Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database Search Method
2.2. Inclusion and Exclusion Criteria
2.3. Study Screening
2.4. Data Extraction
2.5. Risk of Bias Assessment
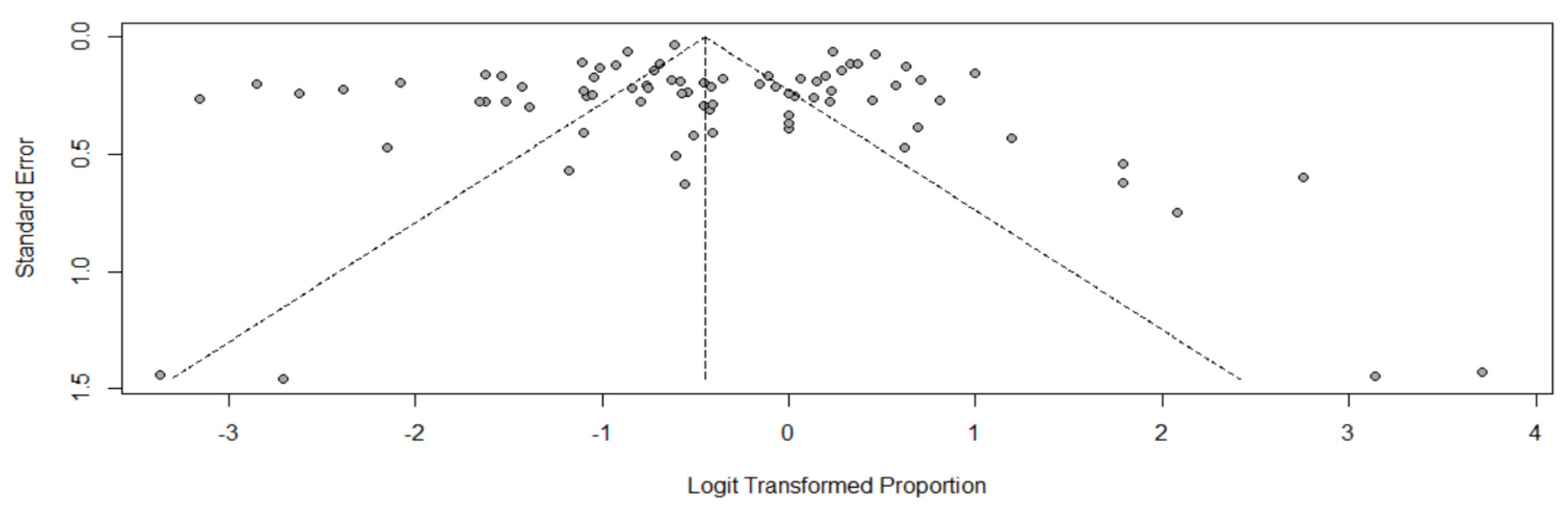
2.6. Data Analysis
3. Results
3.1. ARC Definition
3.2. ARC Prevalence
3.3. ARC Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakke, V.; Sporsem, H.; Von der Lippe, E.; Nordoy, I.; Lao, Y.; Nyrerod, H.C.; Sandvik, L.; Harvig, K.R.; Bugge, J.F.; Helset, E. Vancomycin levels are frequently subtherapeutic in critically ill patients: A prospective observational study. Acta Anaesthesiol. Scand. 2017, 61, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Carrie, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: A prospective observational study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ishii, H.; Shimoshikiryo, T.; Shimomura, T.; Tsuji, D.; Inoue, K.; Kadoiri, T.; Itoh, K. Augmented Renal Clearance in Patients with Febrile Neutropenia is Associated with Increased Risk for Subtherapeutic Concentrations of Vancomycin. Ther. Drug Monit. 2016, 38, 706–710. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Shen, C. Augmented Renal Clearance in Critical Illness: An Important Consideration in Drug Dosing. Pharmaceutics 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 2011, 7, 539–543. [Google Scholar] [CrossRef]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented renal clearance in critically ill patients: Etiology, definition and implications for beta-lactam dose optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Gijsen, M.; Huang, C.Y.; Flechet, M.; Van Daele, R.; Declercq, P.; Debaveye, Y.; Meersseman, P.; Meyfroidt, G.; Wauters, J.; Spriet, I. Development and External Validation of an Online Clinical Prediction Model for Augmented Renal Clearance in Adult Mixed Critically Ill Patients: The Augmented Renal Clearance Predictor. Crit. Care Med. 2020, 48, e1260–e1268. [Google Scholar] [CrossRef]
- Gijsen, M.; Wilmer, A.; Meyfroidt, G.; Wauters, J.; Spriet, I. Can augmented renal clearance be detected using estimators of glomerular filtration rate? Crit. Care 2020, 24, 359. [Google Scholar] [CrossRef]
- Baptista, J.P. Augmented renal clearance. In Antibiotic Pharmacokinetic/Pharmacodynamic Considerations in the Critically Ill; Springer: Singapore, 2017; pp. 125–150. [Google Scholar]
- Bilbao-Meseguer, I.; Rodriguez-Gascon, A.; Barrasa, H.; Isla, A.; Solinis, M.A. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Udy, A.A.; Putt, M.T.; Boots, R.J.; Lipman, J. ARC—Augmented renal clearance. Curr. Pharm. Biotechnol. 2011, 12, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.L.; Shea, K.M.; Roberts, K.M.; Daley, M.J. Implications of Augmented Renal Clearance on Drug Dosing in Critically Ill Patients: A Focus on Antibiotics. Pharmacotherapy 2015, 35, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.; Boots, R.; Senthuran, S.; Stuart, J.; Deans, R.; Lassig-Smith, M.; Lipman, J. Augmented Creatinine Clearance in Traumatic Brain Injury. Anesth. Analg. 2010, 111, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- May, C.C.; Arora, S.; Parli, S.E.; Fraser, J.F.; Bastin, M.T.; Cook, A.M. Augmented Renal Clearance in Patients with Subarachnoid Hemorrhage. Neurocrit. Care 2015, 23, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Morbitzer, K.A.; Jordan, J.D.; Dehne, K.A.; Durr, E.A.; Olm-Shipman, C.M.; Rhoney, D.H. Enhanced Renal Clearance in Patients With Hemorrhagic Stroke. Crit. Care Med. 2019, 47, 800–808. [Google Scholar] [CrossRef]
- Lautrette, A.; Phan, T.-N.; Ouchchane, L.; AitHssain, A.; Tixier, V.; Heng, A.-E.; Souweine, B. High creatinine clearance in critically ill patients with community-acquired acute infectious meningitis. BMC Nephrol. 2012, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Carrie, C.; Bentejac, M.; Cottenceau, V.; Masson, F.; Petit, L.; Cochard, J.F.; Sztark, F. Association between augmented renal clearance and clinical failure of antibiotic treatment in brain-injured patients with ventilator-acquired pneumonia: A preliminary study. Anaesth. Crit. Care Pain Med. 2018, 37, 35–41. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; 4.0.3 (2020-10-10); R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.R.; Thompson, S.G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010, 29, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between augmented renal clearance and clinical outcomes in patients receiving beta-lactam antibiotic therapy by continuous or intermittent infusion: A nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Lazzarotto, D.; Candoni, A.; Dubbini, M.V.; Zannier, M.E.; Fanin, R.; Pea, F. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: Results from a prospective, monocentric, interventional study. J. Antimicrob. Chemother. 2020, 75, 3029–3037. [Google Scholar] [CrossRef]
- Joynt, G.M.; Lipman, J.; Gomersall, C.D.; Young, R.J.; Wong, E.L.; Gin, T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 2001, 47, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster-Lluch, O.; Geronimo-Pardo, M.; Peyro-Garcia, R.; Lizan-Garcia, M. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth. Intensive Care 2008, 36, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Baptista, J.P.; Udy, A.A.; Sousa, E.; Pimentel, J.; Wang, L.; Roberts, J.A.; Lipman, J. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit. Care 2011, 15, R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minville, V.; Asehnoune, K.; Ruiz, S.; Breden, A.; Georges, B.; Seguin, T.; Tack, I.; Jaafar, A.; Saivin, S.; Fourcade, O.; et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: A retrospective observational study. Crit. Care 2011, 15, R49. [Google Scholar] [CrossRef] [Green Version]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef]
- Grootaert, V.; Spriet, I.; Decoutere, L.; Debaveye, Y.; Meyfroidt, G.; Willems, L. Augmented renal clearance in the critically ill: Fiction or fact? Int. J. Clin. Pharm. 2012, 34, 143. [Google Scholar] [CrossRef]
- Carlier, M.; Carrette, S.; Roberts, J.A.; Stove, V.; Verstraete, A.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; Lipman, J.; Wallis, S.C.; et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: Does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit. Care 2013, 17, R84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udy, A.A.; Roberts, J.A.; Shorr, A.F.; Boots, R.J.; Lipman, J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: Identifying at-risk patients. Crit. Care 2013, 17, R35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minkute, R.; Briedis, V.; Steponaviciute, R.; Vitkauskiene, A.; Maciulaitis, R. Augmented renal clearance—An evolving risk factor to consider during the treatment with vancomycin. J. Clin. Pharm. Ther. 2013, 38, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Morton, F.J.A.; Nguyen-Pham, S.; Jarrett, P.; Lassig-Smith, M.; Stuart, J.; Dunlop, R.; Starr, T.; Boots, R.J.; Lipman, J. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013, 14, 250. [Google Scholar] [CrossRef] [Green Version]
- Claus, B.O.M.; Hoste, E.A.; Colpaert, K.; Robays, H.; Decruyenaere, J.; De Waele, J.J. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care 2013, 28, 695–700. [Google Scholar] [CrossRef]
- Baptista, J.P.; Roberts, J.A.; Sousa, E.; Freitas, R.; Deveza, N.; Pimentel, J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: Developing and testing of a dosing nomogram. Crit. Care 2014, 18, 654. [Google Scholar] [CrossRef] [Green Version]
- Baptista, J.P.; Neves, M.; Rodrigues, L.; Teixeira, L.; Pinho, J.; Pimentel, J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J. Nephrol. 2014, 27, 403–410. [Google Scholar] [CrossRef]
- Campassi, M.L.; Gonzalez, M.C.; Masevicius, F.D.; Vazquez, A.R.; Moseinco, M.; Navarro, N.C.; Previgliano, L.; Rubatto, N.P.; Benites, M.H.; Estenssoro, E.; et al. Augmented renal clearance in critically ill patients: Incidence, associated factors and effects on vancomycin treatment. Rev. Bras. Ter. Intensiva 2014, 26, 13–20. [Google Scholar] [CrossRef]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented Renal Clearance in the ICU: Results of a Multicenter Observational Study of Renal Function in Critically Ill Patients with Normal Plasma Creatinine Concentrations. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef]
- Adnan, S.; Ratnam, S.; Kumar, S.; Paterson, D.; Lipman, J.; Roberts, J.; Udy, A.A. Select critically ill patients at risk of augmented renal clearance: Experience in a Malaysian intensive care unit. Anaesth. Intensive Care 2014, 42, 715–722. [Google Scholar] [CrossRef]
- Ruiz, S.; Minville, V.; Asehnoune, K.; Virtos, M.; Georges, B.; Fourcade, O.; Conil, J.M. Screening of patients with augmented renal clearance in ICU: Taking into account the CKD-EPI equation, the age, and the cause of admission. Ann. Intensive Care 2015, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Dias, C.; Gaio, A.R.; Monteiro, E.; Barbosa, S.; Cerejo, A.; Donnelly, J.; Felgueiras, O.; Smielewski, P.; Paiva, J.A.; Czosnyka, M. Kidney-brain link in traumatic brain injury patients? A preliminary report. Neurocrit. Care 2015, 22, 192–201. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Dumoulin, A.; Janssen, A.; Hoste, E.A. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015, 81, 1079–1085. [Google Scholar]
- Steinke, T.; Moritz, S.; Beck, S.; Gnewuch, C.; Kees, M.G. Estimation of creatinine clearance using plasma creatinine or cystatin C: A secondary analysis of two pharmacokinetic studies in surgical ICU patients. BMC Anesthesiol. 2015, 15, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Luo, Y.; Qu, L.; Zhao, C.; Jiang, M. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharmaceut. Biol. 2016, 54, 2802–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Morimoto, S.; Izutani, Y.; Muranishi, K.; Kaneyama, H.; Hoshino, K.; Nishida, T.; Ishikura, H. Augmented renal clearance in Japanese intensive care unit patients: A prospective study. J. Intensive Care 2016, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saour, M.; Klouche, K.; Deras, P.; Damou, A.; Capdevila, X.; Charbit, J. Assessment of modification of diet in renal disease equation to predict reference serum creatinine value in severe trauma patients: Lessons from an obser vational study of 775 cases. Ann. Surg. 2016, 263, 814–820. [Google Scholar] [CrossRef]
- Abdel El Naeem, H.E.M.; Abdelhamid, M.H.E.; Atteya, D.A.M. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt. J. Anaesth. 2017, 33, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Barletta, J.F.; Mangram, A.J.; Byrne, M.; Hollingworth, A.K.; Sucher, J.F.; Ali-Osman, F.R.; Shirah, G.R.; Dzandu, J.K. The importance of empiric antibiotic dosing in critically ill trauma patients: Are we under-dosing based on augmented renal clearance and inaccurate renal clearance estimates? J. Trauma Acute Care Surg. 2016, 81, 1115–1121. [Google Scholar] [CrossRef] [Green Version]
- Declercq, P.; Nijs, S.; D’Hoore, A.; Van Wijngaerden, E.; Wolthuis, A.; de Buck van Overstraeten, A.; Wauters, J.; Spriet, I. Augmented renal clearance in non-critically ill abdominal and trauma surgery patients is an underestimated phenomenon: A point prevalence study. J. Trauma Acute Care Surg. 2016, 81, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, L.; Zoller, M.; Minichmayr, I.K.; Scharf, C.; Maier, B.; Schmitt, M.V.; Hartung, N.; Huisinga, W.; Vogeser, M.; Frey, L.; et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: A prospective observational study. Crit. Care 2017, 21, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnham, J.P.; Micek, S.T.; Kollef, M.H. Augmented renal clearance is not a risk factor for mortality in Enterobacteriaceae bloodstream infections treated with appropriate empiric antimicrobials. PLoS ONE 2017, 12, e0180247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrie, C.; Lannou, A.; Rubin, S.; De Courson, H.; Petit, L.; Biais, M. Augmented renal clearance in critically ill trauma patients: A pathophysiologic approach using renal vascular index. Anaesth. Crit. Care Pain Med. 2019, 38, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Jarrett, P.; Lassig-Smith, M.; Stuart, J.; Starr, T.; Dunlop, R.; Deans, R.; Roberts, J.A.; Senthuran, S.; Boots, R.; et al. Augmented Renal Clearance in Traumatic Brain Injury: A Single-Center Observational Study of Atrial Natriuretic Peptide, Cardiac Output, and Creatinine Clearance. J. Neurotrauma 2017, 34, 137–144. [Google Scholar] [CrossRef]
- Barletta, J.F.; Mangram, A.J.; Byrne, M.; Sucher, J.F.; Hollingworth, A.K.; Ali-Osman, F.R.; Shirah, G.R.; Haley, M.; Dzandu, J.K. Identifying augmented renal clearance in trauma patients: Validation of the Augmented Renal Clearance in Trauma Intensive Care scoring system. J. Trauma Acute Care Surg. 2017, 82, 665–671. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Roberts, J.A.; Carlier, M.; Verstraete, A.G.; Stove, V.; De Waele, J.J. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int. J. Antimicrob. Agents 2018, 51, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Tamatsukuri, T.; Ohbayashi, M.; Kohyama, N.; Kobayashi, Y.; Yamamoto, T.; Fukuda, K.; Nakamura, S.; Miyake, Y.; Dohi, K.; Kogo, M. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2018, 24, 834–840. [Google Scholar] [CrossRef]
- Carrie, C.; Legeron, R.; Petit, L.; Ollivier, J.; Cottenceau, V.; d’Houdain, N.; Boyer, P.; Lafitte, M.; Xuereb, F.; Sztark, F.; et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J. Crit. Care 2018, 48, 66–71. [Google Scholar] [CrossRef]
- Kawano, Y.; Maruyama, J.; Hokama, R.; Koie, M.; Nagashima, R.; Hoshino, K.; Muranishi, K.; Nakashio, M.; Nishida, T.; Ishikura, H. Outcomes in patients with infections and augmented renal clearance: A multicenter retrospective study. PLoS ONE 2018, 13, e0208742. [Google Scholar] [CrossRef] [Green Version]
- Tsai, D.; Udy, A.A.; Stewart, P.C.; Gourley, S.; Morick, N.M.; Lipman, J.; Roberts, J.A. Prevalence of augmented renal clearance and performance of glomerular filtration estimates in Indigenous Australian patients requiring intensive care admission. Anaesth. Intensive Care 2018, 46, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Hirai, K.; Sugiyama, K.; Nakatani, E.; Kimura, M.; Itoh, K. Validation of a Nomogram for Achieving Target trough Concentration of Vancomycin: Accuracy in Patients with Augmented Renal Function. Ther. Drug Monit. 2018, 40, 693–698. [Google Scholar] [CrossRef]
- Ollivier, J.; Carrie, C.; d’Houdain, N.; Djabarouti, S.; Petit, L.; Xuereb, F.; Legeron, R.; Biais, M.; Breilh, D. Are Standard Dosing Regimens of Ceftriaxone Adapted for Critically Ill Patients with Augmented Creatinine Clearance? Antimicrob. Agents Chemother. 2019, 63, e02134-18. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Tai, C.H.; Liao, W.Y.; Wang, C.C.; Kuo, C.H.; Lin, S.W.; Ku, S.C. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: A prospective observational study. Infect. Drug Resist. 2019, 12, 2531–2541. [Google Scholar] [CrossRef] [Green Version]
- Aitullina, A.; Krumina, A.; Purvina, S. Augmented clearance in patients with colistin therapy in intensive care units. Int. J. Clin. Pharm. 2019, 41, 310. [Google Scholar] [CrossRef]
- Weber, N.; Jackson, K.; McWhinney, B.; Ungerer, J.; Kennedy, G.; Lipman, J.; Roberts, J.A. Evaluation of pharmacokinetic/pharmacodynamic and clinical outcomes with 6-hourly empiric piperacillin-tazobactam dosing in hematological malignancy patients with febrile neutropenia. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2019, 25, 503–508. [Google Scholar] [CrossRef]
- Izumisawa, T.; Kaneko, T.; Soma, M.; Imai, M.; Wakui, N.; Hasegawa, H.; Horino, T.; Takahashi, N. Augmented Renal Clearance of Vancomycin in Hematologic Malignancy Patients. Biol. Pharm. Bull. 2019, 42, 2089–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Luo, Y.; Jiang, M.; Zhou, B. Application of vancomycin in patients with augmented renal clearance. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.D.; Talledo, O.; Neely, S.; White, B.; Celii, A.; Cross, A.; Kennedy, R. Vancomycin dosing in critically ill trauma patients: The VANCTIC Study. J. Trauma Acute Care Surg. 2019, 87, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Morbitzer, K.A.; Rhoney, D.H.; Dehne, K.A.; Jordan, J.D. Enhanced renal clearance and impact on vancomycin pharmacokinetic parameters in patients with hemorrhagic stroke. J. Intensive Care 2019, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.B.; Eidelson, S.A.; Sussman, M.S.; Schulman, C.I.; Lineen, E.B.; Iyenger, R.S.; Namias, N.; Proctor, K.G. Risk Factors and Clinical Outcomes Associated with Augmented Renal Clearance in Trauma Patients. J. Surg. Res. 2019, 244, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Bricheux, A.; Lenggenhager, L.; Hughes, S.; Karmime, A.; Lescuyer, P.; Huttner, A. Therapeutic drug monitoring of imipenem and the incidence of toxicity and failure in hospitalized patients: A retrospective cohort study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 383.e381–383.e384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helset, E.; Nordøy, I.; Sporsem, H.; Bakke, V.D.; Bugge, J.F.; Gammelsrud, K.W.; Zucknick, M.; Lippe, E.; von der Lippe, E. Factors increasing the risk of inappropriate vancomycin therapy in ICU patients: A prospective observational study. Acta Anaesthesiol. Scand. 2020, 64, 1295–1304. [Google Scholar] [CrossRef]
- Barrasa, H.; Soraluce, A.; Uson, E.; Sainz, J.; Martin, A.; Sanchez-Izquierdo, J.A.; Maynar, J.; Rodriguez-Gascon, A.; Isla, A. Impact of augmented renal clearance on the pharmacokinetics of linezolid: Advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int. J. Infect. Dis. Off. Publ. Int. Soc. Infect. Dis. 2020, 93, 329–338. [Google Scholar] [CrossRef]
- Lannou, A.; Carrie, C.; Rubin, S.; Cane, G.; Cottenceau, V.; Petit, L.; Biais, M. Salt wasting syndrome in brain trauma patients: A pathophysiologic approach using sodium balance and urinary biochemical analysis. BMC Neurol. 2020, 20, 190. [Google Scholar] [CrossRef]
- Arechiga-Alvarado, N.A.; Medellin-Garibay, S.E.; Milan-Segovia, R.D.C.; Ortiz-Alvarez, A.; Magana-Aquino, M.; Romano-Moreno, S. Population Pharmacokinetics of Amikacin Administered Once Daily in Patients with Different Renal Functions. Antimicrob. Agents Chemother. 2020, 64, e02178-19. [Google Scholar] [CrossRef]
- Carrie, C.; Delzor, F.; Roure, S.; Dubuisson, V.; Petit, L.; Molimard, M.; Breilh, D.; Biais, M. Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy. Antimicrob. Agents Chemother. 2020, 64, e02098-19. [Google Scholar] [CrossRef]
- Saito, K.; Kamio, S.; Ito, K.; Suzuki, N.; Abe, K.; Goto, T. A simple scoring method to predict augmented renal clearance in haematologic malignancies. J. Clin. Pharm. Ther. 2020, 45, 1120–1126. [Google Scholar] [CrossRef]
- Lannou, A.; Carrie, C.; Rubin, S.; De Courson, H.; Biais, M. Renal response after traumatic brain injury: A pathophysiological relationship between augmented renal clearance and salt wasting syndrome? Anaesth. Crit. Care Pain Med. 2020, 39, 239–241. [Google Scholar] [CrossRef]
- Brown, A.; Lavelle, R.; Gerlach, A. Discordance of renal drug dosing using estimated creatinine clearance and measured urine creatinine clearance in hospitalized adults: A retrospective cohort study. Int. J. Crit. Illn. Inj. Sci. 2020, 10, S1–S5. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhu, M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J. Int. Med. Res. 2020, 48, 300060520949076. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Martins, P.J.; Marques, M.; Pimentel, J.M. Prevalence and Risk Factors for Augmented Renal Clearance in a Population of Critically Ill Patients. J. Intensive Care Med. 2020, 35, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Nei, A.M.; Kashani, K.B.; Dierkhising, R.; Barreto, E.F. Predictors of Augmented Renal Clearance in a Heterogeneous ICU Population as Defined by Creatinine and Cystatin C. Nephron 2020, 144, 313–320. [Google Scholar] [CrossRef]
- Ramos, A.; Acharta, F.; Perezlindo, M.; Lovesio, L.; Gauna Antonelli, P.; Dogliotti, A.; Lovesio, C. Factors that predict supranormal glomerular filtration in critical diseases. In Proceedings of the 37th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 21–24 March 2017; Volume 21. [Google Scholar] [CrossRef] [Green Version]
- Eidelson, S.A.; Mulder, M.B.; Rattan, R.; Karcutskie, C.A.; Meizoso, J.P.; Madiraju, S.K.; Lineen, E.B.; Schulman, C.I.; Namias, N. Incidence and Functional Significance of Augmented Renal Clearance in Trauma Patients at High Risk for Venous Thromboembolism. J. Am. Coll. Surg. 2018, 227, S80–S81. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial beta-lactam concentrations in select critically ill patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Drust, A.; Luchtmann, M.; Firsching, R.; Troger, U.; Martens-Lobenhoffer, J.; Bode-Boger, S.M. Recurrent seizures in a levetiracetam-treated patient after subarachnoid hemorrhage: A matter of enhanced renal function? Epilepsy Behav. 2012, 23, 394–395. [Google Scholar] [CrossRef]
- Grootaert, V.; Willems, L.; Debaveye, Y.; Meyfroidt, G.; Spriet, I. Augmented renal clearance in the critically ill: How to assess kidney function. Ann. Pharmacother. 2012, 46, 952–959. [Google Scholar] [CrossRef]
- Morbitzer, K.; Jordan, D.; Sullivan, K.; Durr, E.; Olm-Shipman, C.; Rhoney, D. Enhanced renal clearance and impact on vancomycin trough concentration in patients with hemorrhagic stroke. Pharmacotherapy 2016, 36, e218. [Google Scholar] [CrossRef]
- Neves, M.; Baptista, J.P.; Rodrigues, L.; Pinho, J.; Teixeira, L.; Pimentel, J. Correlation between estimated glomerular filtration rate and measured renal creatinine clearance in critically ill patients with normal serum creatinine. Nephrol. Dial. Transplant. 2013, 28, 345. [Google Scholar] [CrossRef] [Green Version]
- Baptista, J.P.; Silva, N.; Costa, E.; Fontes, F.; Marques, M.; Ribeiro, G.; Pimentel, J. Identification of the critically ill patient with augmented renal clearance: Make do with what you have! Intensive Care Med. 2014, 40, S110. [Google Scholar] [CrossRef]
- Akers, K.S.; Niece, K.L.; Chung, K.K.; Cannon, J.W.; Cota, J.M.; Murray, C.K. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J. Trauma Acute Care Surg. 2014, 77, S163–S170. [Google Scholar] [CrossRef] [Green Version]

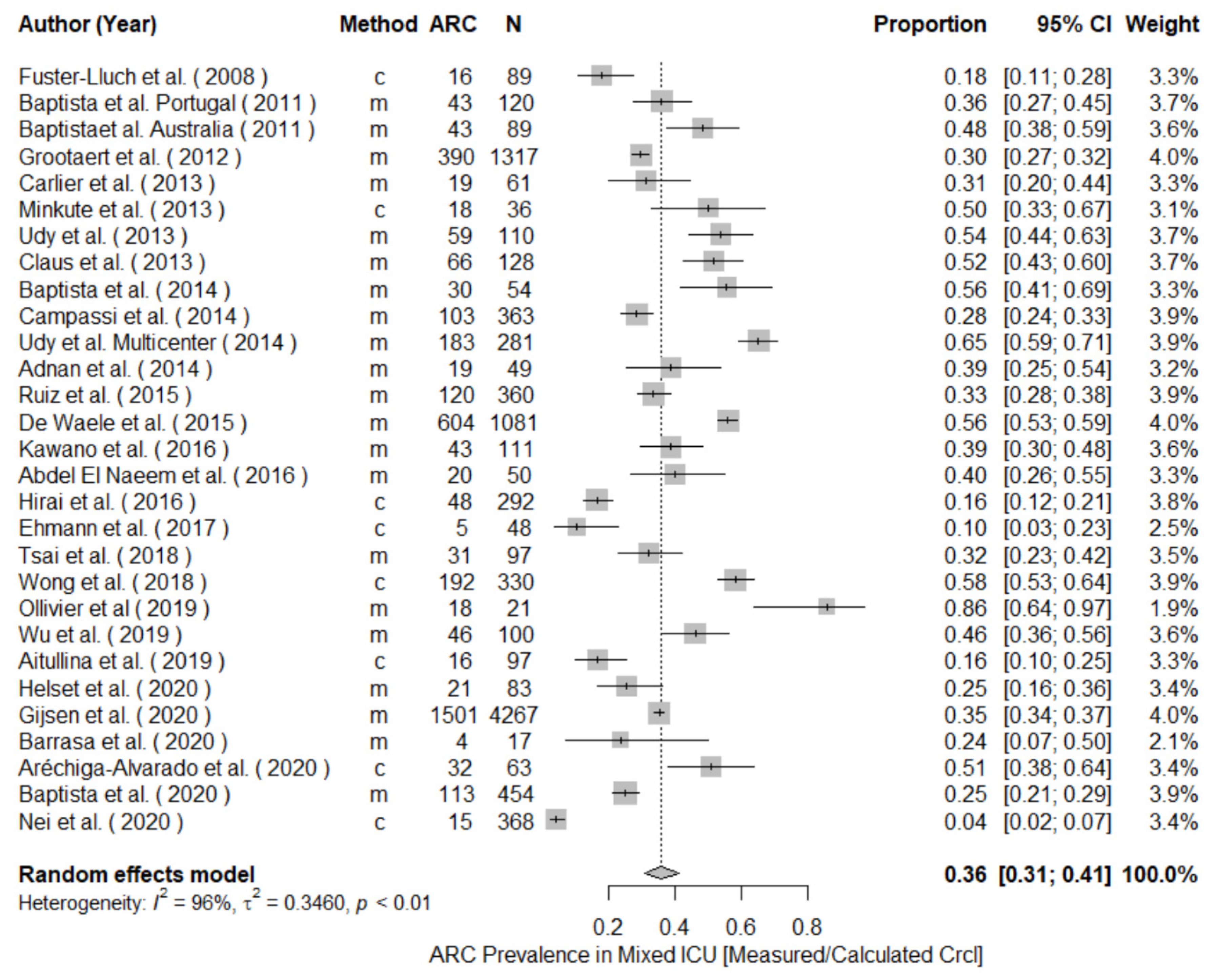

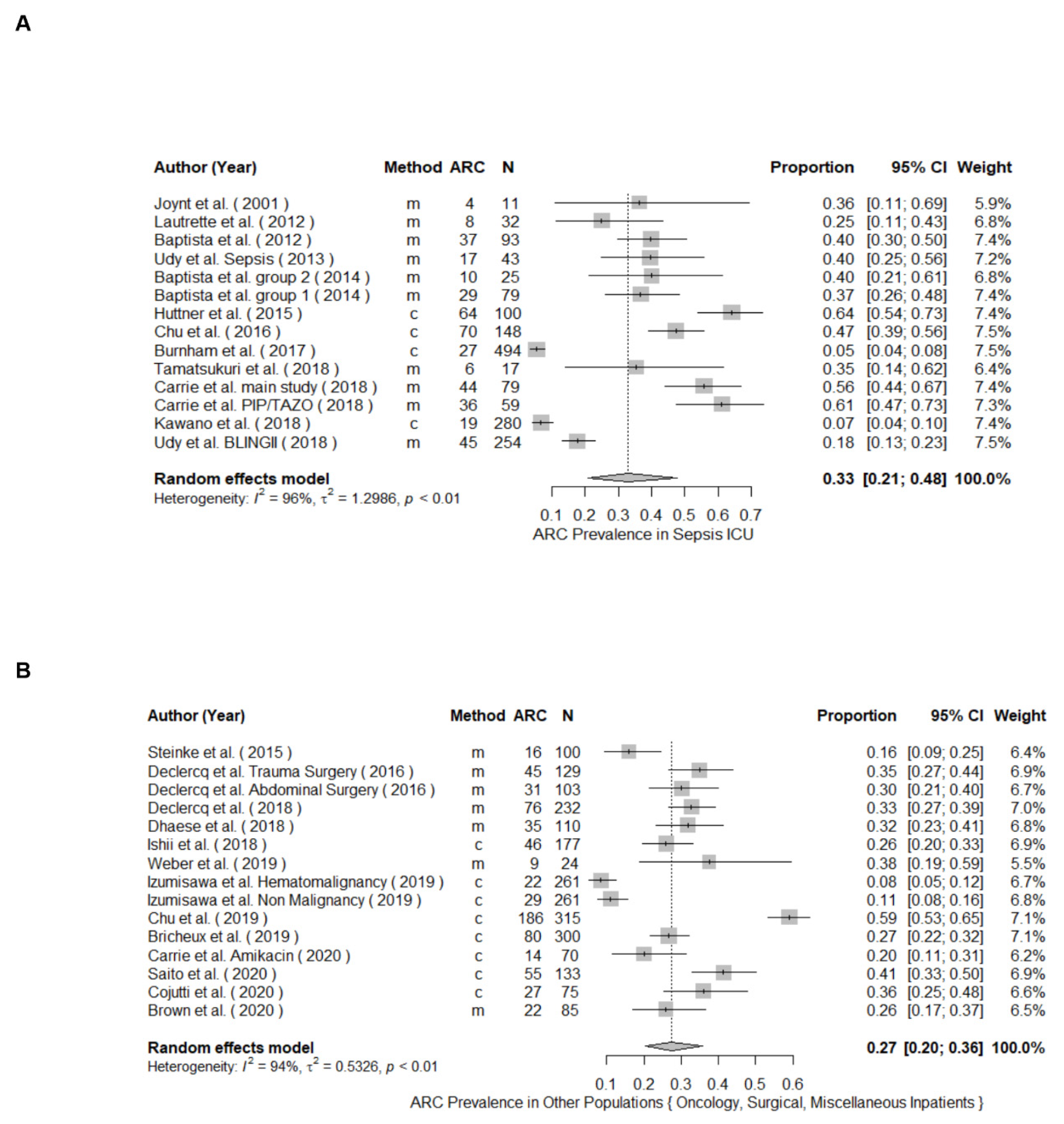

| Author | Year | Population | Study Design | Clearance Determination | ARC Definition | N | Prevalence (%) | Male n (%) | Age * | Main Diagnoses | Identifiable Risk Factors | Renal Impairment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Joynt et al. [29] | 2001 | Sepsis ICU | prospective observational | m | 24 h Urine | 130 | 11 | 36.4 | 7 (63.6) | 45 ± 16 | Sepsis | not reported | Excluded |
| Fuster-Lluch et al. [30] | 2008 | Mixed ICU | prospective observational | c | NKF | 120 | 89 | 18.0 | 67 (75.3) | 60.5 (18–86) | Several | not reported | Excluded |
| Baptista et al. Portugal [31] | 2011 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 120 | 35.8 | 87 (72.5) | 55.9 ± 21.1 | Sepsis, Trauma | not reported | Excluded |
| Baptista et al. Australia [31] | 2011 | Mixed ICU | prospective observational | m | 8 h Urine | 130 | 89 | 48.3 | 64 (71.9) | 40 ± 18.9 | Sepsis, Trauma | not reported | Excluded |
| Minville et al. PolyTrauma [32] | 2011 | Trauma ICU | retrospective observational | m | 24 h Urine | 120 | 144 | 54.9 | 108 (75) | 42 ± 18 | Poly trauma ICU | Age Trauma | Excluded |
| Minville et al. Non-PolyTrauma [32] | 2011 | Trauma ICU | retrospective observational | m | 24 h Urine | 120 | 140 | 19.3 | 88 (62.8) | 58 ± 17 | Non trauma ICU | Age Trauma | Excluded |
| Lautrette et al. [17] | 2012 | Sepsis ICU | retrospective observational | m | 24 h Urine | 140 | 32 | 25.0 | 15 (46.8) | 54 ± 16 | Infectious meningitis | not reported | Included |
| Baptista et al. [33] | 2012 | Sepsis ICU | prospective observational | m | 24 h Urine | 130 | 93 | 39.8 | 69 (74.2) | 58 (34–75) | Trauma, Sepsis, Other. | not reported | Excluded |
| Grootaert et al. [34] | 2012 | Mixed ICU | retrospective observational | m | 24 h Urine | 120 | 1317 | 29.6 | 247 (18.8) | 59 (48–67) | Several | not reported | Unclear |
| Carlier et al. [35] | 2013 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 61 | 31.1 | 51 (85) | 56 (48–67) | Infections | not reported | Excluded |
| Udy et al. Sepsis [36] | 2013 | Sepsis ICU | prospective observational | m | 6 h Urine | 130 | 43 | 39.5 | 22 (51.2) | 46.3 ± 17.1 | Sepsis | Age, Trauma, mod. SOFA | Included |
| Udy et al. Trauma [36] | 2013 | Trauma ICU | prospective observational | m | 6 h Urine | 130 | 28 | 85.7 | 23 (82.1) | 36.4 ± 13.9 | Trauma | Age, Trauma, mod. SOFA | Included |
| Minkute et al. [37] | 2013 | Mixed ICU | retrospective observational | c | C&G | 130 | 36 | 50.0 | 29 (80.5) | 49.75 (21) | Several | not reported | Excluded |
| Udy et al. [38] | 2013 | Mixed ICU | prospective observational | m | 8 h Urine | 120 | 110 | 53.6 | 70 (63.6) | 50.9 ± 16.9 | Several | not reported | Excluded |
| Claus et al. [39] | 2013 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 128 | 51.6 | 86 (67.2) | 59 (49–67.8) | Several | Age, APACHEII, Male sex | Excluded |
| Baptista et al. group 2 [40] | 2014 | Sepsis ICU | prospective observational | m | 8 h Urine | 130 | 25 | 40.0 | 17 (68) | 59.9 ± 17.2 | Several | not reported | Excluded |
| Baptista et al. group 1 [40] | 2014 | Sepsis ICU | retrospective observational | m | 8 h Urine | 130 | 79 | 36.7 | 52 (66) | 57.8 ± 15.5 | Several | not reported | Excluded |
| Baptista et al. [41] | 2014 | Mixed ICU | prospective observational | m | 8 h Urine | 130 | 54 | 55.6 | 39 (72.2) | 54.2 ± 16.9 | Several | not reported | Excluded |
| Campassi et al. [42] | 2014 | Mixed ICU | prospective observational | m | 24 h Urine | 120 | 363 | 28.4 | 103 (28.4) | 56.5 ± 16 | Several | Age, DM | Excluded |
| Udy et al. Multicenter [43] | 2014 | Mixed ICU | prospective observational | m | 8 h Urine | 130 | 281 | 65.1 | 178 (63.3) | 54.4 (52.5–56.4) | Several | not reported | Excluded |
| Adnan et al. [44] | 2014 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 49 | 38.8 | 37 (75.5) | 34 (24–47) | Trauma, others | not reported | Excluded |
| Ruiz et al. [45] | 2015 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 360 | 33.3 | 246 (68.3) | 50 ± 19 | Polytrauma, Non-polytrauma | Age, Polytrauma | Excluded |
| Huttner et al. [46] | 2015 | Sepsis ICU | prospective observational | c | C&G | 130 | 100 | 64.0 | 75 (73.5) | 46 ± 10.55 | Several | not reported | Excluded |
| Dias et al. [47] | 2015 | Neuro ICU | retrospective observational | c | C&G | 130 | 18 | 88.9 | 16 (89) | 41 ± 15.6 | TBI, Polytrauma | not reported | Included |
| May et al. [15] | 2015 | Neuro ICU | prospective observational | m | 24 h Urine | 130 | 20 | 100.0 | 8 (40) | 52.14 ± 10.36 | SAH | not reported | Excluded |
| De Waele et al. [48] | 2015 | Mixed ICU | retrospective observational | m | 24 h Urine | 130 | 1081 | 55.9 | 687 (63.6) | 62 (20.5) | Several | not reported | Excluded |
| Steinke et al. [49] | 2015 | Surgical ICU | retrospective observational | m | 18 h Urine | 130 | 100 | 16.0 | 61 (61) | 66 (57–74) | Infection, others | not reported | Included |
| Chu et al. [50] | 2016 | Sepsis ICU | retrospective observational | c | C&G | 130 | 148 | 47.3 | 97 (65.5) | 55.3 ± 14.9 | Infection | not reported | Excluded |
| Kawano et al. [51] | 2016 | Mixed ICU | prospective observational | m | 8 h Urine | 130 | 111 | 38.7 | 62 (55.9) | 67 (53–770) | Several | Age, DM, Weight, APACHEII, others | Excluded |
| Saour et al. [52] | 2016 | Trauma ICU | retrospective observational | c | MDRD | 120 | 775 | 61.3 | 581 (75) | 37.7 ± 17 | Several | not reported | Excluded |
| Abd El Naeem et al. [53] | 2017 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 50 | 40.0 | 32 (64) | 71 ± 15 | Sepsis, others | not reported | Excluded |
| Barletta et al. [54] | 2016 | Trauma ICU | retrospective observational | m | 12 h Urine | 130 | 65 | 69.2 | 48 (74) | 48 ± 18 | TBI, other traumas | not reported | Unclear |
| Declercq et al. Trauma Surgery [55] | 2016 | Surgical non-ICU | prospective observational | m | 8 h Urine | 130 | 129 | 34.9 | 75 (58) | 62 (46–75) | Trauma surgery | Age, Sex | Excluded |
| Declercq et al. Abdominal Surgery [55] | 2016 | Surgical non-ICU | prospective observational | m | 8 h Urine | 130 | 103 | 30.1 | 76 (74) | 63 (51–71) | Abdominal surgery | Age | Excluded |
| Hirai et al. [3] | 2016 | Mixed ICU | retrospective observational | c | C&G | 130 | 292 | 16.4 | 185 (63.4) | 72 (62.8–82) | Several | Age, Brain injury, others | Excluded |
| Ehmann et al. [56] | 2017 | Mixed ICU | prospective observational | c | C&G | 130 | 48 | 10.4 | 27 (56.3) | 55.5 (32–69.9) | Sepsis, others | not reported | Included |
| Burnham et al. [57] | 2017 | Sepsis ICU | retrospective observational | c | MDRD | 130 | 494 | 5.5 | 260 (52.6) | 59.9 ± 15.8 | Sepsis | Age, sepsis severity, others | Included |
| Carrie et al. RVI [58] | 2018 | Trauma ICU | retrospective observational | m | 24 h Urine | 130 | 30 | 66.7 | 27 (90) | 48 (32–67) | Polytrauma, TBI | not reported | Excluded |
| Udy et al. TBI [59] | 2017 | Neuro ICU | prospective observational | m | 8 h Urine | 150 | 11 | 100.0 | 9 (81.8) | 37 (24–49) | TBI | not reported | Included |
| Barletta et al. ARCTIC [60] | 2017 | Trauma ICU | prospective observational | m | 12 h Urine | 130 | 133 | 66.9 | 101 (76) | 48 ± 19 | TBI, fractures, others | Age, Sex | Excluded |
| Dhaese et al. [61] | 2018 | Surgical ICU | prospective observational | m | 8 h Urine | 130 | 110 | 31.8 | 75 (68.2) | 60 ± 14.4 | Several | not reported | Excluded |
| Tamatsukuri et al. [62] | 2018 | Sepsis ICU | prospective observational | m | 8 h Urine | 130 | 17 | 35.3 | 11 (64.7) | 60 (19.5) | Sepsis | not reported | Excluded |
| Carrie et al. main study [2] | 2018 | Sepsis ICU | prospective observational | m | 24 h Urine | 150 | 79 | 55.7 | 62 (78) | 52 (33–68) | Sepsis | not reported | Excluded |
| Carrie et al. PIP/TAZO [63] | 2018 | Sepsis ICU | prospective observational | m | 24 h Urine | 130 | 59 | 61.0 | 47 (80) | 53 ± 21 | Polytrauma, non-trauma surgery | not reported | Excluded |
| Carrie et al. TBI [18] | 2018 | Neuro ICU | prospective observational | m | 24 h Urine | 130 | 223 | 73.1 | 184 (83) | 36 (23–57) | TBI, VAP | not reported | Included |
| Kawano et al. [64] | 2018 | Sepsis ICU | retrospective observational | c | Japanese equation | 130 | 280 | 6.8 | 145 (51.8) | 74 (64–83) | Infection | Age, Sex, DM, others | Excluded |
| Tsai et al. [65] | 2018 | Mixed ICU | prospective observational | m | 8 h Urine | 130 | 97 | 32.0 | 60 (46) | 50 ± 18 | Sepsis, Trauma, others | not reported | Excluded |
| Wong et al. [66] | 2018 | Mixed ICU | prospective observational | c | C&G | 130 | 330 | 58.2 | 198 (60) | 53.4 ± 17.7 | Infection | not reported | Included |
| Ishii et al. [67] | 2018 | Mixed ICU—Non-ICU | retrospective observational | c | Japanese equation | 120 | 177 | 26.0 | 109 (62) | 73 (63–80) | Tumors, Brain injury | not reported | Excluded |
| Udy et al. BLINGII [27] | 2018 | Sepsis ICU | randomized controlled trial | m | 8 h Urine | 130 | 254 | 17.7 | 151 (59.4) | 63 (52–71) | Infection | not reported | Included |
| Ollivier et al. [68] | 2019 | Mixed ICU | prospective observational | m | 24 h Urine | 150 | 21 | 85.7 | 17 (81) | 36 (27–60) | Trauma, Surgery | not reported | Included |
| Wu et al. [69] | 2019 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 100 | 46.0 | 66 (66) | 60 (47–71) | Several | Age, SOFA, Weight, others | Excluded |
| Aitullina et al. [70] | 2019 | Mixed ICU | retrospective observational | c | not reported | 108 | 97 | 16.5 | 65 (67) | 63 (51–73.5) | Several | not reported | Included |
| Weber et al. [71] | 2019 | Oncology ICU | prospective observational | m | 24 h Urine | 120 | 24 | 37.5 | 14 (58.3) | 59 (39.8–63.5) | Febrile neutropenia | not reported | Excluded |
| Izumisawa et al. Hematomalignancy [72] | 2019 | Oncology Non-ICU & ICU | retrospective observational | c | C&G | 120 | 261 | 8.4 | 146 (55.9) | 65.6 ± 13.6 | Hematologic malignancy | not reported | Excluded |
| Izumisawa et al. Non-Malignancy [72] | 2019 | Oncology Non-ICU & ICU | retrospective observational | c | C&G | 120 | 261 | 11.1 | 175 (67) | 67.2 ± 16.9 | Non malignancy | not reported | Excluded |
| Chu et al. [73] | 2019 | Mixed ICU—Non-ICU | retrospective observational | c | C&G | 130 | 315 | 59.0 | 213 (67.6) | 56.3 (19) | Infection | not reported | Excluded |
| Villanueva et al. [74] | 2019 | Trauma ICU | retrospective observational | c | C&G | 160 | 70 | 50.0 | 57 (81.4) | 47.5 (31–61) | TBI, Spinal injury | not reported | Excluded |
| Morbitzer et al. aSAH [75] | 2019 | Neuro ICU | prospective observational | m | 8 h Urine | 130 | 50 | 94.0 | 16 (32) | 57.2 ± 10.7 | SAH | not reported | Excluded |
| Morbitzer et al. ICH [75] | 2019 | Neuro ICU | prospective observational | m | 8 h Urine | 130 | 30 | 50.0 | 18 (60) | 70 ± 13.7 | ICH | not reported | Excluded |
| Mulder et al. [76] | 2019 | Trauma ICU | retrospective observational | m | 24 h Urine | 130 | 207 | 57.0 | 141 (68) | 45 ± 20 | Trauma | Age, Sex, others | Excluded |
| Bricheux et al. [77]. | 2019 | Hospitalized | retrospective observational | c | C&G | 130 | 300 | 26.7 | 203 (68) | 59 ± 17 | Abdominal infection, Pneumonia | not reported | Unclear |
| Helset et al. [78] | 2020 | Mixed ICU | prospective observational | m | 24 h Urine | 130 | 83 | 25.3 | 61 (73.5) | 54.5 (38–63) | Several | not reported | Unclear |
| Gijsen et al. [7] | 2020 | Mixed ICU | retrospective observational | m | 24 h Urine | 130 | 4267 | 35.2 | 2669 (62.5) | 65 (54–74) | Several | not reported | Excluded |
| Barrasa et al. [79] | 2020 | Mixed ICU | prospective observational | m | 10 h Urine | 130 | 17 | 23.5 | 12 (70.6) | 61.7 | Several | not reported | Included |
| Lannou et al. [80] | 2020 | Neuro ICU | prospective observational | m | 24 h Urine | 130 | 60 | 53.3 | 53 (88) | 48 (32–60) | TBI, Multiple trauma | not reported | Excluded |
| Aréchiga-Alvarado et al. [81] | 2020 | Mixed ICU | prospective observational | c | C&G | 130 | 63 | 50.8 | 56 (88.9) | 33.25 (47.5) | Infection | not reported | Unclear |
| Carrie et al. Amikacin [82] | 2020 | Surgical ICU | retrospective observational | c | C&G | 130 | 70 | 20.0 | 53 (76) | 65 (51–73) | Infection | not reported | Unclear |
| Saito et al. [83] | 2020 | Oncology ICU | retrospective observational | c | own predictive model | 130 | 133 | 41.4 | 80 (60.2) | 64 (25–86) | Haematologic malignancies | Age, Sex, Scr, others | Included |
| Lannou et al. Editorial Letter [84] | 2020 | Neuro ICU | retrospective observational | m | 24 h Urine | 155 | 30 | 76.7 | not reported | 33 (47–57) | Brain trauma | not reported | Included |
| Cojutti et al. [28] | 2020 | Oncology ICU | prospective interventional | c | MDRD | 130 | 75 | 36.0 | 47 (62.7) | 58 (51–66) | Febrile neutropenia | not reported | Included |
| Brown et al. [85] | 2020 | Hospitalized | retrospective observational | m | 8 h Urine | 130 | 85 | 25.9 | 43 (50.6) | 55 (41–70) | Several | not reported | Excluded |
| Chen et al. [86] | 2020 | Neuro ICU | retrospective observational | c | C&G | 130 | 104 | 25.0 | 71 (68.3) | 44.5 (18.5) | Cerebral tumor, Stroke, TBI | not reported | Excluded |
| Baptista et al. [87] | 2020 | Mixed ICU | retrospective observational | m | 8 h Urine | 130 | 454 | 24.9 | 293 (64.5) | 66 (52–76) | Several | Age, Sex, Trauma, others | Included |
| Nei et al. [88] | 2020 | Mixed ICU | retrospective observational | c | CKD-EPI | 130 | 368 | 4.1 | 208 (56.5) | 66.8 (55.7–76.6) | TBI, Trauma, Sepsis, others | Age, ICH, SOFA, Trauma, others | Included |
| Author | Year | Population | Sample Size | Clearance Determination | Identified Risk Factor (s) | Odds Ratio (95% CI) | Study Inclusion in Prevalence Meta-Analysis |
|---|---|---|---|---|---|---|---|
| Hirai et al. [3] | 2016 | Mixed Hospital | 292 | Calculated | Febrile Neutropenia | 2.76 (1.11–6.67) | ✓ |
| Fluid Infusion ≥ 1500 mL/day | 2.53 (1.27–5.16) | ||||||
| Traumatic Brain Injury | 5.11 (1.49–17.57) | ||||||
| Nei et al. [88] | 2020 | Mixed ICU | 368 | Calculated | Charlson Comorbidity Index | 0.80 (0.16–1.00) | ✓ |
| Intracerebral Hemorrhage | 2.82 (1–69.1) | ||||||
| Kawano et al. [51] | 2016 | Mixed ICU | 111 | Measured | Post-Operative Without Sepsis | 0.28 (0.07–1.04) | ✓ |
| Wu et al. [69] | 2019 | Mixed ICU | 100 | Measured | Loop Diuretics | 0.32 (0.11–0.93) | ✓ |
| Age < 50 | 4.02 (1.54–10.51) | ||||||
| Udy et al. [36] | 2013 | Mixed ICU | 71 | Measured | Age </= 50 | 28.6 (4.4–187.2) | ✓ |
| Ramos et al. [89] | 2017 | Mixed ICU | 36 | Measured | 24h Sodium Excretion | 0.99 (0.98–1.00) | ✗ |
| Saito et al. [83] | 2020 | Oncology Hospital | 133 | Calculated | Serum Creatinine | 0.89 (0.83–0.94) | ✓ |
| Leukemia | 9.4 (2.4–36.8) | ||||||
| Fever | 2.4 (0.78–7.1) | ||||||
| Burnham et al. [57] | 2017 | Sepsis ICU | 494 | Calculated | African American Ethnicity | 3.45 (1.40–8.50) | ✗ |
| Sepsis Severity | 0.54 (0.30–0.97) | ||||||
| Mulder et al. [76] | 2019 | Trauma ICU | 207 | Measured | Packed RBC Transfusion | 0.31 (0.15–0.66) | ✓ |
| Eidelson et al. [90] | 2018 | Trauma ICU | 154 | Measured | Admission Hematocrit | 1.18 (1.04–1.33) | ✗ |
| Barletta et al. [60] | 2017 | Trauma ICU | 133 | Measured | Serum Creatinine < 0.7 mg/dL | 12.5 (3–52.6) | ✓ |
| Age < 56 | 58.3 (5.2–658.9) | ||||||
| Age 56–75 | 13.5 (1.2–151.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefny, F.; Stuart, A.; Kung, J.Y.; Mahmoud, S.H. Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 445. https://doi.org/10.3390/pharmaceutics14020445
Hefny F, Stuart A, Kung JY, Mahmoud SH. Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics. 2022; 14(2):445. https://doi.org/10.3390/pharmaceutics14020445
Chicago/Turabian StyleHefny, Fatma, Anna Stuart, Janice Y. Kung, and Sherif Hanafy Mahmoud. 2022. "Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis" Pharmaceutics 14, no. 2: 445. https://doi.org/10.3390/pharmaceutics14020445
APA StyleHefny, F., Stuart, A., Kung, J. Y., & Mahmoud, S. H. (2022). Prevalence and Risk Factors of Augmented Renal Clearance: A Systematic Review and Meta-Analysis. Pharmaceutics, 14(2), 445. https://doi.org/10.3390/pharmaceutics14020445






