Core–Shell Nanoparticles Loading Triptolide Combats Breast Cancer Growth and Lung Metastasis. J. Nanobiotechnol. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Xu, Y.; Li, G.; Hu, J.; Ma, B.; Yu, T.; Su, X.; Wang, Y. Redox and Ph Dual-Responsive Polymeric Micelle with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 18489–18498. [Google Scholar] [CrossRef]
- Xia, D.; Wang, F.; Pan, S.; Yuan, S.; Liu, Y.; Xu, Y. Redox/PH-Responsive Biodegradable Thiol-Hyaluronic Acid/Chitosan Charge-Reversal Nanocarriers for Triggered Drug Release. Polymers 2021, 13, 3785. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Tang, C.; Yin, C. Dual Stimulus-Responsive Chitosan-Based Nanoparticles Co-Delivering Doxorubicin and Quercetin for Cancer Therapy. Mater. Lett. 2021, 305, 130826. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Liu, T. Fabrication of Mixed Polymeric Micelles Based on Stimuli-Responsive Amphiphilic Copolymers for Drug Delivery and Controlled Release. Nano 2020, 15. [Google Scholar] [CrossRef]
- Wang, N.; Liu, C.; Yao, W.; Zhou, H.; Yu, S.; Chen, H.; Qiao, W. A Traceable, GSH/PH Dual-Responsive Nanoparticles with Spatiotemporally Controlled Multiple Drugs Release Ability to Enhance Antitumor Efficacy. Colloids Surf. B Biointerfaces 2021, 205, 111866. [Google Scholar] [CrossRef]
- Jing, X.; Zhi, Z.; Jin, L.; Wang, F.; Wu, Y.; Wang, D.; Yan, K.; Shao, Y.; Meng, L. pH/Redox Dual-Stimuli-Responsive Cross-Linked Polyphosphazene Nanoparticles for Multimodal Imaging-Guided Chemo-Photodynamic Therapy. Nanoscale 2019, 11, 9457–9467. [Google Scholar] [CrossRef]
- Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. PEG-PEI-Modified Gated N-Doped Mesoporous Carbon Nanospheres for pH/NIR Light-Triggered Drug Release and Cancer Phototherapy. J. Mater. Chem. B 2021, 9, 3666–3676. [Google Scholar] [CrossRef]
- Fan, B.; Gillies, E.R. Poly(Ethyl Glyoxylate)-Poly(Ethylene Oxide) Nanoparticles: Stimuli-Responsive Drug Release via End-to-End Polyglyoxylate Depolymerization. Mol. Pharm. 2017, 14, 2548–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Cai, H.; Zhang, H.; Zhu, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-Responsive Polymer-Doxorubicin Conjugate: Antitumor Mechanism and Potential as Nano-Prodrug. Acta Biomater. 2019, 84, 339–355. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric Nanoparticles, Micelles and Polymersomes from Amphiphilic Block Copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Xie, H.; Zhu, A.; Xu, Y.; Zeng, B.; Luo, W.; Dai, L. Polyion Complex Micelles Formed by Azobenzene-Based Polymer with Multi-Responsive Properties. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Renukuntla, J. Thermo- and PH Dual Responsive Polymeric Micelles and Nanoparticles. Chem.-Biol. Interact. 2018, 295, 20–37. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Mechanism for Rapid Self-Assembly of Block Copolymer Nanoparticles. Phys. Rev. Lett. 2003, 91, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; Amaral, M.H.; Lobão, P. Temperature and PH Stimuli-Responsive Polymers and Their Applications in Controlled and Selfregulated Drug Delivery. J. Appl. Pharm. Sci. 2012, 2, 01–10. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Don, T.-M.; Chiu, W.-Y. Synthesis and Properties of Chitosan-Based Thermo- and PH-Responsive Nanoparticles and Application in Drug Release. J. Polym. Sci. 2009, 47, 2798–2810. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution Properties of Poly(N-Isopropylacrylamide). J. Macromol. Sci. Part A-Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.; Zhong, Z. Biomaterials Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Speci Fi c Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, R.; Yang, M.; Jiang, X.; Liu, B. Thermo and PH Dual-Responsive Nanoparticles for Anti-Cancer Drug Delivery. Adv. Mater. 2007, 19, 2988–2992. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Liu, T.Y.; Hardiansyah, A.; Lee, C.F.; Wang, M.S.; Chiu, W.Y. Self-Assembly Behaviors of Thermal- and PH- Sensitive Magnetic Nanocarriers for Stimuli-Triggered Release. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerweck, L.E.; Seetharaman, K. Cellular pH Gradient in Tumor versus Normal Tissue: Potential Exploitation for the Treatment of Cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Stefanadis, C.; Chrysochoou, C.; Markou, D.; Petraki, K.; Panagiotakos, D.B.; Fasoulakis, C.; Kyriakidis, A.; Papadimitriou, C.; Toutouzas, P.K. Increased Temperature of Malignant Urinary Bladder Tumors in Vivo: The Application of a New Method Based on a Catheter Technique. J. Clin. Oncol. 2001, 19, 676–681. [Google Scholar] [CrossRef]
- Remant, B.; Thapa, B.; Xu, P. pH and Redox Dual Responsive Nanoparticle for Nuclear Targeted Drug Delivery. Mol. Pharm. 2012, 9, 2719–2729. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Sankaranarayanan, J.; Morachis, J.M.; Kim, G.; Almutairi, A. Inflammation Responsive Logic Gate Nanoparticles for the Delivery of Proteins. Bioconjugate Chem. 2011, 22, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Sk, M.A.; Mondal, A.; Molla, M.R. Self-Immolative Polyurethane-Based Nanoassemblies: Surface Charge Modulation at Tumor-Relevant PH and Redox-Responsive Guest Release. Langmuir 2020, 36, 8282–8289. [Google Scholar] [CrossRef]
- Sankaranarayanan, J.; Mahmoud, E.A.; Kim, G.; Morachis, M.; Almutairi, A. Multiresponse Strategies to Modulate Burst Degradation and Release from Nanoparticles. ACS Nano 2010, 4, 5930–5936. [Google Scholar] [CrossRef]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor-Made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Zhang, F.; Ni, Q.; Jacobson, O.; Cheng, S.; Liao, A.; Wang, Z.; He, Z.; Yu, G.; Song, J.; Ma, Y.; et al. Polymeric Nanoparticles with a Glutathione-Sensitive Heterodimeric Multifunctional Prodrug for In Vivo Drug Monitoring and Synergistic Cancer Therapy. Angew. Chem.-Int. Ed. 2018, 57, 7066–7070. [Google Scholar] [CrossRef] [PubMed]
- Ao, L.; Wu, C.; Liu, K.; Wang, W.; Fang, L.; Huang, L.; Su, W. Polydopamine-Derivated Hierarchical Nanoplatforms for Efficient Dual-Modal Imaging-Guided Combination in Vivo Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 12544–12552. [Google Scholar] [CrossRef] [PubMed]
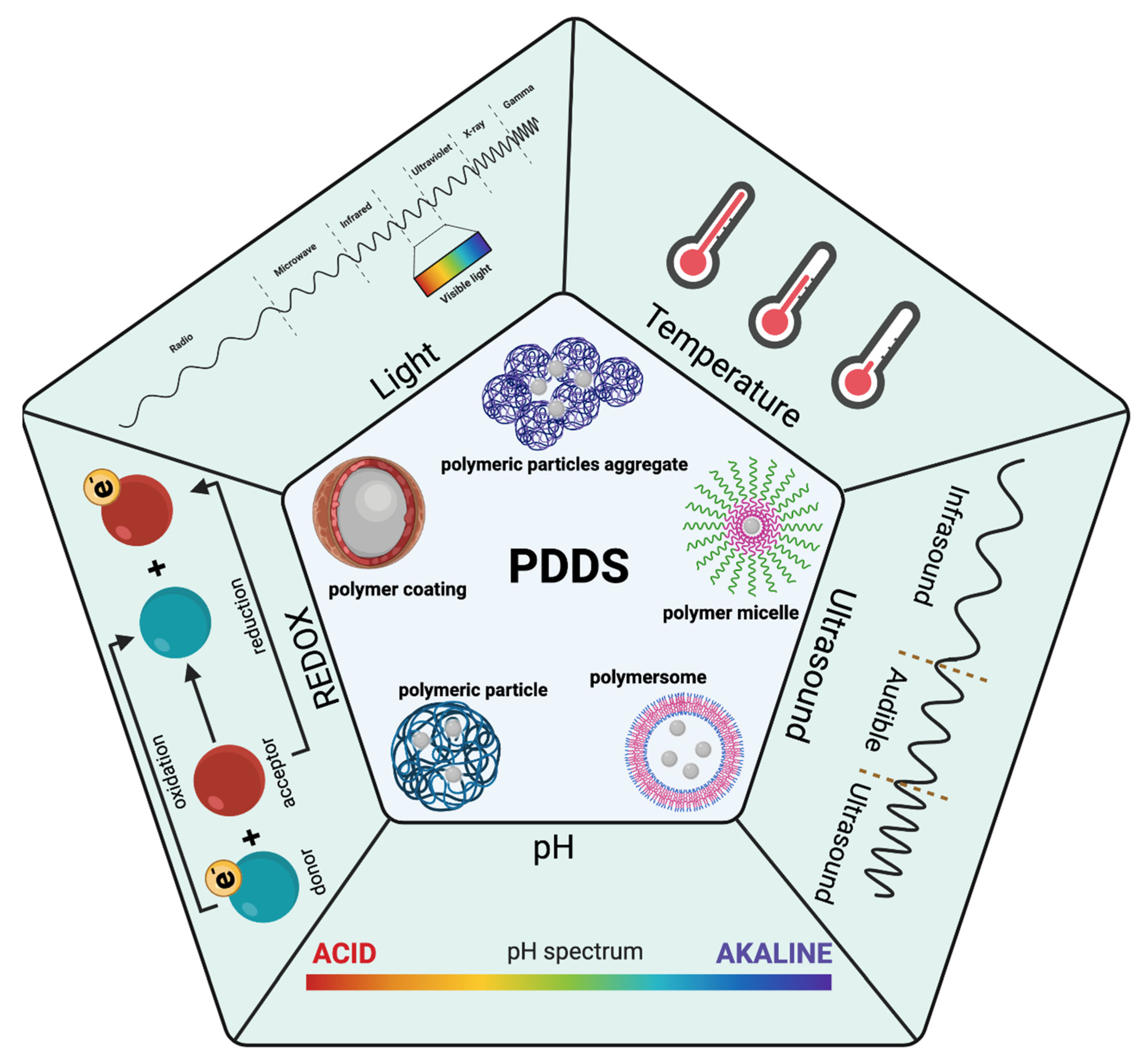

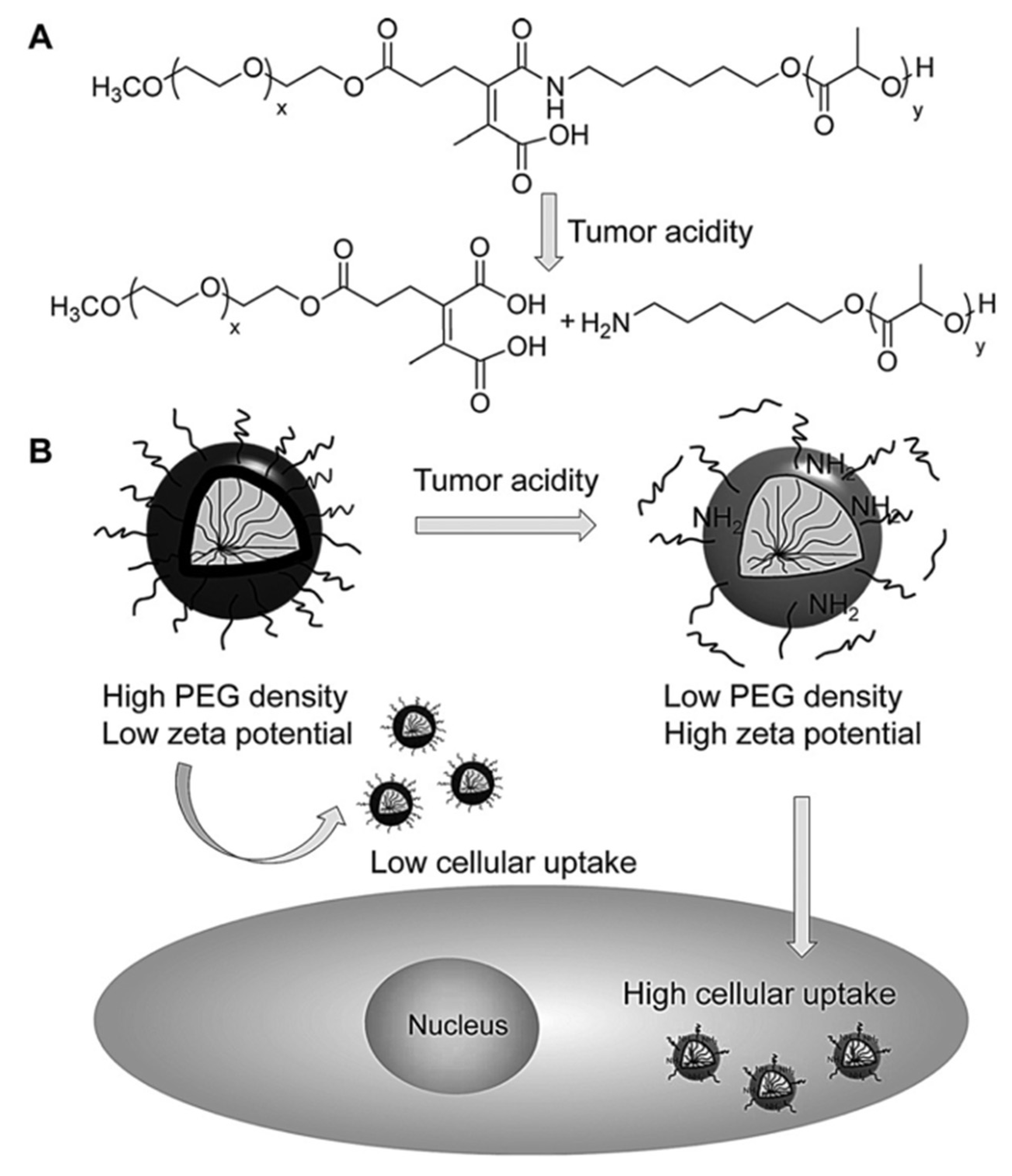
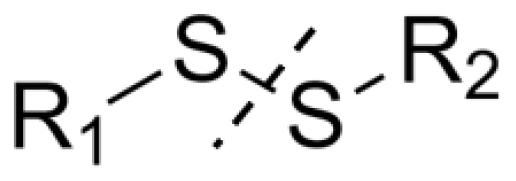
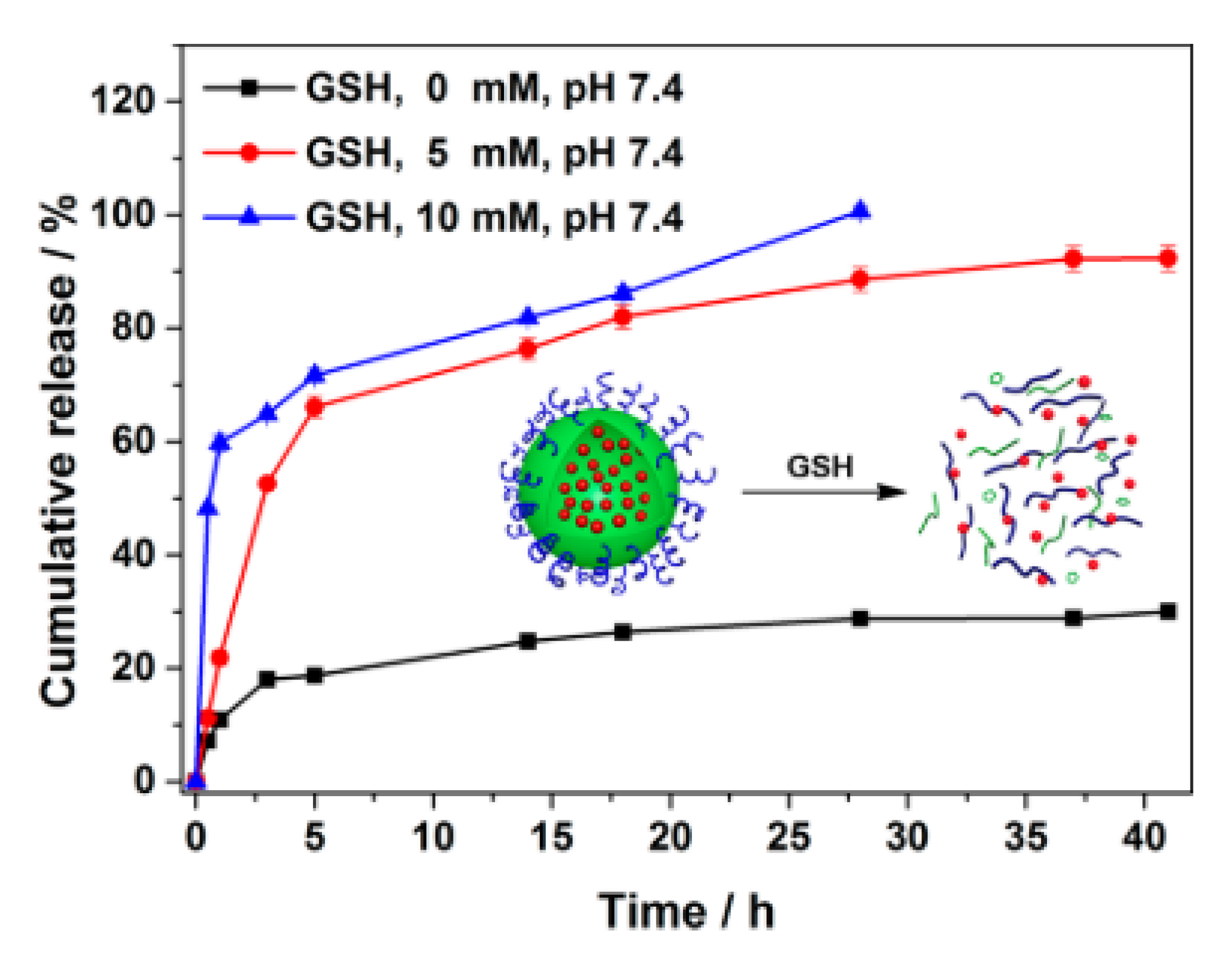




| Stimuli | Active Part | Examples | Ref. |
|---|---|---|---|
| pH | Cleavable bonds | Imine bond: HA-mPEG hyaluronic acid-methoxy Poly(ethylene-glycol) amine (Di)methyl maleate bond: PDLLA-PEG Poly(D,L-lactide)-Poly(ethylene-glycol) | [20,21] |
| Redox potential | Disulfide bond | MPEG-P(BHD-SS)-MPEG Poly(ethylene-glycol)-b-polycarbonate-Poly(ethylene-glycol) | [22] |
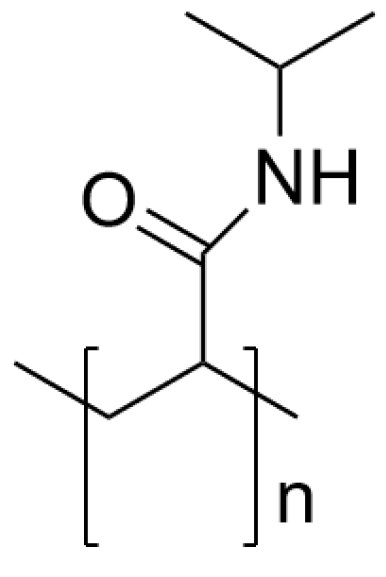
| Temperature | Lower critical solution (LCST) Upper critical solution (UCST) | LCST: PNIPAM Poly-N-isopropylacrylamide UCST: iMAPA Insoluble multi-L-arginyl-poly-L-aspartic | [23,24] |
| Light | Photo-triggered groups | Polydopamine | [25,26] |
| Ultrasound | Disulfide bond Particles aggregates | PLA-S-S-PEG Poly(L-lactide)-S-S-Poly(ethylene-glycol) PLGA aggregates Poly(lactic-co-glycolic acid) | [27,28] |
| Magnetism | Incorporation of magnetic particles | Iron nanoparticles | [29,30] |
| Shear stress | Flexible particles, generally hydrogels | ADEN/THYM polymersomes Adenine/thymine functionalized block co polymers | [31] |
| Cleavable Bond | pH |
|---|---|
| Imine | <5–7 |
| Hydrazone | <5 |
| Hydrazide | <5 |
| Oxime | <5 |
| (di)Methyl maleate | <6.8 |
| Environment | GSH Level |
|---|---|
| Intracellular | 1–10 mM |
| Extracellular | 1–10 μM |
| Brain Cancer | 0.5–3 mM |
| Polymer | Stimuli | Description | Ref. |
|---|---|---|---|
| PMAEFc-ONB-PDMAEMA poly(2-methacryloyloxyethyl ferrocenecarboxylate)-(5-propargylether-2-nitrobenzyl bromoisobutyrate)-poly(di-methylaminoethyl methacrylate) | Light pH Temperature Redox: -oxidative -reduction | pH-responsive and LCST: PDMAEMA. Oxidation/reduction: ferrocenyl groups. Light responsive: o-nitrobenzyl methyl esters. | [104] |
| Poly[HBCEEM-b-(NIPA-r-PEGMA)] (PHNP) 2-(2-((4-(hexyloxy)benzyloxy)carbonyl)ethylthio)ethyl acrylate, N-isopropyl acrylamide, poly(ethylene glycol methyl ether acrylate) | pH Temperature Redox | pH-responsive: HBCEEA. Disulfide bond (S-S): redox responsive. Temperature-responsive: NIPA and PEGMA. | [105] |
| Fc-DEAE-AM poly(2-(3-(N-(2-(diethylamino)ethyl)acrylamido)-propanoyloxy)ethyl ferrocenecarboxylate) | Redox pH CO2 | Redox-responsive: Fc. pH-responsive and CO2: DEAE. | [106] |
| PDA Polydopamine | Light pH Redox (if S-S) | NIR-responsive and pH: dopamine. Redo-responsive: incorporation of disulfide bond (S-S). | [25,88,89,92,107,108] |
| P(MEO2MA-co-OEGMA)-b-P(MAA-co-SPMA) Poly(2-(2-methoxyethoxy)ethylmethacrylate-co-oligo(ethylene glycol) methacrylate)-block-poly(methacrcid-co-spiropyran methacrylate) | pH Light Temperature | UV light-responsive: SP-MC. pH-responsive: P(MAA-co-SPMA). LCST: change based on monomer ratio. | [109] |
| PSB-block-P(NIPAM-A)) poly(sulfobetaine)-b-poly(N-isopropylacrylamide-co-dopamine methacrylamide) iMAPA-HA insoluble Multi-L-arginyl-poly-L-aspartate- hyaluronic acid 700DX-P(NIPAAm/AIPAAm-PMM) poly(N-isopropylacrylamide) -2-aminoisoprpylacrylamide-2-propionic-3-methyl-maleic PAA@PHEMA poly(acrylic acid)-poly(2-hydroxyethylmethacrylate) PBM-b-ND poly(butyl methacrylate)-b-poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) PMAA-b-PNIPAM poly(methacrylic acid)-b-poly(N-isopropylacrylamid poly(NIPAM-co-GMA) poly(N-isopropylacrylamid)-co-glycidyl methacrylate | Temperature pH | Thermo-responsive (LCST): NIPAM. Thermo-responsive (UCST): iMAPA, combined with pH-responsive block: -poly(acrylic acid) PAA, -metal–catecholate, -iMAPA, -N-alkyl groups, -PDPA, -hydrazine units. | [78,110,111,112,113,114,115] |
| HA-VE and PBAEss hyaluronic acid-vitamine E poly(β-amino ester) mPEG-P(TPE-co-AEMA) poly ethylene glycol-poly(tetrapheny-lethene-co-2-azepane ethyl methacrylate) HA-SH-CS thiol-hyaluronic acid-chitosan PPZ Polyphosphazene PEG modified trimethyl chitosan Polyethylene glycol-trimethyl chitosan PAE(-ss-mPEG)-g-Chol poly(-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol PEG-SS-CPT Polyethylene glycol- disulfide bond- camptothecin | pH Redox | Redox-responsive: disulfide bond (S-S). pH-responsive segments: -poly(β-amino ester), -(PAEMA): pH > 6.8 hydrophobic, pH < 6.8 hydrophilic, -polyelectrolyte complexes, -cross-linked polyphosphazene, -trimethyl chitosan, -copolymer poly(-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol. | [116,117,118,119,120,121,122] |
| PEG-PEI-GEM polyethylenimine-graft-poly(ethylene glycol)- gemcitabine | pH Light | Light-responsive: photo-cleavable-o-nitrobenzyl, with a linker of thermosensitive: PEG–PEI. | [123] |
| PEO-PEtG-PEO Poly(ethyl glyoxylate)-Poly(ethylene oxide) | Light (UV) Redox | Redox-responsive: disulfide bond (S-S). Light-responsive: o-nitrobenzyl moiety. | [124] |
| BU-PPG Uraciland-oligomeric polypropylene glycol | Temperature Light | Light-responsive: uracil. Thermoresponsive: oligomeric PPG. | [103] |
| pDHPMA-DOX poly[N-(1, 3-dihydroxypropyl) methacrylamide]-doxorubicin | pH Enzyme | Enzyme-responsive: Gly–Phe–Leu–Gly (GFLG), with a linker of pH-responsive: hydrazone bond. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Ruiz, A.; Ramirez, A.; McEnnis, K. Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharmaceutics 2022, 14, 421. https://doi.org/10.3390/pharmaceutics14020421
López Ruiz A, Ramirez A, McEnnis K. Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharmaceutics. 2022; 14(2):421. https://doi.org/10.3390/pharmaceutics14020421
Chicago/Turabian StyleLópez Ruiz, Aida, Ann Ramirez, and Kathleen McEnnis. 2022. "Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery" Pharmaceutics 14, no. 2: 421. https://doi.org/10.3390/pharmaceutics14020421
APA StyleLópez Ruiz, A., Ramirez, A., & McEnnis, K. (2022). Single and Multiple Stimuli-Responsive Polymer Particles for Controlled Drug Delivery. Pharmaceutics, 14(2), 421. https://doi.org/10.3390/pharmaceutics14020421






