Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges
Abstract
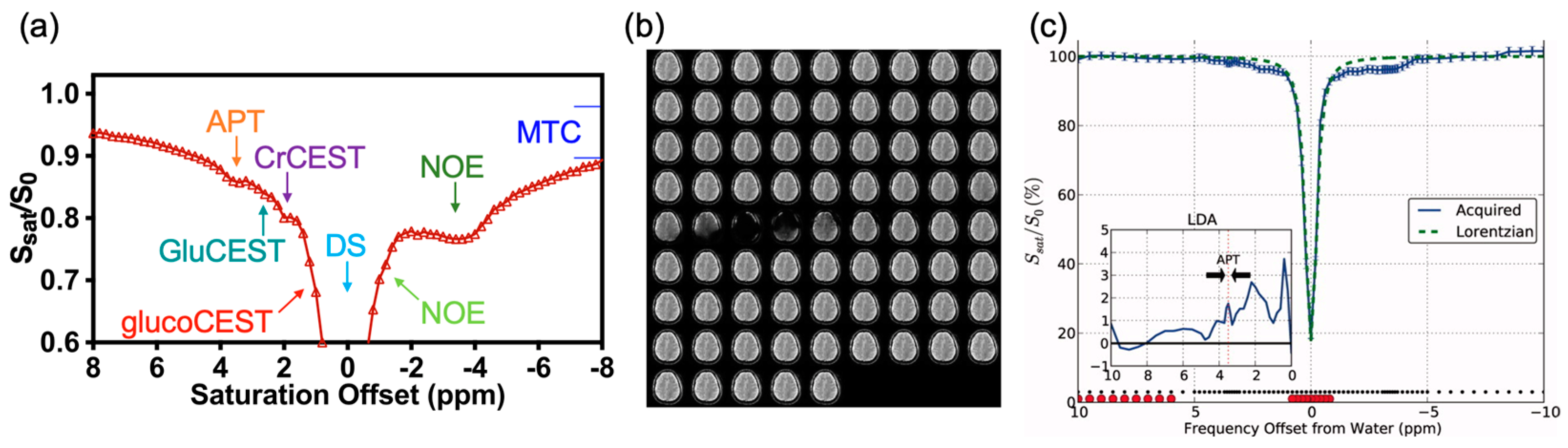
:1. Introduction
2. CEST Imaging of Brain Tumors
2.1. Endogenous Contrast
2.1.1. APT-Weighted (APTw) Contrast
2.1.2. NOE Contrast
2.2. Glioblastoma and Gliomas (Grade II, III)
| Species | Tumor Type (Grade) | B0 (T) | Analysis Method | CEST Contrast | Molecular/Cellular Changes | Ref. |
|---|---|---|---|---|---|---|
| Rat | Glioma, C6 | 3 | DISC-CEST | APT | Cellular and nuclear atypia | Wu Y. et al., 2019 [92] |
| Rat | Gliosarcoma, 9L | 4.7 | MTRasym | APTw | Cellular proteins and peptides | Zhou Z. et al., 2003 [3] |
| Rat | Gliosarcoma, 9L | 4.7 | MTRasym | APTw | pH | Zhou Z. et al., 2003 [48] |
| Rat | Gliosarcoma, 9L SF188/V + glioma | 4.7 | MTRasym | APTw | Treatment effects (radiation therapy), radiation necrosis, mobile cytosolic proteins, and peptides | Zhou J. et al., 2011 [70] |
| Rat | Gliosarcoma, 9L | 4.7 | MTRasym | APTw NOE (−2.5 to −5 ppm) | Mobile proteins, peptides, lipids, and metabolites | Zhou J. et al., 2013 [43] |
| Rat | U87 | 4.7 | MTRasym | APTw | Treatment effects (radiation therapy), radiation necrosis, cellularity, nuclear atypia, and vacuolation | Hong X. et al., 2014 [69] |
| Rat | GBM | 4.7 | EMR | APT, NOE | Mobile proteins and peptides | Heo HY. et al., 2016 [87] |
| Rat | GBM | 4.7 | MTRREX, AREX, CESTR, CESTRnr | APT, 2 ppm | APT: mobile proteins and peptides, 2 ppm: protein and peptide side-chain amide protons and various amine-related protons | Heo HY. et al., 2017 [89] |
| Rat | U87 | 4.7 | MTRasym | APTw | Amide proton mobile amide proton content or the increased amide proton exchange rate | Lee DH. et al. 2017 [90] |
| EMR | APT, NOE | |||||
| Rat | Glioma | 4.7 | DISC-CEST | APT NOE | APT: intracellular mobile proteins/peptides concentration NOE: aliphatic and olefinic protons | Zhou IY. et al., 2017 [26] |
| Rat | Gliosarcoma, 9L | 4.7 | MTRasym | APTw | NA | Heo H. et al., 2019 [91] |
| EMR | APT, NOE | |||||
| Rat | Gliosarcoma, 9L | 9.4 | AREX | APT, NOE | Protein contents | Xu J. et al., 2014 [93] |
| Rat | Gliosarcoma, 9L | 9.4 | Lorentzian | APT (3.6 ppm) NOE (−3.2 ppm) | Amide proton | Cai K. et al., 2015 [115] |
| 2 ppm | Tumor progression and creatine | |||||
| Rat | Gliosarcoma, 9L; glioma, F98 | 9.4 | Lorentzian | 2 ppm | Creatine and tumor aggressiveness | Cai K. et al., 2017 [116] |
| Rat | Gliosarcoma, 9L | 9.4 | MTRasym, AREX | 3 ppm | Amine and protein | Zhang XY. et al., 2017 [117] |
| Rat | ENU1564 (brain metastasis model) | 9.4 | APTR* | APT | Protein concentration and pH | Ray KJ. et al., 2019 [107] |
| Rat | Gliosarcoma, 9L | 9.4 | Lorentzian | 3 ppm | Glutamate | Debnath A. et al., 2020 [118] |
| Rat | Gliosarcoma, 9L | 9.4 | RPT | NOE (−1.6 ppm) | Phospholipids on cell membranes | Zu Z. et al., 2020 [101] |
| Mouse | GBM, patient cells | 7 | MTRasym | APTw | Proliferation, cellular acidification, and treatment effect (TMZ) | Sagiyama K. et al., 2014 [40] |
| Mouse | Glioma, GL261 | 7 | MTRasym | 3 ppm | Amine, pH, cellularity, and necrosis | Harris RJ. et al., 2015 [38] |
| Mouse | U87MG | 9.4 | AACID | AACID (amide at 3.5 ppm, amine at 2.75 ppm) | Intracellular pH and treatment effect | Albatany M. et al., 2019 [66] |
| Human (n = 10) | GBM (IV), oligodendroglioma (III), LGO (II), LGA (II), Meningioma | 3 | MTRasym | APTw | Cellular protein/peptide and intracellular pH | Jones CK. et al., 2006 [4] |
| Human (n = 9) | GMB (IV), AO (III), AA (III), LGO (II), LGA (II) | 3 | MTRasym | APTw | Glioma grading, cytosolic protein and peptide, and intracellular pH | Zhou J. et al., 2008 [60] |
| Human (n = 12) | GBM (IV), astrocytoma (III), oligodendroglioma (III) | 3 | MTRasym | APTw | Viable tumor core, edema, necrosis, mobile protein, and peptide | Wen Z. et al., 2010 [45] |
| Human (n = 14) | GBM (IV), AA (III), LGO (II), LGA (II), LGOA (II) | 3 | MTRasym | APTw | Protein content | Zhou J. et al., 2013 [42] |
| Human (n = 36) | GBM (IV), AO (III), AA (III), AOA (III), LGA (II), LGO (II), LGOA (II) | 3 | MTRasym | APTw | Glioma grading, necrosis, cell density, and proliferation | Togao O. et al., 2014 [39] |
| Human (n = 25) | Glioma (II–IV) | 3 | MTRasym | 3 ppm | An acidic signature, treatment effect (CRT), and PFS | Harris RJ. et al., 2015 [38] |
| Human (n = 26) | GBM (IV), AA (III), AO (III), LGO (II), LGOA (II) | 3 | MTRasym | APTw | Glioma grading | Sakata A. et al., 2015 [65] |
| Human (n = 13) | GBM (IV), Gliomas (low–grade), meningiomas, lymphoma | 3 | MTRasym | APTw | NA | Togao O. et al., 2015 [36] |
| Human (n = 11) | High–grade glioma | 3 | EMR | APT, NOE | NA | Heo HY. et al., 2016 [119] |
| Human (n = 32) | High–grade glioma Lymphomas | 3 | MTRasym | APTw | Differentiate lymphomas from high-grade glioma and protein | Jiang S. et al., 2016 [64] |
| Human (n = 65) | Glioma (II–IV) | 3 | MTRasym | APTw | Proliferation | Park J. et al., 2016 [32] |
| Human (n = 32) | GBM (IV), AA (III), gliomas (low–grade) | 3 | MTRasym | APTw | Cellularity | Ma B. et al., 2016 [68] |
| Human (n = 65) | Glioma (II–IV) | 3 | MTRasym | APTw | Proliferation | Park J. et al., 2016 [32] |
| Human (n = 32) | GBM (IV), AA (III), gliomas (low–grade) | 3 | MTRasym | APTw | Cellularity | Ma B. et al., 2016 [68] |
| Human (n = 7) | AA (III), LGO (II), LGA (II) | 3 | MTRasym | APTw | NA | Zhang Y. et al., 2016 [88] |
| Human (n = 44) | Glioma (II–IV) | 3 | MTRasym | APTw | Glioma grading and proliferation | Bai Y. et al., 2017 [63] |
| Human (n = 46) | Glioma (II–IV) | 3 | MTRasym | APTw | Glioma grading, protein, and peptide | Choi YS. et al., 2017 [31] |
| Human (n = 24) | Glioma (II–IV), edema | 3 | MTRasym | APTw | Cellularity, proliferation, and glioma grading | Jiang S. et al., 2017 [30] |
| Human (n = 27) | Glioma (II) | 3 | MTRasym | APTw | IDH mutation | Jiang S. et al., 2017 [29] |
| Human (n = 42) | Glioma (II–IV) | 3 | MTRasym | APTw | Glioma grading, proliferation, choline, and N-acetylaspartate | Su C. et al., 2017 [27] |
| Human (n = 18) | GBM (IV) | 3 | MTRasym | APTw | MGMT promoter methylation status | Jiang S. et al., 2018 [24] |
| Human (n = 57) | Meningioma | 3 | MTRasym | APTw | Intracellular proteins and peptides | Joo B. et al., 2018 [23] |
| Human (n = 42) | Glioma (II–IV) | 3 | MTRasym | APTw | MGMT prediction | Su L. et al., 2018 [20] |
| Human (n = 21) | GBM (IV), glioma (II), metastases, meningoma, chronic infarction | 3 | MTRasym | APTw | Proteins and peptides | Sun H. et al., 2018 [120] |
| Human (n = 32) | Glioma (II–IV) | 3 | Z-spectral fitted, | APT | Glioma grading and proliferation | Zhang J. et al., 2018 [19] |
| MTRasym | APTw | |||||
| Human (n = 51) | Glioma (II–IV) | 3 | MTRasym | APTw | Glioma grading and mobile cellular proteins | Zou T. et al., 2018 [62] |
| Human (n = 21) | GBM (IV), gliosarcoma (IV), AA (III), | 3 | MTRasym | APTw | Cellularity, proliferation, tumor recurrence, and a marker for active glioma | Jiang S. et al., 2019 [18] |
| Human (n = 71) | Glioma (III and IV) | 3 | MTRasym | APTw | Overall survival, PFS, and IDH mutation | Joo B. et al., 2019 [17] |
| Human (n = 14) | GBM (IV) | 3 | MTRasym | APTw | IDH and pH | Schure JR. et al., 2019 [108] |
| Lorentzian | APT | |||||
| Human (n = 90) | Glioma (II–IV) | 3 | MTRasym | 3 ppm | Cerebral blood volume and IDH mutation | Wang YL. et al., 2019 [72] |
| Human (n = 26) | Glioma (II, IV) Metastasis | 3 | MTRasym | APTw (3.5±0.4 ppm) | Glioma grading, MGMT, and IDH | Durmo F. et al., 2020 [61] |
| Human (n = 59) | Glioma (II, III) | 3 | MTRasym, machine learning | APTw | IDH1 mutation | Han Y. et al., 2020 [71] |
| Human (n = 54) | GBM (IV) | 3 | MTRasym | APTw | Treatment effect (bevacizumab), 12-month progression, PFS, and CBV | Park J. et al., 2020 [13] |
| Human (n = 30) | Glioma (III, IV) | 3 | MTRasym | APTw | Treatment effect (radiotherapy or CRT), tumor recurrence, and protein | Liu J. et al., 2020 [14] |
| Human (n = 46) | Glioma (II–IV) | 3 | MTRasym | APTw | Cellularity and CBV glioma grading | Schon S. et al., 2020 [59] |
| Human (n = 18) | GBM (IV), AA (III), astrocytoma (III), LGO (II), LGA (II) | 3 | MTRasym | APTw | Cytosolic protein content, mobile proteins, and semisolid macromolecules | Warnert EAH. et al., 2021 [11] |
| Lorentzian | APT | |||||
| Human (n = 51) | Glioma (II–IV) | 3 | MTRasym | APTw | Glioma grading (peptide or protein concentrations), cellularity, proliferation, and IDH mutation | Xu Z. et al., 2021 [9] |
| Human (n = 48) | Glioma (II–IV), Brain metastases | 3 | MTRasym, machine learning | APTw | Protein content | Sartoretti E. et al., 2021 [12] |
| Human (n = 19) | GBM, meningioma, brain metastasis | 3 | QUASS | APT, MT&NOE (−1.5 ppm) | −1.5 ppm: proliferation | Wu Y. et al., 2021 [10] |
| Human (n = 48) | High–grade glioma (III,IV) Low–grade glioma (I,II) | 3 | CESTRnr, EMR | APT | Glioma grading (proteins and peptides) | Zhang H. et al., 2021 [8] |
| Human (n = 81) | H3K27M–mutant associated brainstem glioma | 3 | MTRasym | APTw | H3K27M mutation, proliferation, pH, and protein and peptide metabolism | Zhuo Z. et al., 2021 [6] |
| Human (n = 113) | Glioma (II–IV) | 3 | Lorentzian | APT | Glioma grading (cellularity, mobile protein, and peptides), and IDH mutation | Su C. et al., 2022 [5] |
| 2 ppm | Creatine and 1p/19q co-deletion | |||||
| Human (n = 1) | AA (III) | 7 | MTRasym | −3.5 ppm | Cellular density | Jones CK. et al., 2013 [44] |
| Lorentzian | APT (3.3 to 3.7 ppm) NOE (−2 to −5 ppm) | |||||
| Human (n = 2) | GBM (IV), glioma (II or III) | 7 | MTRasym | −3 ppm | Necrosis and the structural integrity of proteins in cells (protein folding) | Zaiss M. et al., 2013 [121] |
| Human (n = 12) | GBM (IV) | 7 | MTRasym | 3.3 ppm | Protein structures proliferation | Paech D. et al., 2014 [41] |
| Human (n = 15) | GBM (IV) | 7 | MTRasym | 3.3 ppm | Cell density and edema | Paech D. et al., 2015 [37] |
| Human (n = 1) | LGO (II) | 7 | AREX | APT, NOE | NA | Windschuh J. et al., 2015 [35] |
| Human (n = 10) | GBM (IV) | 7 | AREX | 3.5 ppm, NOE | Protein and lipid | Zaiss M. et al., 2015 [34] |
| Human (n = 10) | Gliomas (II–IV) | 7 | MTRasym | APTw | Glioma grading | Heo HY. et al., 2016 [33] |
| EMR | APT (3.3 to 3.7 ppm) NOE (−3.3 to −3.7 ppm) | |||||
| Human (n = 11) | GBM (IV) | 7 | MTRasym, dnsAREX | 3.5 ppm | Amide proton and pH | Zaiss M. et al., 2017 [25] |
| Human (n = 31) | Glioma (II–IV) | 7 | MTRasym, dnsAREX | APT (3.5 ppm) | Glioma grading, IDH mutation, and MGMT promoter methylation status | Paech D. et al., 2018 [22] |
| Human (n = 20) | GBM (IV) | 7 | Lorentzian | NOE | Treatment effect (First-line therapy) | Regnery S. et al., 2018 [21] |
| MTRasym | APTw | |||||
| dnsAREX | APT | |||||
| Human (n = 12) | GBM (IV), LGO (II), LGA (II) | 7 | AREX | NOE | Treatment effect (CRT) | Meissner JE. et al., 2019 [67] |
| dnsAREX | APT | |||||
| Human (n = 26) | GBM (IV), AA (III) | 7 | AREX, dnsAREX | APT | Overall survival and PFS, amino acid, and protein | Paech D. et al., 2019 [16] |
| Human (n = 1) | GBM | 9.4 | Lorentzian | 3.5 ppm, NOE (−1.6, −3.5 ppm), 2 ppm, 2.7 ppm | Proteins and lipids | Zaiss M. et al., 2018 [98] |
2.3. Multiple CEST Contrast in Brain Tumors
3. Non-Metallic CEST Contrast Agents for Brain Tumor Imaging
4. Imaging Drugs and Drug Delivery
4.1. Imaging Drugs and Drug Delivery Using CEST MRI

4.2. Theranostic Applications
5. Technical Part
5.1. CEST Acquisition
5.2. CEST Post-Processing
5.2.1. Z-Spectra and B0/B1 Correction
5.2.2. Z-Spectra Analysis
- (1)
- MTRasym analysis
- (2)
- Lorentzian difference analysis (LDA)
- (3)
- Multi-pool Lorentzian fitting
- (4)
- Polynomial and Lorentzian line-shape fitting (PLOF)
- (5)
- Three-offset method
5.2.3. Inverse Z-Spectra Analysis
5.2.4. Deep Learning-Based Analysis Methods
- (1)
- Deep learning-based Z-spectra analysis
- (2)
- Deep learning-based CEST fingerprinting
6. Promises and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanvito, F.; Castellano, A.; Falini, A. Advancements in Neuroimaging to Unravel Biological and Molecular Features of Brain Tumors. Cancers 2021, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.; Pomper, M.G.; Golay, X.; Zhou, J. Amide proton transfer imaging of human brain tumors at 3 T. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Xu, S.; Lin, D.; He, H.; Chen, Z.; Damen, F.C.; Ke, C.; Lv, X.; Cai, K. Multi-parametric Z-spectral MRI may have a good performance for glioma stratification in clinical patients. Eur. Radiol. 2022, 32, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Qu, L.; Zhang, P.; Duan, Y.; Cheng, D.; Xu, X.; Sun, T.; Ding, J.; Xie, C.; Liu, X.; et al. Prediction of H3K27M-mutant brainstem glioma by amide proton transfer-weighted imaging and its derived radiomics. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4426–4436. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.; Peng, Y. Amide Proton Transfer-Weighted MR Imaging of Pediatric Central Nervous System Diseases. Magn. Reson. Imaging Clin. N. Am. 2021, 29, 631–641. [Google Scholar] [CrossRef]
- Zhang, H.; Yong, X.; Ma, X.; Zhao, J.; Shen, Z.; Chen, X.; Tian, F.; Chen, W.; Wu, D.; Zhang, Y. Differentiation of low- and high-grade pediatric gliomas with amide proton transfer imaging: Added value beyond quantitative relaxation times. Eur. Radiol. 2021, 31, 9110–9119. [Google Scholar] [CrossRef]
- Xu, Z.; Ke, C.; Liu, J.; Xu, S.; Han, L.; Yang, Y.; Qian, L.; Liu, X.; Zheng, H.; Lv, X.; et al. Diagnostic performance between MR amide proton transfer (APT) and diffusion kurtosis imaging (DKI) in glioma grading and IDH mutation status prediction at 3T. Eur. J. Radiol. 2021, 134, 109466. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Yang, Q.; Zou, L.; Zhang, F.; Qian, L.; Liu, X.; Zheng, H.; Luo, D.; Sun, P.Z. Fast and equilibrium CEST imaging of brain tumor patients at 3T. Neuroimage Clin. 2021, 33, 102890. [Google Scholar] [CrossRef]
- Warnert, E.A.H.; Wood, T.C.; Incekara, F.; Barker, G.J.; Vincent, A.J.P.; Schouten, J.; Kros, J.M.; van den Bent, M.; Smits, M.; Tamames, J.A.H. Mapping tumour heterogeneity with pulsed 3D CEST MRI in non-enhancing glioma at 3T. MAGMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Sartoretti, T.; Wyss, M.; Reischauer, C.; van Smoorenburg, L.; Binkert, C.A.; Sartoretti-Schefer, S.; Mannil, M. Amide proton transfer weighted (APTw) imaging based radiomics allows for the differentiation of gliomas from metastases. Sci. Rep. 2021, 11, 5506. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, S.Y.; Jung, S.C.; Kim, J.H.; Heo, H.Y. Identification of Early Response to Anti-Angiogenic Therapy in Recurrent Glioblastoma: Amide Proton Transfer-weighted and Perfusion-weighted MRI compared with Diffusion-weighted MRI. Radiology 2020, 295, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Chen, Y.; Lv, X.; Lv, Y.; Zhou, J.; Xi, S.; Dou, W.; Qian, L.; Zheng, H.; et al. Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J. Magn. Reson. Imaging 2020, 51, 1154–1161. [Google Scholar] [CrossRef]
- Chen, L.; Schär, M.; Chan, K.W.; Huang, J.; Wei, Z.; Lu, H.; Qin, Q.; Weiss, R.G.; van Zijl, P.C.; Xu, J. In vivo imaging of phosphocreatine with artificial neural networks. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Paech, D.; Dreher, C.; Regnery, S.; Meissner, J.E.; Goerke, S.; Windschuh, J.; Oberhollenzer, J.; Schultheiss, M.; Deike-Hofmann, K.; Bickelhaupt, S.; et al. Relaxation-compensated amide proton transfer (APT) MRI signal intensity is associated with survival and progression in high-grade glioma patients. Eur. Radiol. 2019, 29, 4957–4967. [Google Scholar] [CrossRef]
- Joo, B.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J.; Lee, S.K. Amide proton transfer imaging might predict survival and IDH mutation status in high-grade glioma. Eur. Radiol. 2019, 29, 6643–6652. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Lim, M.; Heo, H.Y.; Zhang, Y.; Blair, L.; Wen, Z.; Holdhoff, M.; Lin, D.; Huang, P.; et al. Identifying Recurrent Malignant Glioma after Treatment Using Amide Proton Transfer-Weighted MR Imaging: A Validation Study with Image-Guided Stereotactic Biopsy. Clin. Cancer Res. 2019, 25, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, W.; Tain, R.; Zhou, X.J.; Cai, K. Improved Differentiation of Low-Grade and High-Grade Gliomas and Detection of Tumor Proliferation Using APT Contrast Fitted from Z-Spectrum. Mol. Imaging Biol. 2018, 20, 623–631. [Google Scholar] [CrossRef]
- Su, L.; Gao, P.; Lin, S.; Wu, B.; Qin, W.; Lin, Y.; Xue, J. Predicting O6-Methylguanine-DNA Methyltransferase Protein Expression in Primary Low- and High-Grade Gliomas Using Certain Qualitative Characteristics of Amide Proton Transfer-Weighted Magnetic Resonance Imaging. World Neurosurg. 2018, 116, e814–e823. [Google Scholar] [CrossRef]
- Regnery, S.; Adeberg, S.; Dreher, C.; Oberhollenzer, J.; Meissner, J.E.; Goerke, S.; Windschuh, J.; Deike-Hofmann, K.; Bickelhaupt, S.; Zaiss, M.; et al. Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients. Oncotarget 2018, 9, 28772–28783. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of IDH mutation and MGMT methylation status in glioma patients using relaxation-compensated multipool CEST MRI at 7.0 T. Neurol Oncol. 2018, 20, 1661–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, B.; Han, K.; Choi, Y.S.; Lee, S.K.; Ahn, S.S.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J. Amide proton transfer imaging for differentiation of benign and atypical meningiomas. Eur. Radiol. 2018, 28, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Rui, Q.; Wang, Y.; Heo, H.Y.; Zou, T.; Yu, H.; Zhang, Y.; Wang, X.; Du, Y.; Wen, X.; et al. Discriminating MGMT promoter methylation status in patients with glioblastoma employing amide proton transfer-weighted MRI metrics. Eur. Radiol. 2018, 28, 2115–2123. [Google Scholar] [CrossRef]
- Zaiss, M.; Windschuh, J.; Goerke, S.; Paech, D.; Meissner, J.E.; Burth, S.; Kickingereder, P.; Wick, W.; Bendszus, M.; Schlemmer, H.P.; et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn. Reson. Med. 2017, 77, 196–208. [Google Scholar] [CrossRef]
- Zhou, I.Y.; Wang, E.; Cheung, J.S.; Lu, D.; Ji, Y.; Zhang, X.; Fulci, G.; Sun, P.Z. Direct saturation-corrected chemical exchange saturation transfer MRI of glioma: Simplified decoupling of amide proton transfer and nuclear overhauser effect contrasts. Magn. Reson. Med. 2017, 78, 2307–2314. [Google Scholar] [CrossRef]
- Su, C.; Liu, C.; Zhao, L.; Jiang, J.; Zhang, J.; Li, S.; Zhu, W.; Wang, J. Amide Proton Transfer Imaging Allows Detection of Glioma Grades and Tumor Proliferation: Comparison with Ki-67 Expression and Proton MR Spectroscopy Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Bai, Y.; Lin, Y.; Hong, X.; Liu, T.; Ma, L.; Haacke, E.M.; Zhou, J.; Wang, J.; Wang, M. Amide proton transfer magnetic resonance imaging in detecting intracranial hemorrhage at different stages: A comparative study with susceptibility weighted imaging. Sci. Rep. 2017, 7, 45696. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Zhang, Y.; Heo, H.Y.; Wen, Z.; Blair, L.; Qin, H.; Lim, M.; Quinones-Hinojosa, A.; Weingart, J.D.; et al. Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur. J. Cancer 2017, 83, 9–18. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ahn, S.S.; Lee, S.K.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J. Amide proton transfer imaging to discriminate between low- and high-grade gliomas: Added value to apparent diffusion coefficient and relative cerebral blood volume. Eur. Radiol. 2017, 27, 3181–3189. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Park, K.J.; Kim, S.J.; Kim, J.H.; Smith, S.A. Pre- and Posttreatment Glioma: Comparison of Amide Proton Transfer Imaging with MR Spectroscopy for Biomarkers of Tumor Proliferation. Radiology 2016, 278, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Jones, C.K.; Hua, J.; Yadav, N.; Agarwal, S.; Zhou, J.; van Zijl, P.C.; Pillai, J.J. Whole-brain amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging in glioma patients using low-power steady-state pulsed chemical exchange saturation transfer (CEST) imaging at 7T. J. Magn. Reson. Imaging 2016, 44, 41–50. [Google Scholar] [CrossRef]
- Zaiss, M.; Windschuh, J.; Paech, D.; Meissner, J.E.; Burth, S.; Schmitt, B.; Kickingereder, P.; Wiestler, B.; Wick, W.; Bendszus, M.; et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 2015, 112, 180–188. [Google Scholar] [CrossRef]
- Windschuh, J.; Zaiss, M.; Meissner, J.E.; Paech, D.; Radbruch, A.; Ladd, M.E.; Bachert, P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Togao, O.; Hiwatashi, A.; Keupp, J.; Yamashita, K.; Kikuchi, K.; Yoshiura, T.; Suzuki, Y.; Kruiskamp, M.J.; Sagiyama, K.; Takahashi, M.; et al. Scan-rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J. Magn. Reson. Imaging 2015, 42, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Paech, D.; Burth, S.; Windschuh, J.; Meissner, J.E.; Zaiss, M.; Eidel, O.; Kickingereder, P.; Nowosielski, M.; Wiestler, B.; Sahm, F.; et al. Nuclear Overhauser Enhancement imaging of glioblastoma at 7 Tesla: Region specific correlation with apparent diffusion coefficient and histology. PLoS ONE 2015, 10, e0121220. [Google Scholar] [CrossRef]
- Harris, R.J.; Cloughesy, T.F.; Liau, L.M.; Prins, R.M.; Antonios, J.P.; Li, D.; Yong, W.H.; Pope, W.B.; Lai, A.; Nghiemphu, P.L.; et al. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neurol Oncol. 2015, 17, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Togao, O.; Yoshiura, T.; Keupp, J.; Hiwatashi, A.; Yamashita, K.; Kikuchi, K.; Suzuki, Y.; Suzuki, S.O.; Iwaki, T.; Hata, N.; et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neurol Oncol. 2014, 16, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Sagiyama, K.; Mashimo, T.; Togao, O.; Vemireddy, V.; Hatanpaa, K.J.; Maher, E.A.; Mickey, B.E.; Pan, E.; Sherry, A.D.; Bachoo, R.M.; et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 4542–4547. [Google Scholar] [CrossRef] [Green Version]
- Paech, D.; Zaiss, M.; Meissner, J.E.; Windschuh, J.; Wiestler, B.; Bachert, P.; Neumann, J.O.; Kickingereder, P.; Schlemmer, H.P.; Wick, W.; et al. Nuclear overhauser enhancement mediated chemical exchange saturation transfer imaging at 7 Tesla in glioblastoma patients. PLoS ONE 2014, 9, e104181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, H.; Lim, M.; Blair, L.; Quinones-Hinojosa, A.; Messina, S.A.; Eberhart, C.G.; Pomper, M.G.; Laterra, J.; Barker, P.B.; et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. J. Magn. Reson. Imaging 2013, 38, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Hong, X.; Zhao, X.; Gao, J.H.; Yuan, J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn. Reson. Med. 2013, 70, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.K.; Huang, A.; Xu, J.; Edden, R.A.; Schar, M.; Hua, J.; Oskolkov, N.; Zaca, D.; Zhou, J.; McMahon, M.T.; et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage 2013, 77, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.; Hu, S.; Huang, F.; Wang, X.; Guo, L.; Quan, X.; Wang, S.; Zhou, J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 2010, 51, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.; Aletras, A.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef]
- Zhou, J.; Van Zijl, P.C. Chemical exchange saturation transfer imaging and spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 48, 109–136. [Google Scholar] [CrossRef]
- Zhou, J.; Payen, J.-F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Sherry, A.D.; Woods, M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu. Rev. Biomed. Eng. 2008, 10, 391–411. [Google Scholar] [CrossRef] [Green Version]
- Van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Song, X.; Chan, K.W.; McMahon, M.T. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013, 26, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.; Bachert, P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: A review of theoretical approaches and methods. Phys. Med. Biol. 2013, 58, R221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Payen, J.F.; van Zijl, P.C. The interaction between magnetization transfer and blood-oxygen-level-dependent effects. Magn. Reson. Med. 2005, 53, 356–366. [Google Scholar] [CrossRef]
- van Zijl, P.C.M.; Brindle, K.; Lu, H.; Barker, P.B.; Edden, R.; Yadav, N.; Knutsson, L. Hyperpolarized MRI, functional MRI, MR spectroscopy and CEST to provide metabolic information in vivo. Curr. Opin. Chem. Biol. 2021, 63, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Rev. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Schon, S.; Cabello, J.; Liesche-Starnecker, F.; Molina-Romero, M.; Eichinger, P.; Metz, M.; Karimov, I.; Preibisch, C.; Keupp, J.; Hock, A.; et al. Imaging glioma biology: Spatial comparison of amino acid PET, amide proton transfer, and perfusion-weighted MRI in newly diagnosed gliomas. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1468–1475. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Blakeley, J.O.; Hua, J.; Kim, M.; Laterra, J.; Pomper, M.G.; van Zijl, P.C. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn. Reson. Med. 2008, 60, 842–849. [Google Scholar] [CrossRef] [Green Version]
- Durmo, F.; Rydhog, A.; Testud, F.; Latt, J.; Schmitt, B.; Rydelius, A.; Englund, E.; Bengzon, J.; van Zijl, P.; Knutsson, L.; et al. Assessment of Amide proton transfer weighted (APTw) MRI for pre-surgical prediction of final diagnosis in gliomas. PLoS ONE 2020, 15, e0244003. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Yu, H.; Jiang, C.; Wang, X.; Jiang, S.; Rui, Q.; Mei, Y.; Zhou, J.; Wen, Z. Differentiating the histologic grades of gliomas preoperatively using amide proton transfer-weighted (APTW) and intravoxel incoherent motion MRI. NMR Biomed. 2018, 31, e3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Lin, Y.; Zhang, W.; Kong, L.; Wang, L.; Zuo, P.; Vallines, I.; Schmitt, B.; Tian, J.; Song, X.; et al. Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 2017, 8, 5834–5842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Yu, H.; Wang, X.; Lu, S.; Li, Y.; Feng, L.; Zhang, Y.; Heo, H.Y.; Lee, D.H.; Zhou, J.; et al. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur. Radiol. 2016, 26, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakata, A.; Okada, T.; Yamamoto, A.; Kanagaki, M.; Fushimi, Y.; Okada, T.; Dodo, T.; Arakawa, Y.; Schmitt, B.; Miyamoto, S.; et al. Grading glial tumors with amide proton transfer MR imaging: Different analytical approaches. J. Neurooncol. 2015, 122, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Albatany, M.; Ostapchenko, V.G.; Meakin, S.; Bartha, R. Brain tumor acidification using drugs simultaneously targeting multiple pH regulatory mechanisms. J. Neurooncol. 2019, 144, 453–462. [Google Scholar] [CrossRef]
- Meissner, J.E.; Korzowski, A.; Regnery, S.; Goerke, S.; Breitling, J.; Floca, R.O.; Debus, J.; Schlemmer, H.P.; Ladd, M.E.; Bachert, P.; et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J. Magn. Reson. Imaging 2019, 50, 1268–1277. [Google Scholar] [CrossRef]
- Ma, B.; Blakeley, J.O.; Hong, X.; Zhang, H.; Jiang, S.; Blair, L.; Zhang, Y.; Heo, H.Y.; Zhang, M.; van Zijl, P.C.; et al. Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J. Magn. Reson. Imaging 2016, 44, 456–462. [Google Scholar] [CrossRef]
- Hong, X.; Liu, L.; Wang, M.; Ding, K.; Fan, Y.; Ma, B.; Lal, B.; Tyler, B.; Mangraviti, A.; Wang, S.; et al. Quantitative multiparametric MRI assessment of glioma response to radiotherapy in a rat model. Neuro Oncol. 2014, 16, 856–867. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Tryggestad, E.; Wen, Z.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Wang, W.; Yang, Y.; Sun, Y.Z.; Xiao, G.; Tian, Q.; Zhang, J.; Cui, G.B.; Yan, L.F. Amide Proton Transfer Imaging in Predicting Isocitrate Dehydrogenase 1 Mutation Status of Grade II/III Gliomas Based on Support Vector Machine. Front. Neurosci. 2020, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yao, J.; Chakhoyan, A.; Raymond, C.; Salamon, N.; Liau, L.M.; Nghiemphu, P.L.; Lai, A.; Pope, W.B.; Nguyen, N.; et al. Association between Tumor Acidity and Hypervascularity in Human Gliomas Using pH-Weighted Amine Chemical Exchange Saturation Transfer Echo-Planar Imaging and Dynamic Susceptibility Contrast Perfusion MRI at 3T. AJNR Am. J. Neuroradiol. 2019, 40, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, L.R.; Randtke, E.A.; High, R.A.; Jones, K.M.; Howison, C.M.; Pagel, M.D. A comparison of exogenous and endogenous CEST MRI methods for evaluating in vivo pH. Magn. Reson. Med. 2018, 79, 2766–2772. [Google Scholar] [CrossRef] [PubMed]
- Sotirios, B.; Demetriou, E.; Topriceanu, C.C.; Zakrzewska, Z. The role of APT imaging in gliomas grading: A systematic review and meta-analysis. Eur. J. Radiol. 2020, 133, 109353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Nakajo, M.; Yoneyama, T.; Takumi, K.; Kumagae, Y.; Fukukura, Y.; Yoshiura, T. Amide proton transfer imaging of tumors: Theory, clinical applications, pitfalls, and future directions. Jpn. J. Radiol. 2019, 37, 109–116. [Google Scholar] [CrossRef]
- Jones, K.M.; Pollard, A.C.; Pagel, M.D. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J. Magn. Reson. Imaging 2018, 47, 11–27. [Google Scholar] [CrossRef]
- Vinogradov, E.; Sherry, A.D.; Lenkinski, R.E. CEST: From basic principles to applications, challenges and opportunities. J. Magn. Reson. 2013, 229, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Kogan, F.; Hariharan, H.; Reddy, R. Chemical Exchange Saturation Transfer (CEST) Imaging: Description of Technique and Potential Clinical Applications. Curr Radiol Rep 2013, 1, 102–114. [Google Scholar] [CrossRef] [Green Version]
- Ferris, S.P.; Hofmann, J.W.; Solomon, D.A.; Perry, A. Characterization of gliomas: From morphology to molecules. Virchows. Arch. 2017, 471, 257–269. [Google Scholar] [CrossRef]
- Huang, J.; Han, X.; Chen, L.; Xu, X.; Xu, J.; Chan, K.W. Relayed nuclear Overhauser enhancement imaging with magnetization transfer contrast suppression at 3 T. Magn. Reson. Med. 2021, 85, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Goerke, S.; Soehngen, Y.; Deshmane, A.; Zaiss, M.; Breitling, J.; Boyd, P.S.; Herz, K.; Zimmermann, F.; Klika, K.D.; Schlemmer, H.P. Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn. Reson. Med. 2019, 82, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Di Gregorio, E.; Abategiovanni, R.; Ceccon, A.; Assfalg, M.; Molinari, H.; Aime, S. Chemical exchange saturation transfer (CEST): An efficient tool for detecting molecular information on proteins’ behaviour. Analyst 2014, 139, 2687–2690. [Google Scholar] [CrossRef]
- Chen, L.; Wei, Z.; Chan, K.W.; Cai, S.; Liu, G.; Lu, H.; Wong, P.C.; van Zijl, P.C.; Li, T.; Xu, J. Protein aggregation linked to Alzheimer’s disease revealed by saturation transfer MRI. Neuroimage 2019, 188, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.N.; Yang, X.; Li, Y.; Li, W.; Liu, G.; Van Zijl, P.C. Detection of dynamic substrate binding using MRI. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Tryggestad, E.; Zhou, T.; Armour, M.; Wen, Z.; Fu, D.X.; Ford, E.; van Zijl, P.C.; Zhou, J. Assessment of MRI parameters as imaging biomarkers for radiation necrosis in the rat brain. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e431–e436. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.Y.; Zhang, Y.; Lee, D.H.; Hong, X.; Zhou, J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla. Magn. Reson. Med. 2016, 75, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Heo, H.Y.; Jiang, S.; Lee, D.H.; Bottomley, P.A.; Zhou, J. Highly accelerated chemical exchange saturation transfer (CEST) measurements with linear algebraic modeling. Magn. Reson. Med. 2016, 76, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.Y.; Lee, D.H.; Zhang, Y.; Zhao, X.; Jiang, S.; Chen, M.; Zhou, J. Insight into the quantitative metrics of chemical exchange saturation transfer (CEST) imaging. Magn. Reson. Med. 2017, 77, 1853–1865. [Google Scholar] [CrossRef]
- Lee, D.H.; Heo, H.Y.; Zhang, K.; Zhang, Y.; Jiang, S.; Zhao, X.; Zhou, J. Quantitative assessment of the effects of water proton concentration and water T1 changes on amide proton transfer (APT) and nuclear overhauser enhancement (NOE) MRI: The origin of the APT imaging signal in brain tumor. Magn. Reson. Med. 2017, 77, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.Y.; Zhang, Y.; Jiang, S.; Zhou, J. Influences of experimental parameters on chemical exchange saturation transfer (CEST) metrics of brain tumors using animal models at 4.7T. Magn. Reson. Med. 2019, 81, 316–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chen, Y.; Zhao, Y.; Yang, S.; Zhao, J.; Zhou, J.; Chen, Z.; Sun, P.Z.; Zheng, H. Direct radiofrequency saturation corrected amide proton transfer tumor MRI at 3 T. Magn. Reson. Med. 2019, 81, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zaiss, M.; Zu, Z.; Li, H.; Xie, J.; Gochberg, D.F.; Bachert, P.; Gore, J.C. On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed. 2014, 27, 406–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-Y.; Wang, F.; Afzal, A.; Xu, J.; Gore, J.C.; Gochberg, D.F.; Zu, Z. A new NOE-mediated MT signal at around −1.6 ppm for detecting ischemic stroke in rat brain. Magn. Reson. Imaging 2016, 34, 1100–1106. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yadav, N.N.; Bar-Shir, A.; Jones, C.K.; Chan, K.W.; Zhang, J.; Walczak, P.; McMahon, M.T.; van Zijl, P.C. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med./Soc. Magn. Reson. Med. 2013, 71, 1798–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yadav, N.N.; Zeng, H.; Jones, C.K.; Zhou, J.; van Zijl, P.C.; Xu, J. Magnetization transfer contrast-suppressed imaging of amide proton transfer and relayed nuclear overhauser enhancement chemical exchange saturation transfer effects in the human brain at 7T. Magn. Reson. Med. 2016, 75, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, J.; Lai, J.H.; Chen, Z.; Lee, C.Y.; Mak, H.K.; Chan, K.H.; Chan, K.W. Relayed nuclear Overhauser effect weighted (rNOEw) imaging identifies multiple sclerosis. Neuro Image Clin. 2021, 32, 102867. [Google Scholar] [CrossRef]
- Zaiss, M.; Schuppert, M.; Deshmane, A.; Herz, K.; Ehses, P.; Fullbier, L.; Lindig, T.; Bender, B.; Ernemann, U.; Scheffler, K. Chemical exchange saturation transfer MRI contrast in the human brain at 9.4T. Neuro Image 2018, 179, 144–155. [Google Scholar] [CrossRef]
- Glang, F.; Deshmane, A.; Prokudin, S.; Martin, F.; Herz, K.; Lindig, T.; Bender, B.; Scheffler, K.; Zaiss, M. DeepCEST 3T: Robust MRI parameter determination and uncertainty quantification with neural networks—Application to CEST imaging of the human brain at 3T. Magn. Reson. Med. 2020, 84, 450–466. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.; Deshmane, A.; Schuppert, M.; Herz, K.; Glang, F.; Ehses, P.; Lindig, T.; Bender, B.; Ernemann, U.; Scheffler, K. DeepCEST: 9.4 T Chemical exchange saturation transfer MRI contrast predicted from 3 T data—A proof of concept study. Magn. Reson. Med. 2019, 81, 3901–3914. [Google Scholar] [CrossRef] [Green Version]
- Zu, Z.; Lin, E.C.; Louie, E.A.; Xu, J.; Li, H.; Xie, J.; Lankford, C.L.; Chekmenev, E.Y.; Swanson, S.D.; Does, M.D.; et al. Relayed nuclear Overhauser enhancement sensitivity to membrane Cho phospholipids. Magn. Reson. Med. 2020, 84, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.K.; Shi, G.; Homer, R.; Harsh, G.; Atlas, S.W.; Bednarski, M.D. Magnetic resonance image-guided proteomics of human glioblastoma multiforme. J. Magn. Reson. Imaging 2003, 18, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.L.; Cotran, R.S. Pathologic Basis of Disease; Saunders: Christchurch, New Zealand, 1979. [Google Scholar]
- Perlman, O.; Ito, H.; Herz, K.; Shono, N.; Nakashima, H.; Zaiss, M.; Chiocca, E.A.; Cohen, O.; Rosen, M.S.; Farrar, C.T. Quantitative imaging of apoptosis following oncolytic virotherapy by magnetic resonance fingerprinting aided by deep learning. Nat. Biomed. Eng. 2021, 1–10. [Google Scholar] [CrossRef]
- Ross, B.D.; Higgins, R.J.; Boggan, J.E.; Knittel, B.; Garwood, M. 31P NMR spectroscopy of the in vivo metabolism of an intracerebral glioma in the rat. Magn. Reson. Med. 1988, 6, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Maintz, D.; Heindel, W.; Kugel, H.; Jaeger, R.; Lackner, K.J. Phosphorus-31 MR spectroscopy of normal adult human brain and brain tumours. NMR Biomed. 2002, 15, 18–27. [Google Scholar] [CrossRef]
- Ray, K.J.; Simard, M.A.; Larkin, J.R.; Coates, J.; Kinchesh, P.; Smart, S.C.; Higgins, G.S.; Chappell, M.A.; Sibson, N.R. Tumor pH and Protein Concentration Contribute to the Signal of Amide Proton Transfer Magnetic Resonance Imaging. Cancer Res. 2019, 79, 1343–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schure, J.R.; Shrestha, M.; Breuer, S.; Deichmann, R.; Hattingen, E.; Wagner, M.; Pilatus, U. The pH sensitivity of APT-CEST using phosphorus spectroscopy as a reference method. NMR Biomed. 2019, 32, e4125. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Han, K.; Zhou, J.; Zhao, Y.; Choi, Y.S.; Lee, S.-K.; Ahn, S.S. Characterizing amide proton transfer imaging in haemorrhage brain lesions using 3T MRI. Eur. Radiol. 2017, 27, 1577–1584. [Google Scholar] [CrossRef] [Green Version]
- Grossman, R.; Tyler, B.; Brem, H.; Eberhart, C.G.; Wang, S.; Fu, D.-X.; Wen, Z.; Zhou, J. Growth properties of SF188/V+ human glioma in rats in vivo observed by magnetic resonance imaging. J. Neurooncol. 2012, 110, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, K.; Nakajo, M.; Yoneyama, T.; Fukukura, Y.; Hirano, H.; Goto, Y.; Sasaki, M.; Akamine, Y.; Keupp, J.; Yoshiura, T. Histogram analysis of amide proton transfer–weighted imaging: Comparison of glioblastoma and solitary brain metastasis in enhancing tumors and peritumoral regions. Eur. Radiol. 2019, 29, 4133–4140. [Google Scholar] [CrossRef]
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015, 47, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Alfaro-Munoz, K.D.; Heathcock, L.E.; van Thuijl, H.F.; Gilbert, M.R.; Armstrong, T.S.; Sulman, E.P.; Cahill, D.P.; Vera-Bolanos, E.; et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015, 129, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateishi, K.; Wakimoto, H.; Cahill, D.P. IDH1 Mutation and World Health Organization 2016 Diagnostic Criteria for Adult Diffuse Gliomas: Advances in Surgical Strategy. Neurosurgery 2017, 64, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Singh, A.; Poptani, H.; Li, W.; Yang, S.; Lu, Y.; Hariharan, H.; Zhou, X.J.; Reddy, R. CEST signal at 2ppm (CEST@2ppm) from Z-spectral fitting correlates with creatine distribution in brain tumor. NMR Biomed. 2015, 28, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, K.; Tain, R.W.; Zhou, X.J.; Damen, F.C.; Scotti, A.M.; Hariharan, H.; Poptani, H.; Reddy, R. Creatine CEST MRI for Differentiating Gliomas with Different Degrees of Aggressiveness. Mol. Imaging Biol. 2017, 19, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Wang, F.; Li, H.; Xu, J.; Gochberg, D.F.; Gore, J.C.; Zu, Z. CEST imaging of fast exchanging amine pools with corrections for competing effects at 9.4T. NMR Biomed. 2017, 30, e3715. [Google Scholar] [CrossRef]
- Debnath, A.; Hariharan, H.; Nanga, R.P.R.; Reddy, R.; Singh, A. Glutamate-Weighted CEST Contrast After Removal of Magnetization Transfer Effect in Human Brain and Rat Brain with Tumor. Mol. Imaging Biol. 2020, 22, 1087–1101. [Google Scholar] [CrossRef]
- Heo, H.Y.; Zhang, Y.; Jiang, S.; Lee, D.H.; Zhou, J. Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 3 Tesla. Magn. Reson. Med. 2016, 75, 1630–1639. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xin, J.; Zhou, J.; Lu, Z.; Guo, Q. Applying Amide Proton Transfer MR Imaging to Hybrid Brain PET/MR: Concordance with Gadolinium Enhancement and Added Value to [(18)F]FDG PET. Mol. Imaging Biol. 2018, 20, 473–481. [Google Scholar] [CrossRef]
- Zaiss, M.; Kunz, P.; Goerke, S.; Radbruch, A.; Bachert, P. MR imaging of protein folding in vitro employing nuclear-Overhauser-mediated saturation transfer. NMR Biomed. 2013, 26, 1815–1822. [Google Scholar] [CrossRef]
- Chan, K.W.; Bulte, J.W.; McMahon, M.T. Diamagnetic chemical exchange saturation transfer (diaCEST) liposomes: Physicochemical properties and imaging applications. WIREs Nanomed. Nanobiotechnol. 2013, 6, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Han, Z.; Liu, G. Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives. Pharmaceuticals 2020, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferrauto, G.; Delli Castelli, D.; Di Gregorio, E.; Terreno, E.; Aime, S. LipoCEST and cellCEST imaging agents: Opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Pagel, M.M. The Pursuit of Theranostics with CEST MRI. Theranostics 2016, 6, 1601–1602. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.N.; Brasher, P.M.A.; Sevick, R.J.; Rewcastle, N.B.; Forsyth, P.A. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002, 59, 947. [Google Scholar] [CrossRef]
- Cowper, S.E.; Robin, H.S.; Steinberg, S.M.; Su, L.D.; Gupta, S.; LeBoit, P.E. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000, 356, 1000–1001. [Google Scholar] [CrossRef]
- FDA. FDA Drug Safety Podcast: FDA Warns That gadolinium-Based Contrast Agents (GBCAs) Are Retained in the Body; Requires New Class Warnings. Available online: https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-drug-safety-podcast-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body-requires (accessed on 26 January 2022).
- Behzadi, A.H.; Zhao, Y.; Farooq, Z.; Prince, M.R. Immediate allergic reactions to gadolinium-based contrast agents: A systematic review and meta-analysis. Radiology 2018, 286, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; McMahon, M.; Kato, Y.; Liu, G.; Bulte, J.; Bhujwalla, Z.; Artemov, D.; van Zijl, P. Natural D-Glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. / Soc. Magn. Reson. Med. 2012, 68, 1764–1773. [Google Scholar] [CrossRef] [Green Version]
- Nasrallah, F.A.; Pagès, G.; Kuchel, P.W.; Golay, X.; Chuang, K.H. Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI. J. Cereb. Blood Flow. Metab. 2013, 33, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Weygand, J.; Hwang, K.-P.; Mohamed, A.S.R.; Ding, Y.; Fuller, C.D.; Lai, S.Y.; Frank, S.J.; Zhou, J. Magnetic Resonance Imaging of Glucose Uptake and Metabolism in Patients with Head and Neck Cancer. Sci. Rep. 2016, 6, 30618. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.A.; Li, Y.; Lal, B.; Yadav, N.N.; Xu, X.; Xu, J.; Laterra, J.; van Zijl, P.C.M. CEST MRI of 3-O-methyl-D-glucose uptake and accumulation in brain tumors. Magn. Reson. Med. 2019, 81, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, M.; Horev, J.; Tsarfaty, I.; Navon, G. Molecular imaging of tumors and metastases using chemical exchange saturation transfer (CEST) MRI. Sci. Rep. 2013, 3, 3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker-Samuel, S.; Ramasawmy, R.; Torrealdea, F.; Rega, M.; Rajkumar, V.; Johnson, S.P.; Richardson, S.; Gonçalves, M.; Parkes, H.G.; Arstad, E.; et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 2013, 19, 1067–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Xu, J.; Knutsson, L.; Liu, J.; Liu, H.; Li, Y.; Lal, B.; Laterra, J.; Artemov, D.; Liu, G.; et al. The effect of the mTOR inhibitor rapamycin on glucoCEST signal in a preclinical model of glioblastoma. Magn. Reson. Med. 2019, 81, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lai, J.H.; Han, X.; Chen, Z.; Xiao, P.; Liu, Y.; Chen, L.; Xu, J.; Chan, K.W. Sensitivity schemes for dynamic glucose-enhanced magnetic resonance imaging to detect glucose uptake and clearance in mouse brain at 3 T. NMR Biomed. 2022, 35, e4640. [Google Scholar] [CrossRef]
- Huang, J.; van Zijl, P.C.; Han, X.; Dong, C.M.; Cheng, G.W.; Tse, K.-H.; Knutsson, L.; Chen, L.; Lai, J.H.; Wu, E.X. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer’s disease detected by dynamic glucose-enhanced MRI. Sci. Adv. 2020, 6, eaba3884. [Google Scholar] [CrossRef]
- Xu, X.; Chan, K.W.; Knutsson, L.; Artemov, D.; Xu, J.; Liu, G.; Kato, Y.; Lal, B.; Laterra, J.; McMahon, M.T.; et al. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn. Reson. Med. 2015, 74, 1556–1563. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yadav, N.N.; Knutsson, L.; Hua, J.; Kalyani, R.; Hall, E.; Laterra, J.; Blakeley, J.; Strowd, R.; Pomper, M.; et al. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography 2015, 1, 105–114. [Google Scholar] [CrossRef]
- Herz, K.; Lindig, T.; Deshmane, A.; Schittenhelm, J.; Skardelly, M.; Bender, B.; Ernemann, U.; Scheffler, K.; Zaiss, M. T1ρ-based dynamic glucose-enhanced (DGEρ) MRI at 3 T: Method development and early clinical experience in the human brain. Magn. Reson. Med. 2019, 82, 1832–1847. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sehgal, A.A.; Yadav, N.N.; Laterra, J.; Blair, L.; Blakeley, J.; Seidemo, A.; Coughlin, J.M.; Pomper, M.G.; Knutsson, L.; et al. d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucose enhanced (DGE) MRI at 3T: Early experience in healthy volunteers and brain tumor patients. Magn. Reson. Med. 2020, 84, 247–262. [Google Scholar] [CrossRef]
- Zu, Z.; Spear, J.; Li, H.; Xu, J.; Gore, J.C. Measurement of regional cerebral glucose uptake by magnetic resonance spin-lock imaging. Magn. Reson. Imaging 2014, 32, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Iordanova, B.; Hitchens, T.K.; Modo, M.; Wang, P.; Mehrens, H.; Kim, S.G. Chemical exchange-sensitive spin-lock (CESL) MRI of glucose and analogs in brain tumors. Magn. Reson. Med. 2018, 80, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Zu, Z.; Jiang, X.; Xu, J.; Gore, J.C. Spin-lock imaging of 3-o-methyl-D glucose (3oMG) in brain tumors. Magn. Reson. Med. 2018, 80, 1110–1117. [Google Scholar] [CrossRef]
- Haris, M.; Singh, A.; Mohammed, I.; Ittyerah, R.; Nath, K.; Nanga, R.P.R.; Debrosse, C.; Kogan, F.; Cai, K.; Poptani, H.; et al. In vivo magnetic resonance imaging of tumor protease activity. Sci. Rep. 2014, 4, 6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.Y.; Luo, L.; McMahon, M.T.; Vogelstein, B.; van Zijl, P.C.M.; Zhou, S.; et al. CEST theranostics: Label-free MR imaging of anticancer drugs. Oncotarget 2016, 7, 6369–6378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Moake, M.; Har-el, Y.E.; Long, C.M.; Chan, K.W.; Cardona, A.; Jamil, M.; Walczak, P.; Gilad, A.A.; Sgouros, G.; et al. In vivo multicolor molecular MR imaging using diamagnetic chemical exchange saturation transfer liposomes. Magn. Reson. Med. 2012, 67, 1106–1113. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.M.; Har-el, Y.E.; McMahon, M.T.; Zhou, J.; Sherry, A.D.; Sgouros, G.; Bulte, J.W.; van Zijl, P.C. Size-induced enhancement of chemical exchange saturation transfer (CEST) contrast in liposomes. J. Am. Chem. Soc. 2008, 130, 5178–5184. [Google Scholar] [CrossRef] [Green Version]
- Terreno, E.; Castelli, D.D.; Milone, L.; Rollet, S.; Stancanello, J.; Violante, E.; Aime, S. First ex-vivo MRI co-localization of two LIPOCEST agents. Contrast. Media Mol. Imaging 2008, 3, 38–43. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Airan, R.; Han, Z.; Xu, J.; Chan, K.W.Y.; Xu, Y.; Bulte, J.W.M.; van Zijl, P.C.M.; McMahon, M.T.; et al. CT and CEST MRI bimodal imaging of the intratumoral distribution of iodinated liposomes. Quant. Imaging Med. Surg. 2019, 9, 1579–1591. [Google Scholar] [CrossRef]
- Chan, K.W.; Yu, T.; Qiao, Y.; Liu, Q.; Yang, M.; Patel, H.; Liu, G.; Kinzler, K.W.; Vogelstein, B.; Bulte, J.W.; et al. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. J. Control Release 2014, 180, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yu, T.; Wang, Y.Y.; Lai, S.K.; Zeng, Q.; Miao, B.; Tang, B.C.; Simons, B.W.; Ensign, L.M.; Liu, G.; et al. Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Adv Healthc Mater 2013, 3, 1044–1052. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Chan, K.W.; Anonuevo, A.; Song, X.; Schuster, B.S.; Chattopadhyay, S.; Xu, Q.; Oskolkov, N.; Patel, H.; Ensign, L.M.; et al. Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: Demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomed. Nanotechnol. Biol. Med. 2015, 11, 401–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, L.H.; Xiao, P.; Huang, J.; HAN, X.; Chan, K.W. CEST Imaging of Nose-to-Brain Drug Delivery using Iohexol liposomes at 3T. In Proceedings of the ISMRM & SMRT Annual Meeting & Exhibition, Online, 15–20 May 2021. No. 0720. [Google Scholar]
- Yuan, Y.; Zhang, J.; Qi, X.; Li, S.; Liu, G.; Siddhanta, S.; Barman, I.; Song, X.; McMahon, M.T.; Bulte, J.W.M. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 2019, 18, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.Y.; Liu, G.; Song, X.; Kim, H.; Yu, T.; Arifin, D.R.; Gilad, A.A.; Hanes, J.; Walczak, P.; van Zijl, P.C.M.; et al. MRI-detectable pH nanosensors incorporated into hydrogels for in vivo sensing of transplanted-cell viability. Nat. Mater. 2013, 12, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lock, L.L.; Li, Y.; Mao, X.; Chen, H.; Staedtke, V.; Bai, R.; Ma, W.; Lin, R.; Li, Y.; Liu, G.; et al. One-Component Supramolecular Filament Hydrogels as Theranostic Label-Free Magnetic Resonance Imaging Agents. ACS Nano 2017, 11, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venur, V.A.; Peereboom, D.M.; Ahluwalia, M.S. Current medical treatment of glioblastoma. Cancer Treat. Res. 2015, 163, 103–115. [Google Scholar] [CrossRef]
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert. Rev. Anticancer Ther. 2013, 13, 1453–1461. [Google Scholar] [CrossRef]
- Tyler, B.; Fowers, K.D.; Li, K.W.; Recinos, V.R.; Caplan, J.M.; Hdeib, A.; Grossman, R.; Basaldella, L.; Bekelis, K.; Pradilla, G.; et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J. Neurosurg. 2010, 113, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Kim, B.; Chun, C.; Lee, S.H.; Song, S.-C. MRI-monitored long-term therapeutic hydrogel system for brain tumors without surgical resection. Biomaterials 2012, 33, 4836–4842. [Google Scholar] [CrossRef]
- Bastiancich, C.; Vanvarenberg, K.; Ucakar, B.; Pitorre, M.; Bastiat, G.; Lagarce, F.; Préat, V.; Danhier, F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J. Control. Release 2016, 225, 283–293. [Google Scholar] [CrossRef]
- Vellimana, A.K.; Recinos, V.R.; Hwang, L.; Fowers, K.D.; Li, K.W.; Zhang, Y.; Okonma, S.; Eberhart, C.G.; Brem, H.; Tyler, B.M. Combination of paclitaxel thermal gel depot with temozolomide and radiotherapy significantly prolongs survival in an experimental rodent glioma model. J. Neuro-Oncol. 2013, 111, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Huang, J.; To, A.K.W.; Lai, J.H.C.; Xiao, P.; Wu, E.X.; Xu, J.; Chan, K.W.Y. CEST MRI detectable liposomal hydrogels for multiparametric monitoring in the brain at 3T. Theranostics 2020, 10, 2215–2228. [Google Scholar] [CrossRef]
- Han, X.; Lai, J.H.C.; Huang, J.; Park, S.W.; Liu, Y.; Chan, K.W.Y. Imaging Self-Healing Hydrogels and Chemotherapeutics Using CEST MRI at 3 T. ACS Appl. Bio Mater. 2021, 4, 5605–5616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhai, Y.; Jin, Z.; Li, C.; Sun, P.Z.; Wu, Y. Preliminary demonstration of in vivo quasi-steady-state CEST postprocessing—Correction of saturation time and relaxation delay for robust quantification of tumor MT and APT effects. Magn. Reson. Med. 2021, 86, 943–953. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, P.C.M.; Lam, W.W.; Xu, J.; Knutsson, L.; Stanisz, G.J. Magnetization Transfer Contrast and Chemical Exchange Saturation Transfer MRI. Features and analysis of the field-dependent saturation spectrum. Neuro Image 2018, 168, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.G. The dynamics of water-protein interactions. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 29–53. [Google Scholar] [CrossRef]
- Poblador Rodriguez, E.; Moser, P.; Dymerska, B.; Robinson, S.; Schmitt, B.; van der Kouwe, A.; Gruber, S.; Trattnig, S.; Bogner, W. A comparison of static and dynamic∆ B0 mapping methods for correction of CEST MRI in the presence of temporal B0 field variations. Magn. Reson. Med. 2019, 82, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Stancanello, J.; Terreno, E.; Castelli, D.D.; Cabella, C.; Uggeri, F.; Aime, S. Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast Media Mol. Imaging 2008, 3, 136–149. [Google Scholar] [CrossRef]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; Van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.Z.; Farrar, C.T.; Sorensen, A.G. Correction for artifacts induced by B0 and B1 field inhomogeneities in pH-sensitive chemical exchange saturation transfer (CEST) imaging. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2007, 58, 1207–1215. [Google Scholar] [CrossRef]
- Sui, R.; Chen, L.; Li, Y.; Huang, J.; Chan, K.W.; Xu, X.; van Zijl, P.C.; Xu, J. Whole-brain amide CEST imaging at 3T with a steady-state radial MRI acquisition. Magn. Reson. Med. 2021, 86, 893–906. [Google Scholar] [CrossRef]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and B1 (WASABI)—Application to field-inhomogeneity correction of CEST MRI data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Khlebnikov, V.; Windschuh, J.; Siero, J.C.; Zaiss, M.; Luijten, P.R.; Klomp, D.W.; Hoogduin, H. On the transmit field inhomogeneity correction of relaxation-compensated amide and NOE CEST effects at 7 T. NMR Biomed. 2017, 30, e3687. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.Z.; Benner, T.; Kumar, A.; Sorensen, A.G. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 2008, 60, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.K.; Polders, D.; Hua, J.; Zhu, H.; Hoogduin, H.J.; Zhou, J.; Luijten, P.; van Zijl, P.C. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn. Reson. Med. 2012, 67, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Zaiss, M.; Schmitt, B.; Bachert, P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J. Magn. Reson. 2011, 211, 149–155. [Google Scholar] [CrossRef]
- Zhou, I.Y.; Wang, E.; Cheung, J.S.; Zhang, X.; Fulci, G.; Sun, P.Z. Quantitative chemical exchange saturation transfer (CEST) MRI of glioma using Image Downsampling Expedited Adaptive Least-squares (IDEAL) fitting. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Barker, P.B.; Weiss, R.G.; van Zijl, P.C.; Xu, J. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magn. Reson. Med. 2019, 81, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wei, Z.; Cai, S.; Li, Y.; Liu, G.; Lu, H.; Weiss, R.G.; van Zijl, P.C.; Xu, J. High-resolution creatine mapping of mouse brain at 11.7 T using non-steady-state chemical exchange saturation transfer. NMR Biomed. 2019, 32, e4168. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, H.; Xu, X.; Yadav, N.N.; Cai, S.; Puts, N.A.; Barker, P.B.; Li, T.; Weiss, R.G.; van Zijl, P.C. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. NMR Biomed. 2017, 30, e3834. [Google Scholar] [CrossRef]
- Jin, T.; Wang, P.; Zong, X.; Kim, S.G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear Overhauser effect at 9.4 T. Magn. Reson. Med. 2013, 69, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.Z.; Benner, T.; Copen, W.A.; Sorensen, A.G. Early experience of translating pH-weighted MRI to image human subjects at 3 Tesla. Stroke 2010, 41, S147–S151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Wang, F.; Li, H.; Xu, J.; Gochberg, D.F.; Gore, J.C.; Zu, Z. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR Biomed. 2017, 30, e3716. [Google Scholar] [CrossRef]
- Huang, J.; Lai, J.H.; Tse, K.-H.; Cheng, G.W.; Liu, Y.; Chen, Z.; Han, X.; Chen, L.; Xu, J.; Chan, K.W. Deep neural network based CEST and AREX processing: Application in imaging a model of Alzheimer’s disease at 3 T. Magn. Reson. Med. 2022, 87, 1529–1545. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1-corrected steady-state pulsed CEST-MRI–application to pH-weighted MRI of acute stroke. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Cohen, O.; Huang, S.; McMahon, M.T.; Rosen, M.S.; Farrar, C.T. Rapid and quantitative chemical exchange saturation transfer (CEST) imaging with magnetic resonance fingerprinting (MRF). Magn. Reson. Med. 2018, 80, 2449–2463. [Google Scholar] [CrossRef]
- Kim, B.; Schär, M.; Park, H.; Heo, H.-Y. A deep learning approach for magnetization transfer contrast MR fingerprinting and chemical exchange saturation transfer imaging. Neuro Image 2020, 221, 117165. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Kang, B.; Kim, B.; Schär, M.; Park, H.; Heo, H.Y. Unsupervised learning for magnetization transfer contrast MR fingerprinting: Application to CEST and nuclear Overhauser enhancement imaging. Magn. Reson. Med. 2021, 85, 2040–2054. [Google Scholar] [CrossRef]
- Svolos, P.; Kousi, E.; Kapsalaki, E.; Theodorou, K.; Fezoulidis, I.; Kappas, C.; Tsougos, I. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: A review and future perspectives. Cancer Imaging 2014, 14, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Field, A.S.; Alexander, A.L.; Wu, Y.C.; Hasan, K.M.; Witwer, B.; Badie, B. Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2004, 20, 555–562. [Google Scholar] [CrossRef]
- Dangouloff-Ros, V.; Deroulers, C.; Foissac, F.; Badoual, M.; Shotar, E.; Grévent, D.; Calmon, R.; Pagès, M.; Grill, J.; Dufour, C. Arterial spin labeling to predict brain tumor grading in children: Correlations between histopathologic vascular density and perfusion MR imaging. Radiology 2016, 281, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Warmuth, C.; Gunther, M.; Zimmer, C. Quantification of blood flow in brain tumors: Comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 2003, 228, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Heye, A.K.; Culling, R.D.; Hernández, M.d.C.V.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuro Image Clin. 2014, 6, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Shiroishi, M.S.; Castellazzi, G.; Boxerman, J.L.; D’Amore, F.; Essig, M.; Nguyen, T.B.; Provenzale, J.M.; Enterline, D.S.; Anzalone, N.; Dörfler, A. Principles of T2*-weighted dynamic susceptibility contrast MRI technique in brain tumor imaging. J. Magn. Reson. Imaging 2015, 41, 296–313. [Google Scholar] [CrossRef]
- Law, M.; Yang, S.; Babb, J.S.; Knopp, E.A.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. Am. J. Neuroradiol. 2004, 25, 746–755. [Google Scholar]
- Wainwright, D.A.; Nigam, P.; Thaci, B.; Dey, M.; Lesniak, M.S. Recent developments on immunotherapy for brain cancer. Expert Opin. Emerg. Drugs 2012, 17, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, H.; Morikawa, M.; Iwanaga, S.; Kaminogo, M.; Ochi, M.; Hayashi, K. Differentiation between high-grade glioma and metastatic brain tumor using single-voxel proton MR spectroscopy. Eur. Radiol. 2001, 11, 1784–1791. [Google Scholar] [CrossRef]
- Ott, D.; Hennig, J.; Ernst, T. Human brain tumors: Assessment with in vivo proton MR spectroscopy. Radiology 1993, 186, 745–752. [Google Scholar] [CrossRef]
- Dunet, V.; Pomoni, A.; Hottinger, A.; Nicod-Lalonde, M.; Prior, J.O. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: Systematic review and meta-analysis. Neuro-oncology 2015, 18, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Chao, S.T.; Suh, J.H.; Raja, S.; Lee, S.Y.; Barnett, G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int. J. Cancer 2001, 96, 191–197. [Google Scholar] [CrossRef]
- Herz, K.; Mueller, S.; Perlman, O.; Zaitsev, M.; Knutsson, L.; Sun, P.Z.; Zhou, J.; van Zijl, P.; Heinecke, K.; Schuenke, P. Pulseq-CEST: Towards multi-site multi-vendor compatibility and reproducibility of CEST experiments using an open-source sequence standard. Magn. Reson. Med. 2021, 86, 1845–1858. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Niu, W.; Lai, C.; Ding, Q.; Chen, W.; Liang, S.; Zhou, J.; Wu, D.; Zhang, Y. Improved chemical exchange saturation transfer imaging with real-time frequency drift correction. Magn. Reson. Med. 2019, 81, 2915–2923. [Google Scholar] [CrossRef]
- Mueller, S.; Stirnberg, R.; Akbey, S.; Ehses, P.; Scheffler, K.; Stöcker, T.; Zaiss, M. Whole brain snapshot CEST at 3T using 3D-EPI: Aiming for speed, volume, and homogeneity. Magn. Reson. Med. 2020, 84, 2469–2483. [Google Scholar] [CrossRef]
- Villano, D.; Romdhane, F.; Irrera, P.; Consolino, L.; Anemone, A.; Zaiss, M.; Dastrù, W.; Longo, D.L. A fast multislice sequence for 3D MRI-CEST pH imaging. Magn. Reson. Med. 2021, 85, 1335–1349. [Google Scholar] [CrossRef]
- Zaiss, M.; Ehses, P.; Scheffler, K. Snapshot-CEST: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3D CEST MRI at 9.4 T. NMR Biomed. 2018, 31, e3879. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Chen, Z.; Park, S.-W.; Lai, J.H.C.; Chan, K.W.Y. Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics 2022, 14, 451. https://doi.org/10.3390/pharmaceutics14020451
Huang J, Chen Z, Park S-W, Lai JHC, Chan KWY. Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics. 2022; 14(2):451. https://doi.org/10.3390/pharmaceutics14020451
Chicago/Turabian StyleHuang, Jianpan, Zilin Chen, Se-Weon Park, Joseph H. C. Lai, and Kannie W. Y. Chan. 2022. "Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges" Pharmaceutics 14, no. 2: 451. https://doi.org/10.3390/pharmaceutics14020451
APA StyleHuang, J., Chen, Z., Park, S.-W., Lai, J. H. C., & Chan, K. W. Y. (2022). Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics, 14(2), 451. https://doi.org/10.3390/pharmaceutics14020451







