Multiple Light-Activated Photodynamic Therapy of Tetraphenylethylene Derivative with AIE Characteristics for Hepatocellular Carcinoma via Dual-Organelles Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of 1-(2-Hydroxyethyl)-4-methylpyridinium Bromide (Compound 1)
2.3. 1-[2-Hydroxyethyl]-4-[4-(1,2,2-triphenylvinyl)styryl]pyridinium Bromide (TPE-Py-OH)
2.4. Cellular Uptake and Subcellular Distribution
2.5. Cytotoxicity Studies In Vitro
2.6. Intracellular ROS Detection
2.7. Calcein-AM and Propidium Iodine (PI) Staining Assay
2.8. Flow Cytometry Analysis
2.9. Mitochondrial Membrane Potential (Δψm) Measurement
2.10. Long-Term Cell Tracking and In Vitro PDT
2.11. Animal Model
2.12. In Vivo Multiple Light-Activated PDT
3. Results and Discussions
3.1. Synthesis and Characterization
3.2. Aggregation and Micellization of TPE-Py-OH
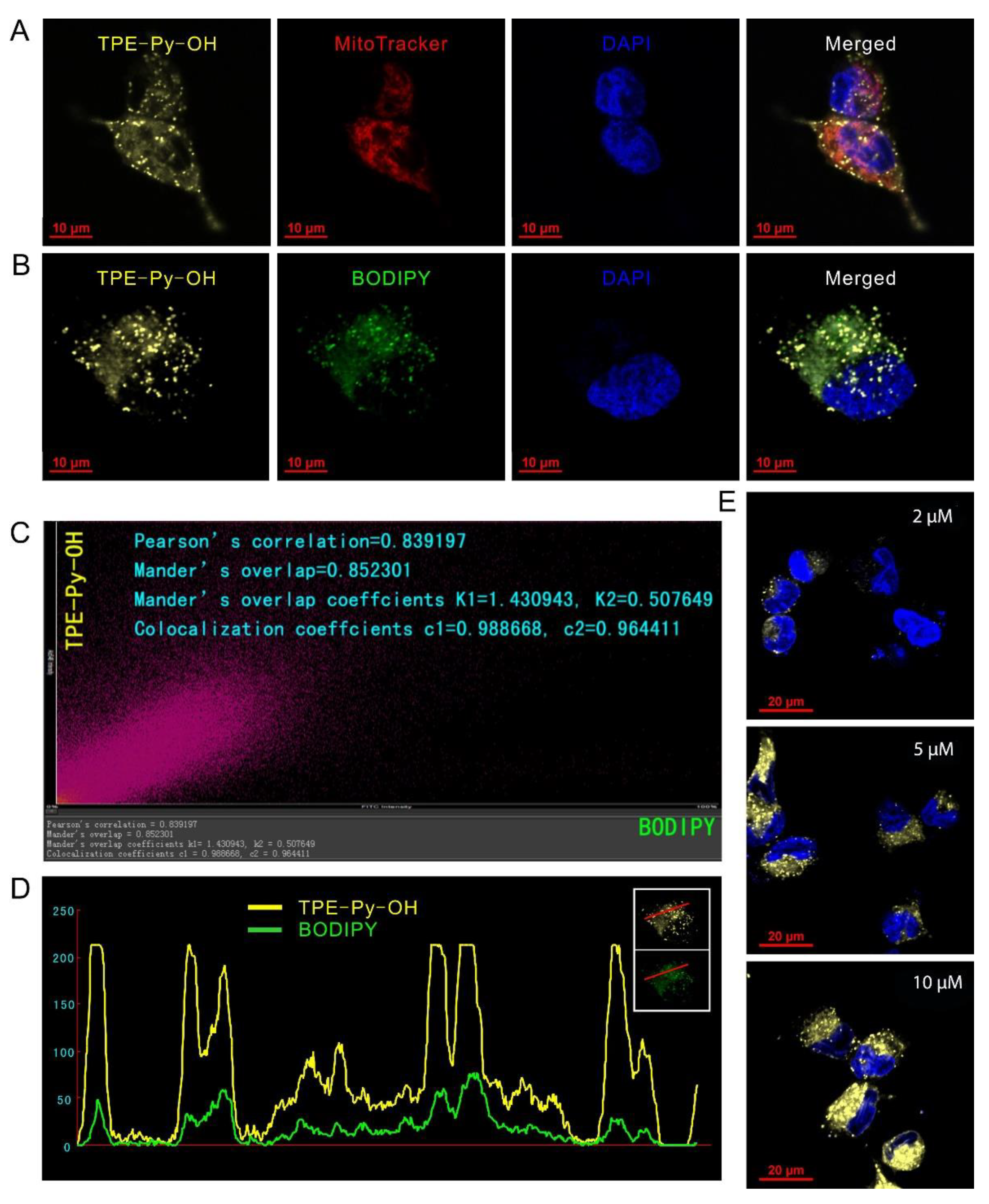
3.3. Dual-Organelles Targeting and Cell Imaging of TPE-Py-OH
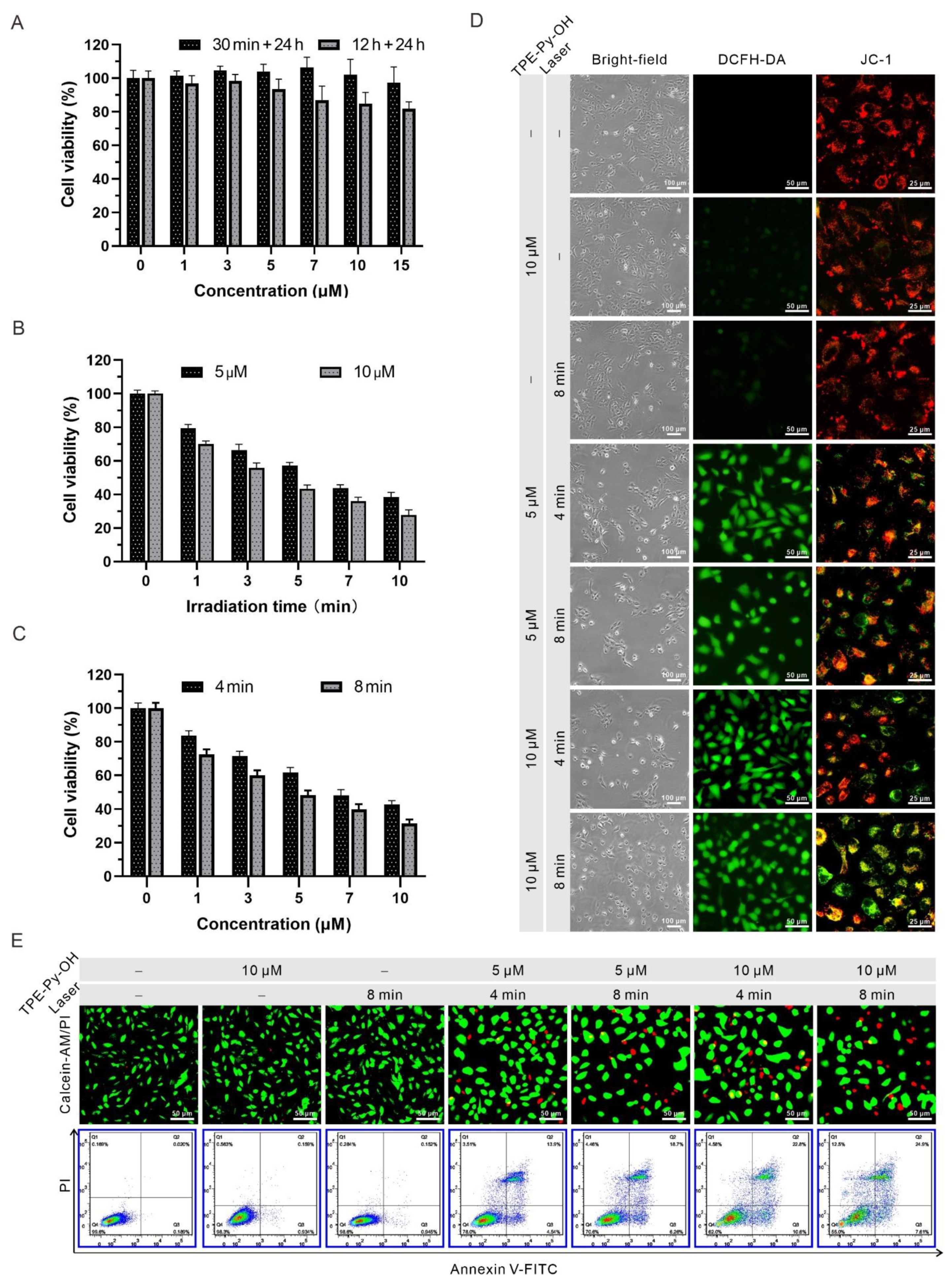
3.4. In Vitro Anti-Cancer Efficacy of TPE-Py-OH
3.5. In Vitro Long-Term Tracking and PDT of TPE-Py-OH
3.6. In Vivo Multiple Light-Activated PDT Effect of TPE-Py-OH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. 4), iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiong, Y.; Sun, J.; Wang, G.; Li, W.; Tang, T.; Li, J. The efficacy of microwave ablation versus liver resection in the treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. Int. J. Surg. 2020, 77, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kudo, M. Image Guidance in Ablation for Hepatocellular Carcinoma: Contrast-Enhanced Ultrasound and Fusion Imaging. Front. Oncol. 2021, 11, 593636. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Morales, O.; Mordon, S.; Delhem, N.; Boleslawski, E. Could Photodynamic Therapy Be a Promising Therapeutic Modality in Hepatocellular Carcinoma Patients? A Critical Review of Experimental and Clinical Studies. Cancers 2021, 13, 5176. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Huang, J.; Song, S.; Chen, H.; Zhang, Z. Cancer-Targeted Nanotheranostics: Recent Advances and Perspectives. Small 2016, 12, 4936–4954. [Google Scholar] [CrossRef]
- Gonzalez-Carmona, M.A.; Bolch, M.; Jansen, C.; Vogt, A.; Sampels, M.; Mohr, R.U.; van Beekum, K.; Mahn, R.; Praktiknjo, M.; Nattermann, J.; et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment. Pharmacol. Ther. 2019, 49, 437–447. [Google Scholar] [CrossRef]
- Song, W.; Kuang, J.; Li, C.X.; Zhang, M.; Zheng, D.; Zeng, X.; Liu, C.; Zhang, X.Z. Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. ACS Nano 2018, 12, 1978–1989. [Google Scholar] [CrossRef]
- Maeding, N.; Verwanger, T.; Krammer, B. Boosting Tumor-Specific Immunity Using PDT. Cancers 2016, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.; Dai, J.; Dong, X.; Wang, Q.; Meng, Z.; Guo, J.; Yu, Y.; Wang, S.; Xia, F.; Zhao, Z.; et al. Improving Image-Guided Surgical and Immunological Tumor Treatment Efficacy by Photothermal and Photodynamic Therapies Based on a Multifunctional NIR AIEgen. Adv. Mater. 2021, 33, e2101158. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, L.; Yi, W.; Fan, W.; Wen, Y.; Miao, X.; Xiong, L. Combination of Fluorescence-Guided Surgery With Photodynamic Therapy for the Treatment of Cancer. Mol. Imaging 2017, 16, 1536012117722911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akopov, A.; Rusanov, A.; Gerasin, A.; Kazakov, N.; Urtenova, M.; Chistyakov, I. Preoperative endobronchial photodynamic therapy improves resectability in initially irresectable (inoperable) locally advanced non small cell lung cancer. Photodiagnosis Photodyn. Ther. 2014, 11, 259–264. [Google Scholar] [CrossRef]
- Jia, R.; Xu, H.; Wang, C.; Su, L.; Jing, J.; Xu, S.; Zhou, Y.; Sun, W.; Song, J.; Chen, X.; et al. NIR-II emissive AIEgen photosensitizers enable ultrasensitive imaging-guided surgery and phototherapy to fully inhibit orthotopic hepatic tumors. J. Nanobiotechnology 2021, 19, 419. [Google Scholar] [CrossRef]
- Feng, Z.; Birong, W.; Zhengfeng, Z.; Siqin, W.; Chuxing, C.; Dang, S.; Min, L. Photodynamic therapy: A next alternative treatment strategy for hepatocellular carcinoma? World J. Gastrointest. Surg. 2021, 13, 1523–1525. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, Q.C.; Xu, S.D.; Yuan, Y.Y.; Cheng, X.; Jiang, S.; Kenry; Yu, Q.H.; Song, Z.F.; Liu, B.; et al. Theranostic Nanodots with Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma. Theranostics 2019, 9, 1264–1279. [Google Scholar] [CrossRef]
- He, W.; Zhang, T.; Bai, H.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Recent Advances in Aggregation-Induced Emission Materials and Their Biomedical and Healthcare Applications. Adv. Healthc. Mater. 2021, e2101055. [Google Scholar] [CrossRef]
- Zhao, N.; Li, P.; Zhuang, J.; Liu, Y.; Xiao, Y.; Qin, R.; Li, N. Aggregation-Induced Emission Luminogens with the Capability of Wide Color Tuning, Mitochondrial and Bacterial Imaging, and Photodynamic Anticancer and Antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 11227–11237. [Google Scholar] [CrossRef]
- Qi, J.; Ou, H.; Liu, Q.; Ding, D. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate 2021, 2, 95–113. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. 2020, 132, 9952–9970. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Aggregation-induced emission probes for cancer theranostics. Drug Discov. Today 2017, 22, 1288–1294. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Yuan, Y.Y.; Wu, Y.Z.; Song, Z.F.; Tang, B.Z.; Liu, B.; Zheng, Q.C. One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano 2017, 11, 3922–3932. [Google Scholar] [CrossRef]
- Wu, W.; Shi, L.; Duan, Y.; Xu, S.; Gao, X.; Zhu, X.; Liu, B. Metabolizable Photosensitizer with Aggregation-Induced Emission for Photodynamic Therapy. Chem. Mater. 2021, 33, 5974–5980. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Wu, M.; Middha, E.; Wu, W.; Daqiqeh Rezaei, S.; Liu, B.; Tan, Y.N. Gold Nanostars-AIE Theranostic Nanodots with Enhanced Fluorescence and Photosensitization Towards Effective Image-Guided Photodynamic Therapy. Nano-Micro Lett. 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, R.; Wang, S.; Xie, Y.; Chen, H.; Huang, R.; Shao, L.; Zhu, Q.; Liu, Y. Highly Efficient Multifunctional Organic Photosensitizer with Aggregation-Induced Emission for In Vivo Bioimaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 54783–54793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J. Am. Chem. Soc. 2014, 136, 11707–11715. [Google Scholar] [CrossRef]
- Feng, G.; Qin, W.; Hu, Q.; Tang, B.Z.; Liu, B. Cellular and Mitochondrial Dual-Targeted Organic Dots with Aggregation-Induced Emission Characteristics for Image-Guided Photodynamic Therapy. Adv. Healthc. Mater. 2015, 4, 2667–2676. [Google Scholar] [CrossRef]
- Chen, S.; Huang, B.; Pei, W.; Wang, L.; Xu, Y.; Niu, C. Mitochondria-Targeting Oxygen-Sufficient Perfluorocarbon Nanoparticles for Imaging-Guided Tumor Phototherapy. Int. J. Nanomed. 2020, 15, 8641–8658. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.L.; Ba, X.X.; Hua, S.Y.; Jiang, P.; Jiang, F.L.; Liu, Y. Multifunction in One Molecule: Mitochondrial Imaging and Photothermal & Photodynamic Cytotoxicity of Fast-Response Near-Infrared Fluorescent Probes with Aggregation-Induced Emission Characteristics. ACS Appl. Mater. Interfaces 2021, 13, 7945–7954. [Google Scholar] [CrossRef]
- Yu, K.; Pan, J.; Husamelden, E.; Zhang, H.; He, Q.; Wei, Y.; Tian, M. Aggregation-induced Emission Based Fluorogens for Mitochondria-targeted Tumor Imaging and Theranostics. Chem. Asian J. 2020, 15, 3942–3960. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, J.F.; Shang, D.; Chai, C.X.; Li, Y.Z.; Sun, H.Y.; Li, Y.Q.; Gao, M.; Li, M. Mitochondria-anchoring and AIE-active photosensitizer for self-monitored cholangiocarcinoma therapy. Mater. Chem. Front. 2020, 4, 3201–3208. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Chen, Y.; Zhao, E.; Hong, Y.; Chen, S.; Lam, J.W.Y.; Chen, Y.; Hou, J.; Tang, B.Z. Amphiphilic Tetraphenylethene-Based Pyridinium Salt for Selective Cell-Membrane Imaging and Room-Light-Induced Special Reactive Oxygen Species Generation. ACS Appl. Mater. Interfaces 2019, 11, 10567–10577. [Google Scholar] [CrossRef]
- Thiam, A.R.; Beller, M. The why, when and how of lipid droplet diversity. J. Cell Sci. 2017, 130, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef]
- Tabero, A.; Garcia-Garrido, F.; Prieto-Castaneda, A.; Palao, E.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Villanueva, A.; de la Moya, S.; Ortiz, M.J. BODIPYs revealing lipid droplets as valuable targets for photodynamic theragnosis. Chem. Commun. 2020, 56, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.; Tang, B.Z. A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Zhao, N.; Li, M.; Yan, Y.; Lam, J.W.Y.; Zhang, Y.L.; Zhao, Y.S.; Wong, K.S.; Tang, B.Z. A tetraphenylethene-substituted pyridinium salt with multiple functionalities: Synthesis, stimuli-responsive emission, optical waveguide and specific mitochondrion imaging. J. Mater. Chem. C 2013, 1, 4640–4646. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, M.; Hong, Y.; Lam, J.W.; Zheng, Q.; Tang, B.Z. Dual-modal MRI contrast agent with aggregation-induced emission characteristic for liver specific imaging with long circulation lifetime. ACS Appl. Mater. Interfaces 2014, 6, 10783–10791. [Google Scholar] [CrossRef]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, R.; Wan, Q.; Hu, R.; Ma, Y.; Wang, Z.; Hou, J.; Zhang, W.; Tang, B.Z. Trojan Horse-Like Nano-AIE Aggregates Based on Homologous Targeting Strategy and Their Photodynamic Therapy in Anticancer Application. Adv. Sci. 2021, e2102561. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Ta, N.; Zhang, T.; Gao, W. The Multifaceted Regulation of Mitochondria in Ferroptosis. Life 2021, 11, 222. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, C.; Zhou, T.; Zhu, J.; Tang, Y.; Xiong, J.; Min, X.; Qin, Q.; Li, M.; Zhao, N.; Wan, C. Multiple Light-Activated Photodynamic Therapy of Tetraphenylethylene Derivative with AIE Characteristics for Hepatocellular Carcinoma via Dual-Organelles Targeting. Pharmaceutics 2022, 14, 459. https://doi.org/10.3390/pharmaceutics14020459
Chai C, Zhou T, Zhu J, Tang Y, Xiong J, Min X, Qin Q, Li M, Zhao N, Wan C. Multiple Light-Activated Photodynamic Therapy of Tetraphenylethylene Derivative with AIE Characteristics for Hepatocellular Carcinoma via Dual-Organelles Targeting. Pharmaceutics. 2022; 14(2):459. https://doi.org/10.3390/pharmaceutics14020459
Chicago/Turabian StyleChai, Chuxing, Tao Zhou, Jianfang Zhu, Yong Tang, Jun Xiong, Xiaobo Min, Qi Qin, Min Li, Na Zhao, and Chidan Wan. 2022. "Multiple Light-Activated Photodynamic Therapy of Tetraphenylethylene Derivative with AIE Characteristics for Hepatocellular Carcinoma via Dual-Organelles Targeting" Pharmaceutics 14, no. 2: 459. https://doi.org/10.3390/pharmaceutics14020459
APA StyleChai, C., Zhou, T., Zhu, J., Tang, Y., Xiong, J., Min, X., Qin, Q., Li, M., Zhao, N., & Wan, C. (2022). Multiple Light-Activated Photodynamic Therapy of Tetraphenylethylene Derivative with AIE Characteristics for Hepatocellular Carcinoma via Dual-Organelles Targeting. Pharmaceutics, 14(2), 459. https://doi.org/10.3390/pharmaceutics14020459






