Abstract
Drug-eluting stents (DESs) are commonly used for the treatment of coronary artery disease. The evolution of the drug-eluting layer on the surface of the metal stent plays an important role in DES functionality. Here, the use of biodegradable polymers has emerged as an attractive strategy because it minimizes the occurrence of late thrombosis after stent implantation. Furthermore, understanding the drug-release behavior of DESs is also important for improving the safety and efficacy of stent treatments. Drug release from biodegradable polymers has attracted extensive research attention because biodegradable polymers with different properties show different drug-release behaviors. Molecular weight, composition, glass transition temperature, crystallinity, and the degradation rate are important properties affecting the behavior of polymers. Sirolimus is a conventional anti-proliferation drug and is the most widely used drug in DESs. Sirolimus-release behavior affects endothelialization and thrombosis formation after DES implantation. In this review, we focus on sirolimus release from biodegradable polymers, including synthetic and natural polymers widely used in the medical field. We hope this review will provide valuable up-to-date information on this subject and contribute to the further development of safe and efficient DESs.
1. Introduction
Percutaneous coronary interaction (PCI) is a minimally invasive non-surgical approach for treating vascular disease. Balloon-expanded bare-material stents (BMSs) are an innovative PCI technology that was developed in the 1980s [1]. BMSs are usually fabricated with metals such as stainless steel, cobalt-chromium, and platinum-chromium. These alloys exhibit properties suitable for supporting the stent shape and durability. However, the placement of BMSs causes endothelial injury and leads to in-stent restenosis [2,3,4]. Accordingly, drug-eluting stents (DESs) were developed as a means to reduce the rate of restenosis [5].
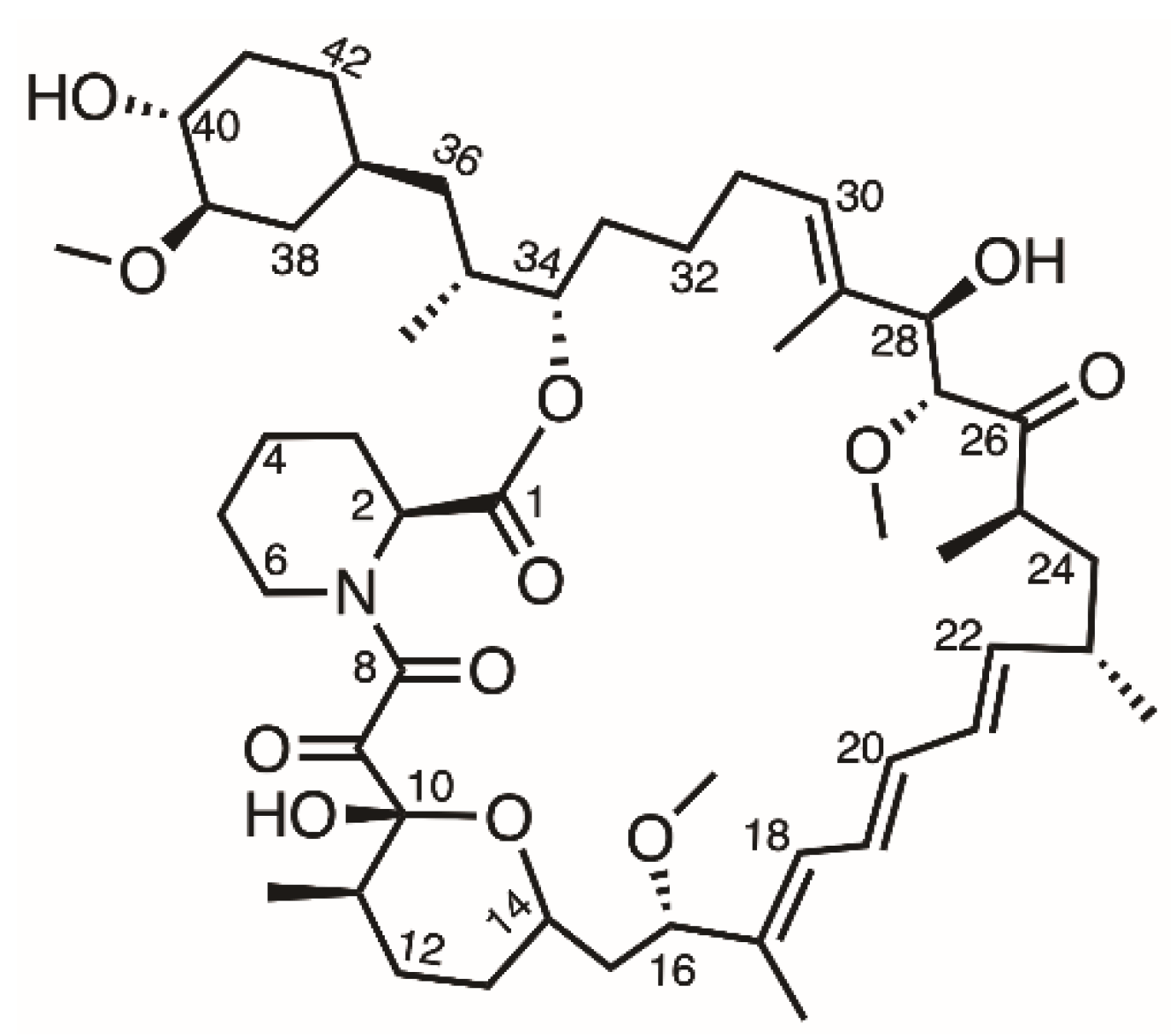
The first-generation DES (Cypher, Cordis Corporation, Hialeah, FL, USA) was composed of a stainless-steel platform and a sirolimus-eluting durable-polymer layer [6]. Sirolimus is an antiproliferative drug that forms a complex with FK binding protein (FKBP-12) that then interacts with mammalian target of rapamycin (mTOR), which is involved in cell growth and proliferation (Figure 1) [7]. Due to the high permeability and poor water solubility (about 2.6 µg/mL) of sirolimus, it is classified as belonging to the BCS (biopharmaceutics classification system) class II drug category [8]. However, compared with other anti-proliferative drugs, sirolimus has better kinetics, a wider therapeutic index, and does not induce cell death even at higher concentrations [9]. Therefore, loading sirolimus onto a DES is a widely used method to inhibit the overgrowth of cells and reduce restenosis after stent implantation.
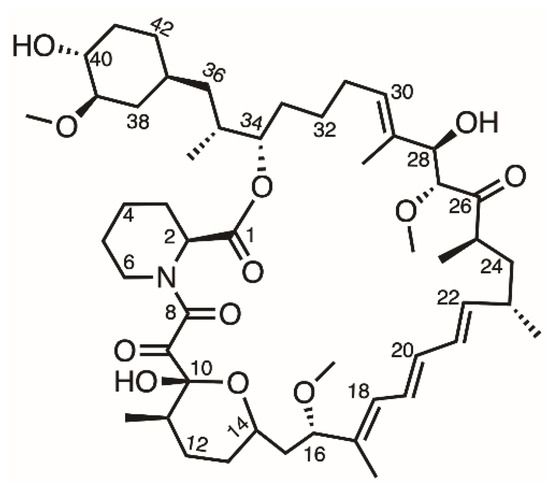
Figure 1.
Structure of sirolimus.
In the same year as the above system, a paclitaxel-eluting stent (Taxus, Boston Scientific, Marlborough, MA, USA) was approved as another first-generation DES. However, several studies showed that paclitaxel-eluting stents present a higher risk of in-stent lumen loss than sirolimus-eluting stents [10]. Therefore, sirolimus became recognized as the more suitable drug for stent treatment.
For long-term DES implantation (one or more years), newly occurring atherosclerotic processes called neoatherosclerosis and very-late in-stent restenosis were reported [11]. In addition to the health conditions of patients, the drug-release behavior and the biocompatibilities of the polymer and strut material of the platform emerged as significant risk factors [12,13]. Therefore, second-generation DESs were developed in 2008. Xience V (Abbott Vascular, Chicago, IL, USA) employed everolimus as the antiproliferative drug [14,15]. The platform material was changed to cobalt-chromium with a strut thickness of 81 µm (that of the Cypher stent was 140 µm), which was found to be more suitable for stent application. It was also reported that these thin-strut DESs resulted in 1.5 times less restenosis than thick-strut DESs [16]. Furthermore, in comparison with stainless steel, cobalt-chromium exhibits better flexibility, mechanical strength, and corrosion resistance.
Both the first- and second-generation DESs have durable polymers as their drug-eluting layers. However, it was reported that the durable polymer evoked a hypersensitivity reaction and thrombus formation after complete drug release [17]. To reduce the risk of thrombosis, patients were obliged to take antiplatelet drugs for a period of time [18]. Therefore, a new generation of DESs based on biodegradable polymers was developed to overcome the long-term risks involved with durable-polymer-coated DESs.
Biodegradable polymeric nanomaterials have been used for controlled drug delivery for many years. Such materials prolong the action of a loaded therapeutic agent and exhibit excellent biocompatibility [19]. Accordingly, a series of randomized trials demonstrated that biodegradable-polymer-coated DESs exhibit higher efficacy and safety than first-generation DESs and are non-inferior to second-generation DESs [20].
Regulating the drug-release behavior of DESs is a key factor to improving their performance. When applied correctly, it can inhibit excess cell growth, which is the main contributing factor to in-stent restenosis, without affecting normal endothelial functions [21]. However, biodegradable polymers with different properties present different drug-release behaviors. Molecular weight, composition, glass transition temperature, crystallinity, hydrophobicity, and the degradation rate are important properties affecting the behavior of polymers [22]. Additionally, the coating process employed, such as conformal coating or abluminal coating, and the solvent used also affect the drug-release behavior of biodegradable polymers.
After the development of second-generation DESs, studies on everolimus (a sirolimus analogue) were performed. However, several large randomized trials showed no significant differences in the rate of stent thrombosis and target lesion revascularization between the sirolimus-eluting stents and everolimus-eluting stents [23,24]. Therefore, the traditional antiproliferative-agent sirolimus is still generally used.
In this review, we summarize biodegradable polymers of poly-lactic acid, poly-D,L-lactic acid, and poly(lactic-co-glycolic acid), which have been used in the DES field over the past 20 years, and focus on the sirolimus release behavior not only from these synthetic polymers but also from natural polymers that are widely used in the medical field. In addition, we review several factors that affect sirolimus release from the polymers, such as the coating process, loading ratio of the drug, release medium, shear stress, pH, temperature, etc.
2. Synthetic Biodegradable Polymers
Biodegradable polymers are widely used in biomedical and pharmaceutical fields. They can be roughly divided into synthetic and natural biodegradable polymers [25]. Synthetic biodegradable polymers are commonly fabricated via condensation polymerization or ring-opening polymerization of monomers. Therefore, it is possible to control their molecular weights and physicochemical features by changing the monomer ratio and the fabrication process [26].
Here, we introduce several synthetic biodegradable polymers derived from lactic acid and glycolic acid that have been approved by the US Food and Drug Administration (FDA) for stent applications, and we discuss the sirolimus-release behavior of these polymers.
2.1. Poly-L-Lactic Acid
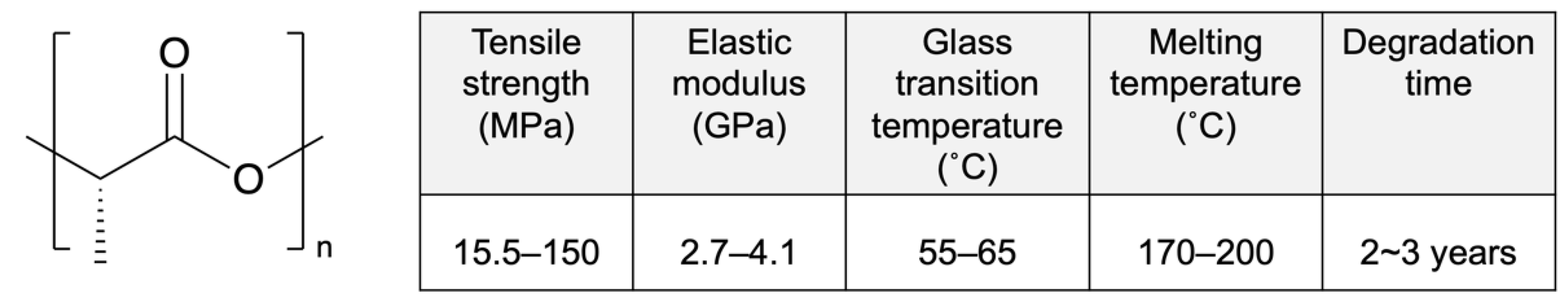
Poly-L-lactic acid (PLLA) is a biodegradable polymer that attracts ongoing research attention because of its excellent biocompatibility, high mechanical strength, and low cost (Figure 2) [27]. It is a semi-crystalline polymer with random and amorphous segments that is known for its high degree of crystallinity. The amorphous segments and molecular weight determine its degradation rate and influence its mechanical properties [28]. High-molecular-weight PLLA is used for clinal applications, especially stents, owing to its excellent mechanical properties [29]. Moreover, unlike low-molecular-weight PLLA (~80 kD), high-molecular-weight PLLA does not actively induce acute or chronic inflammation [30].
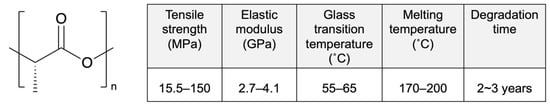
Figure 2.
Structure and properties of poly-L-lactic acid (PLLA). Reprinted with permission from [31], published by Elsevier, 2016.
PLLA is employed as a drug-eluting layer or the platform of DESs approved by Conformité Européenne (CE) Mark and the FDA, as shown in Table 1. The molecular weight significantly affects the performance of DESs, but most information related to molecular weight is confidential. Excel (Jiwei Co. Ltd., Dongguan, China), composed of PLLA with sirolimus as the eluting layer, has shown superior outcomes in realtion to major adverse cardiac event compared with durable-polymer-coated DESs [32]. According to three-year clinical trials, the safety and efficacy of Excel in combination with six-month antiplatelet therapy has been demonstrated, and further evaluations are ongoing. Orisiro (Biotronik, Berlin, Germany) and BioMime (Meril Life Sciences, Gujarat, India) have PLLA and a PLLA co-polymer with poly-glycolic acid, respectively, for drug loading on metallic stents. After releasing the drug completely, the coating polymer degrades relatively quickly, whereas the metal platform remains in the vasculum. According to one-year clinical trials, Orisiro and BioMime present lower thrombosis rates than second-generation DESs [33]. Further trials are ongoing.

Table 1.
DESs with poly-L-lactic acid (PLLA).
In our pervious study, we determined that high-crystallinity PLLA is an inferior drug-carrier because the lack of drug distribution in the crystalline phase leads to a burst drug release [34]. The burst release of the drug impairs the endothelization of DESs and leads to in-stent thrombosis. To decelerate the drug release from the polymer and improve the endothelization of DESs, several coating methods have been investigated. Illner et al. reported the fabrication of PLLA matrices with sirolimus via electrospinning [35]. PLLA was dissolved in a mixed solvent of chloroform and 2,2,2-trifluoroethanol (1/4, v/v). Compared with PLLA films, the use of PLLA fibers inhibited the burst release of sirolimus. It was demonstrated that the lower crystallinity of PLLA fibers led to better drug distribution in the PLLA structure. Thus, optimal drug release can be achieved by controlling the structure of the PLLA layer.
In recent years, PLLA has also been used as a platform for stents, as shown in Table 2. Here, we briefly mention bioabsorbable stents composed of PLLA, termed bioresorbable scaffolds (BRSs). The first-in-man application of a fully biodegradable stent was achieved with the Igaki-Tamai stent (Kyoto Medical Planning Co. Ltd., Kyoto, Japan), which is made of PLLA without drug loading [36]. Even though it showed good short-term results, low primary patency rates at 12 months were indicated by several non-randomized trials [37]. Therefore, the approach of loading the anti-proliferation drug on the stents is still general for stent treatment. The platforms for Absorb BVS (Abbott Vascular, Chicago, IL, USA), Xinsorb (Huaan Biotch., Laiwu, China) and MeRes 100 (Meril Life Sciences, Gujarat, India) are also made of PLLA and are coated with poly-D,L-lactic acid, containing siolimus as the drug-eluting layer [38,39]. However, stents made of PLLA have poorer mechanical properties than those made of metallic materials, and the thicker struts of PLLA DESs lead to a higher incidence of in-stent thrombosis [40]. As a result, the high incidence of in-stent thrombosis associated with Absorb BVS led to its withdrawal from the market [41].

Table 2.
Bioresorbable scaffolds (BRSs) with poly-L-lactic acid (PLLA).
2.2. Poly-D,L-Lactic Acid Used for Biodegradable DESs
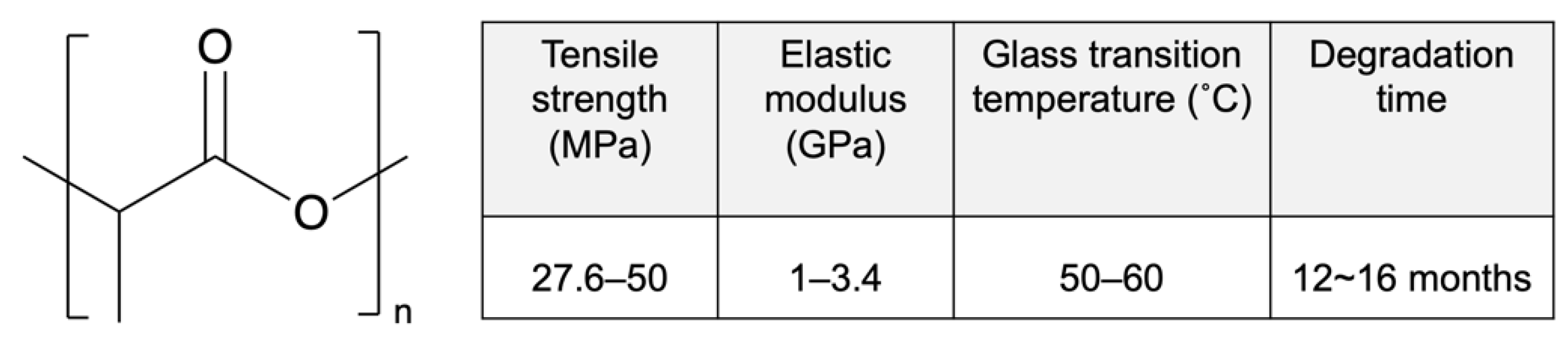
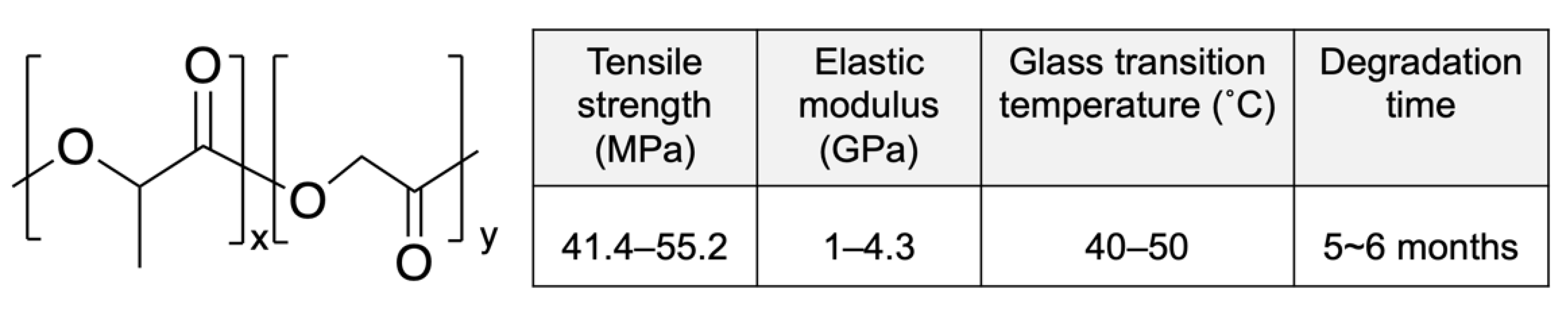
Poly-D,L-lactic acid (PDLLA) is an amorphous polymer consisting of L-lactic acid and D-lactic acid monomers that is widely used in the DES field. PDLLA exhibits lower crystallinity and faster degradation than PLLA (Figure 3), and it is easier to fabricate, with less macroscopic phase separation because of its lower crystalline fraction [42]. In addition, the amorphous structure of PDLLA results in favorable distribution of the drug in the PDLLA structure for a prolonged release.
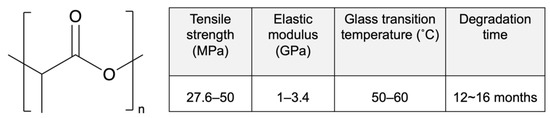
Figure 3.
Structure and properties of poly-D,L-lactic acid (PDLLA). Reprinted with permission from [31], published by Elsevier, 2016.
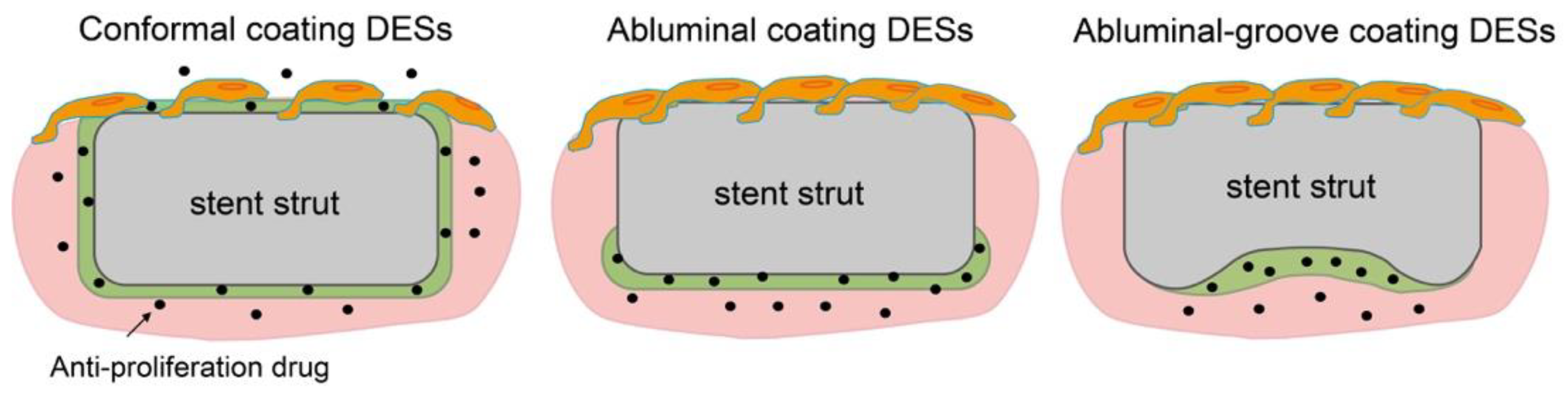
Table 3 shows several kinds of DESs fabricated with PDLLA that have been approved by CE Mark or are currently under evaluation (not limited to sirolimus). Regrettably, as above, the molecular weight information for PDLLA is confidential. Nobori (Terumo, Japan) has PDLLA and biolimus (a sirolimus analogue) coated on the outer surface by means of a novel method called abluminal coating [43]. Here, the drug-eluting layer is formed only on the side that is in contact with the blood vessel, as shown in Figure 4. This technique has been reported to be more suitable for endothelization than conformal coating [44]. Moreover, rapid endothelization also allows for shorter antiplatelet therapy, which results in a reduced risk of major bleeding. According to five-year clinical outcomes, Nobori presents lower risks of cardiac death and stent thrombosis than Orsiro, but further clinical results are pending [45]. Yukon Choice PC (Translumina Gmbh, Hechingen, Germany) is coated with PDLLA and shellac resin for sirolimus loading, and it has a microporous surface [46]. The microporous surface is expected to be better for endothelization. Compared with Cypher and Xience, Yukon Choice PC presents a lower incidence of stent thrombosis according to its five-year clinical outcomes. Firehawk (MicroPort Medical, Amsterdam, Netherlands) features abluminal-groove coating with PDLLA and sirolimus. The grooves on the outer surface of the stent prevent redundant drug loading and allow the targeted release of sirolimus, as shown in Figure 4 [47]. According to clinical outcomes, the safety and efficacy of Firehawk are non-inferior to those of Xience, but long-term clinical assessment remains necessary. Ultimaster (Terumo, Tokyo, Japan) is coated with the co-polymer poly(D,L-lactide-co-caprolactone and sirolimus using a gradient coating method [48]. The gradient coating decreases crack formation on the layer after expansion and reduces redundant drug loading. In our previous studies, we observed that expansion of PDLLA-coated stents causes critical defects, cracking, and desorption of the layer [40,49]. The gradient coating is expected to resolve this problem. Combo (OrbusNeich Medical, Hoevelaken, Netherlands) was designed to promote rapid endothelial formation with two therapeutic coating layers. An anti-restenosis abluminal layer and a pro-healing luminal layer contain sirolimus and anti-CD34+ antibodies, respectively, to promote endothelialization. Compared with Cypher, Combo shows better endothelial cell adhesion and lower rates of neointimal hyperplasia, but further evaluations are ongoing [50]. Thus, in this section, we demonstrated that an abluminal coating reduces redundant drug loading for better endothelization outcomes, commonly seen for new-generation DESs, and that they show lower rates of thrombosis and allow more effective treatment.

Table 3.
DESs with poly-D,L-lactic acid (PDLLA).
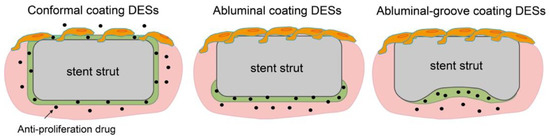
Figure 4.
Schematics of endothelization on conformal coating, abluminal coating, and abluminal-groove coating DESs.
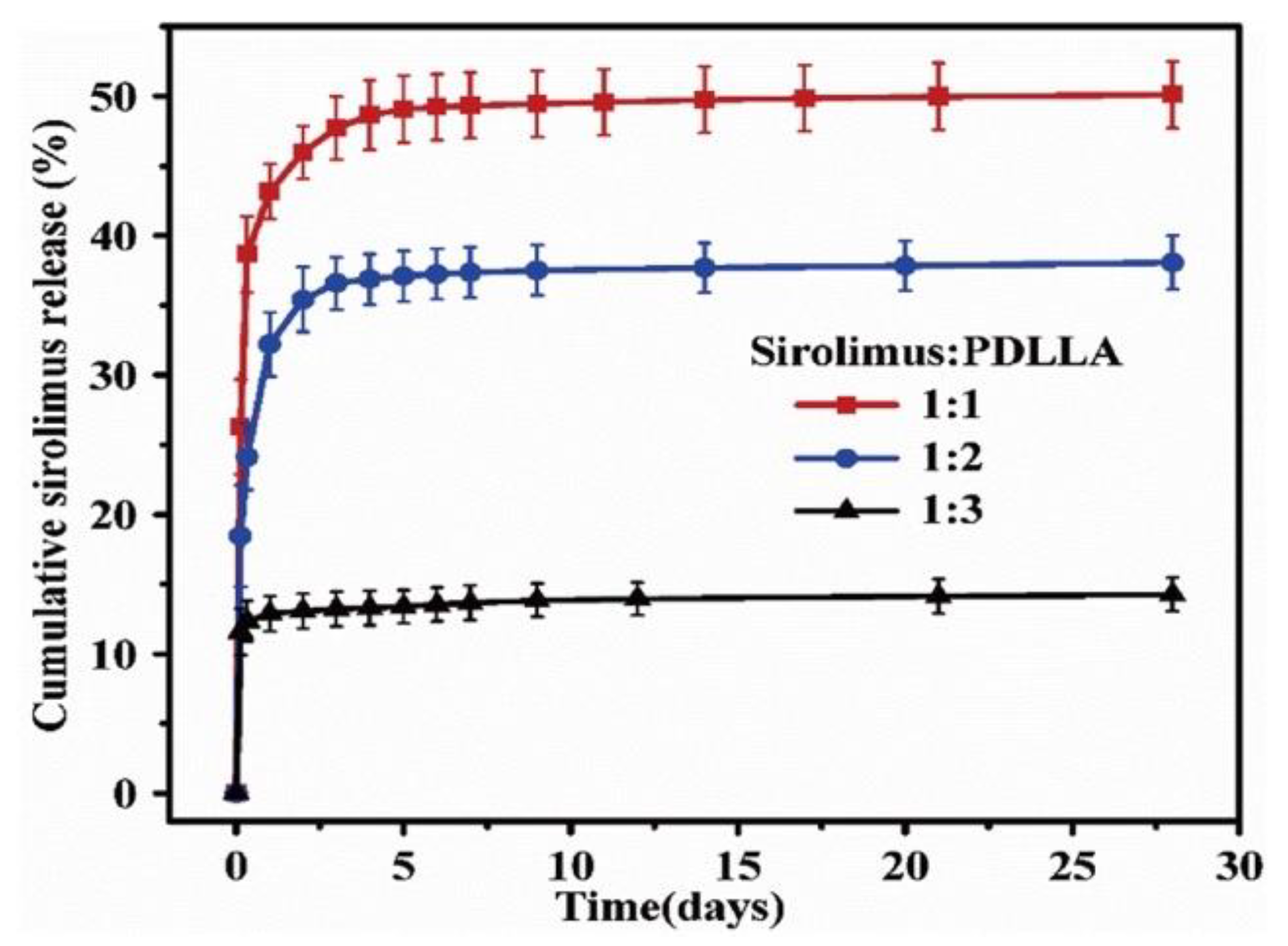
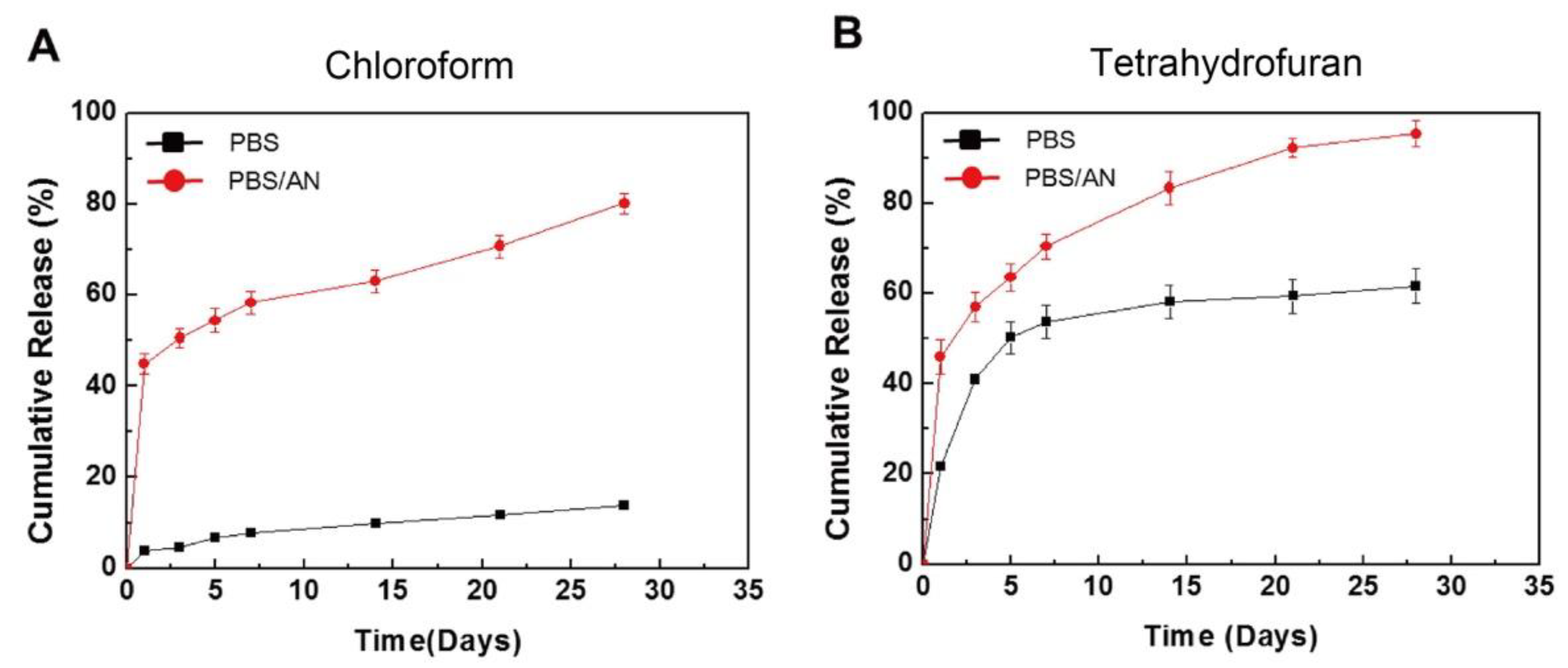
To control the drug release from a polymer, not only the coating method, but also the drug/polymer ratio and organic solvent used are important. For instance, Li et al. reported the effects of various ratios of sirolimus and PDLLA on the release rate [51]. As shown in Figure 5, the sirolimus-release profiles exhibit two phases, i.e., a burst release for 1–3 d, followed by a slower sustained-release period for 28 d. Clearly, increasing the amount of sirolimus in the PDLLA accelerates the burst release. After seven days, all three coatings exhibit extremely slow release, which is due to the slow degradation rate of PDLLA and the diffusion-controlled release mechanism. In addition, Kim et al. studied layers of sirolimus and PDLLA prepared with different solvents, i.e., chloroform and tetrahydrofuran [52]. As shown in Figure 6, in PBS medium, the sirolimus-elution rate for the layer prepared with chloroform is slower than that prepared with tetrahydrofuran. Furthermore, the sirolimus release is accelerated by adding acetonitrile to the PBS medium. This is because sirolimus molecules become aggregated in chloroform, especially at higher concentrations. Therefore, the layer prepared with tetrahydrofuran exhibits better drug dispersion and thus a more controllable drug release.
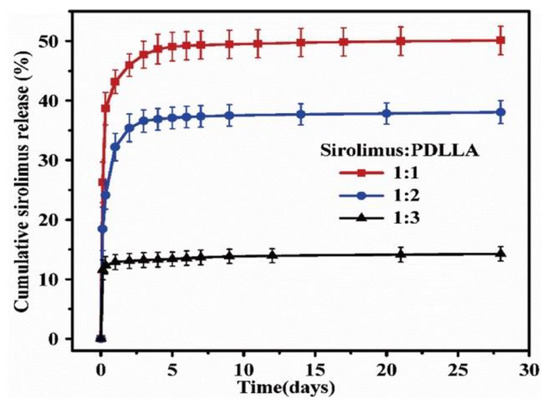
Figure 5.
Cumulative sirolimus release profiles for poly-D,L-lactic acid (PDLLA) coatings with three different drug/polymer ratios in phosphate buffered saline solution at 37 °C. Adapted with permission from [51]; published by Elsevier, 2018.
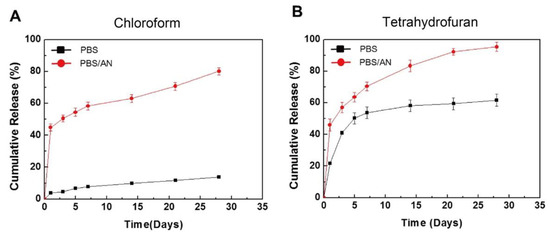
Figure 6.
In vitro cumulative release of sirolimus from poly-D,L-lactic acid (PDLLA) layers in phosphate buffered saline (PBS) or PBS with acetonitrile (AN), prepared using an ultrasonic spray-coating system with (A) chloroform or (B) tetrahydrofuran. Adapted with permission from [52]; published by Elsevier, 2017.
2.3. Poly(lactic-co-glycolic acid)
Poly(lactic-co-glycolic acid) (PLGA), is a co-polymer composed of poly-lactic acid (PLA) and poly-glycolic acid (PGA). The physicochemical properties of PLGA can be controlled by changing the molar ratio of lactic acid and glycolic acid in the polymer chains [53]. When the crystalline glycolic acid is co-polymerized with lactic acid, the crystallinity of PGA is reduced. Therefore, a high content of glycolic acid in PLGA leads to fast degradation. However, as an exception, lactic acid/glycolic acid at a ratio of 50:50 exhibits the fastest degradation [54]. The structure and properties of PLGA (85L:15G) are shown in Figure 7. Compared with PLLA and PDLLA, PLGA has a lower glass transition temperature (Tg) and faster degradation.
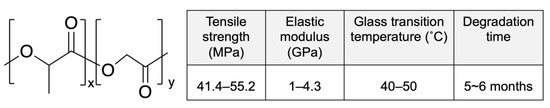
Figure 7.
Structure and properties of poly(lactic-co-glycolic acid) (PLGA) (x-85L:y-15G). Adapted with permission from [31]; published by Elsevier, 2016.
Several kinds of DESs featuring a PLGA layer that have been approved by CE Mark or under ongoing evaluation are shown in Table 4 (not limited to sirolimus). Tivoli (Essen Tech., China) has a conformal coating with PLGA and sirolimus. The safety and efficacy of Tivoli for one year compared with durable-polymer-coated DESs has been confirmed by clinical trials [55]. However, compared with Xinsorb, incidences of late-target lesion failure and thrombosis are higher at 12 months [56]. Synergy (Boston Scientific, USA) consists of a thin-strut platinum-chromium stent platform with a PLGA and everolimus coating. The coating method used the abluminal-rollcoat method to reduce the total polymer burden and eliminate long-term exposure to late thrombosis [57]. According to large one-year real-life-population clinical trials, Synergy appears to be safe and effective, with low rates of restenosis (compared with those for Orisiro, Xience, and Ultimaster) owing to its thin-strut and abluminal coating. However, long-term studies are necessary [58]. Mistent (Micell Technologies, North Carolina, USA) is similar to Tivoli. It is coated with PLGA and crystalline sirolimus using a dry-powder electrostatic coating process. The unique characteristic of Mistent is that the PLGA coating layer degrades within 90 days and the sirolimus is released completely within 45 days. However, the sirolimus is present in the tissue for 270 days, even though the polymer has disappeared [59]. This is due to the unique coating process and the crystalline properties of sirolimus. According to three-year clinical trials, early safety and efficacy have been confirmed when compared with Xinence [60]. In addition, the stent thrombosis risk for Mistent is significantly lower than that for Tivoli at 12 months. BuMa (Sino Medical, Rotterdam, Netherlands) comprises a stent platform with a PLGA and sirolimus coating, and an electro-grafted layer of poly-butyl methacrylate (PBMA) is added between the polymer and the stent platform for resistance to flaking, peeling, and cracking [61]. According to two-year clinical trials, BuMa exhibits superior endothelization and presents a lower incidence of stent thrombosis compared with Excel [62]. Further clinical trials are ongoing. Compared with PLLA and PDLLA, the fast degradation of the PLGA coating is expected to reduce the incidence of very-late thrombosis. However, several studies have demonstrated that the fast degradation of PLGA induces arterial inflammation owing to its acidic products and consequent pH effects [63].

Table 4.
DESs with poly(lactic-co-glycolic acid) (PLGA).
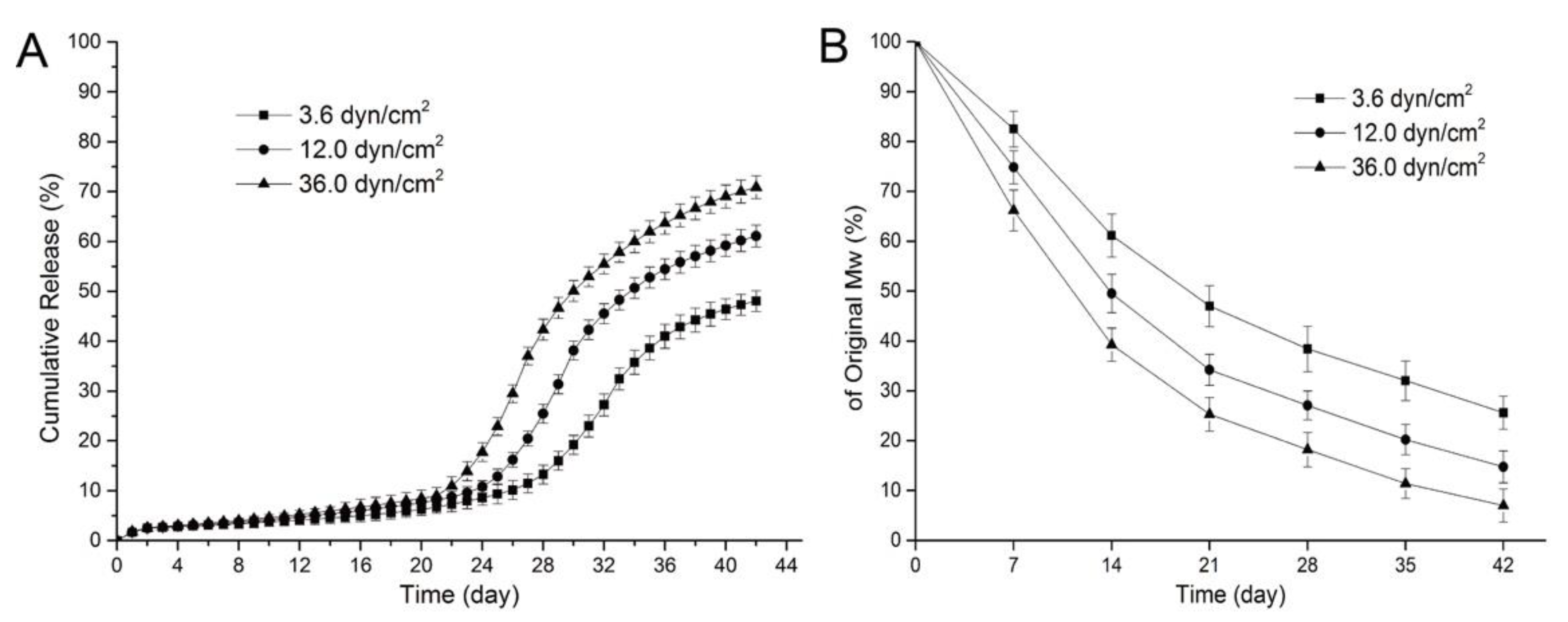
To reduce the inflammation induced by PLGA, the controlled-degradation and burst release of the drug from PLGA were studied. The degradation and drug release for PLGA depend on its physical properties, such as the monomer ratio, crystallinity, and molecular weight [64,65]. Moreover, the influence of environmental factors such as the degradation media, enzymes, and mechanical stress should also be considered. For instance, Zheng et al. investigated the effect of fluid shear stress on the degradation rate and sirolimus release from a PLGA film [66]. As shown in Figure 8A, all the samples showed a slow release of sirolimus for 19 d. After 20 d, an acceleration of the drug release was observed, and higher shear stress caused an earlier and faster sirolimus release from the PLGA film. This is because the higher shear stress leads to faster degradation of PLGA and affects drug diffusion and release (Figure 8B). Moreover, Abbasnezhad et al. investigated the effects of the medium flow rate on the drug release behavior of drug-loaded PLGA (LA/GA 50:50) film [67]. Increasing the flow rate of the medium leads to decreased mechanical stress for the PLGA film and significantly accelerates the burst drug release (~4-fold) from the PLGA film. Therefore, it is not only the intrinsic properties of drug-loaded polymers, but also their mechanical environment that influence the drug-release behavior of DESs [68,69].
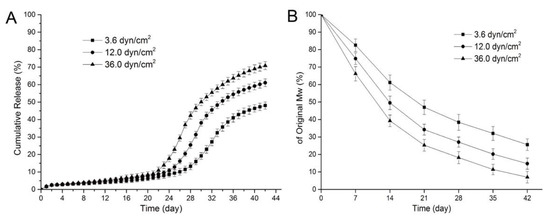
Figure 8.
Release curves for sirolimus from poly(lactic-co-glycolic acid) (PLGA) for various shear stresses (A) and variations in the molecular weight of sirolimus-carrying PLGA films over time under various shear stresses (B). Adapted with permission from [66]; published by MDPI, 2017.
3. Natural Biodegradable Polymers
Compared with synthetic biodegradable polymers, natural biodegradable polymers exhibit higher biocompatibility owing to their having similar macromolecular structures to natural molecules. However, they are sensitive to environmental factors such as temperature, pH, and mechanical stress [70]. Natural biodegradable polymers can be classified as proteins, polysaccharides, or polynucleotides [71]. Here, we focus on the proteins collagen and silk fibroin, which have been studied for biomedical applications because of their unique mechanical strengths, controllable degradation, and stability.
3.1. Collagen
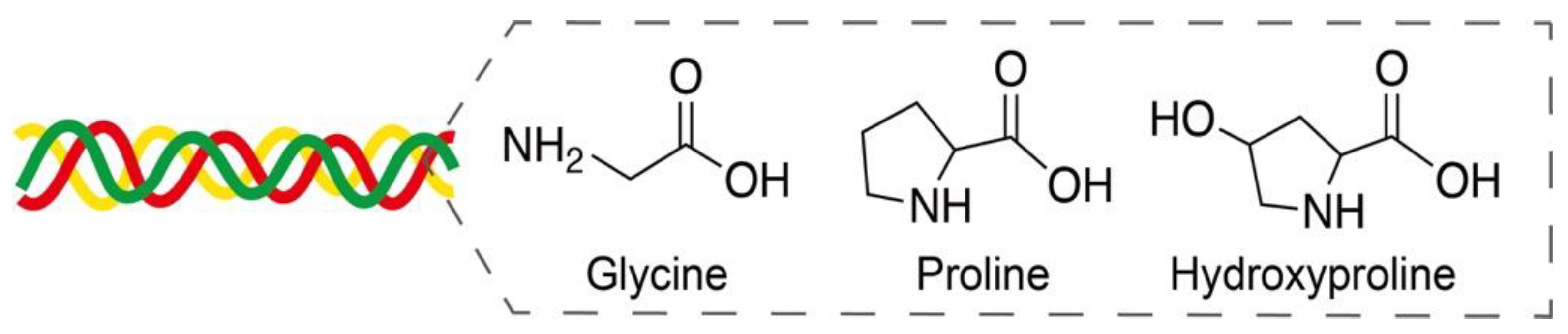
Collagen, as the major component of extracellular matrices, provides mechanical support to connective tissues and is widely used in tissue engineering, wound healing, and bone/nerve regeneration applications [72]. Collagen is mainly composed of glycine, proline, and hydroxyproline in a triplex helix structure (Figure 9) [73]. Nearly 28 types of collagens have been identified, and type-I collagen is the most common in tissues. Owing to its excellent biocompatibility and degradation, collagen layers on the surface of metal stents are expected to provide improved thrombosis prevention and accelerated endothelialization after implantation [74].
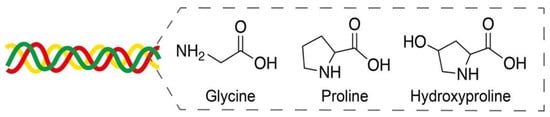
Figure 9.
Amino acids glycine, proline, and hydroxyproline in a triplex helix collagen structure. Adapted with permission from [73]; published by MDPI, 2020.
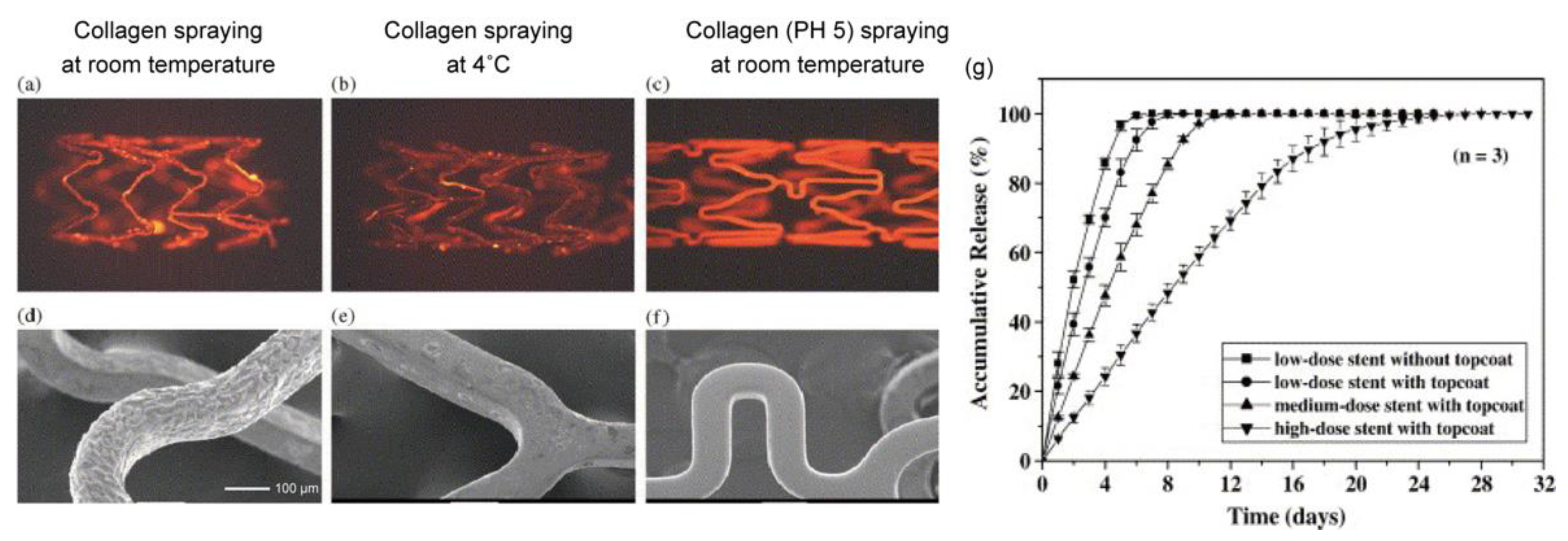
Chen et al. first reported a stainless-steel stent coated with collagen (type-I) and sirolimus that was prepared with a spray method, and the optimal coating conditions were investigated [75]. As shown in Figure 10, collagen at pH 5.0 provided a uniform coating on the stents. Conversely, neutral collagen (pH 7.0) gradually gelled in the air brush and thus interfered with the spraying process, and collagen under low-temperature conditions reduced adhesion on the stents. To slow the sirolimus release from the collagen, an additional topcoat of collagen was applied. The release behavior was determined by the content of sirolimus in the collagen, with a higher dose of sirolimus in the collagen layer exhibiting a significantly slower drug release. Interestingly, this behavior is opposite to the sirolimus release from PDLLA (Figure 5). This is a noteworthy difference between synthetic and natural biodegradable polymers. Based on other studies on the drug release from collagen, we speculated that the interaction between the collagen and the sirolimus results in a slower drug release [76,77]. Although it is possible to control the drug release from collagen, several studies have demonstrated that collagen causes platelet adhesion, activation, and aggregation to induce further thrombosis formation, which is a concern in terms of late restenosis after complete drug release [78]. To address this problem, Yang et al. synthesized a recombinant human type-III collagen containing peptide triplets that provides potent cell adhesion activity and inhibits platelet adhesion [79]. After implanting the recombinant collagen-coated stents in the abdominal aortas of rabbits, the promotion of in-situ endothelialization and the inhibition of neointima hyperplasia were observed in a three-month evaluation in vivo. Since the anti-proliferation drug was not loaded in the collagen coating, it is expected that a highly biocompatible stent without an anti-proliferation drug could exhibit good performance.
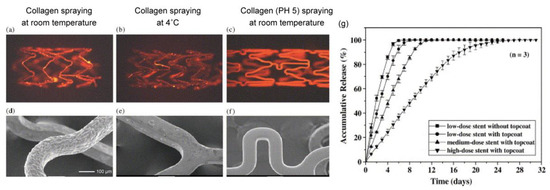
Figure 10.
Fluorescence microscopic images and SEM micrographs of metallic stents spray-coated with collagen using different processes. (a,d) Neutral aqueous collagen spraying at room temperature; (b,e) neutral aqueous collagen spraying at 4 °C; and (c,f) aqueous collagen (pH 5.0) spraying at room temperature. (g) Cumulative release profiles for sirolimus from different types of the sirolimus-loaded stents. Adapted with permission from [75]; published by Elsevier, 2005.
3.2. Silk Fibroin
Among the natural polymers other than collagen used for tissue engineering applications, structural protein silk fibroin has shown great potential. The advantages of using silk for artificial blood vessels are its appropriate mechanical properties, predictable degradation products, and good biocompatibility [80]. Silk fibroin consists of heavy chains (∼390 kDa) and light chains (∼25 kDa) linked by disulfide bonds. The structure of the heavy chain consists of 12 hydrophobic domains, with 11 hydrophilic domains. The heavy chain forms β-sheet structures, which are mainly responsible for the excellent mechanical properties of silk fibroin [81].
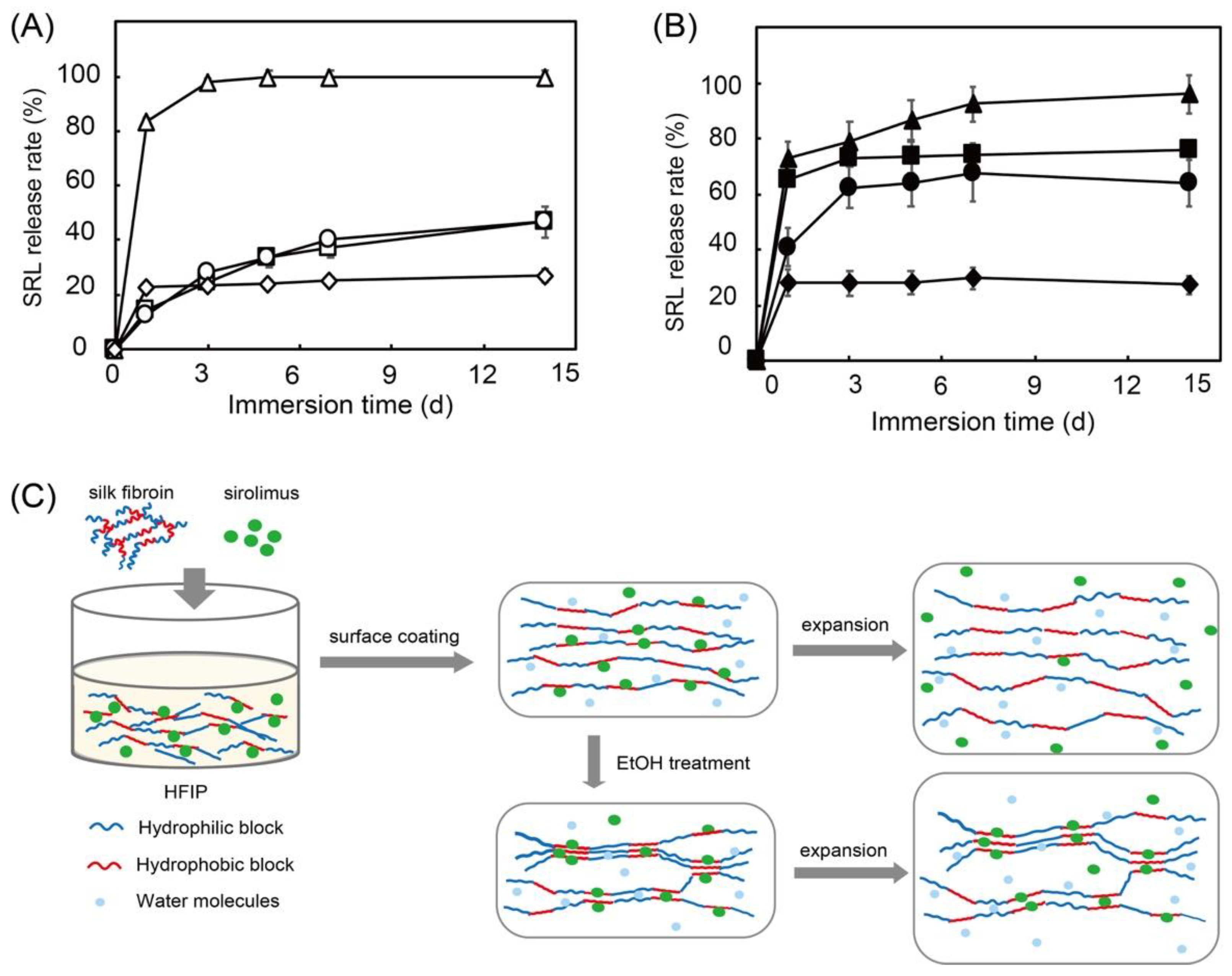
In a previous study, we used sirolimus-loaded silk fibroin as a surface coating for stents and evaluated the sirolimus release with/without balloon expansion [82]. Stents need to be expanded using a balloon catheter for placement in blood vessels. This process causes mechanical stress on the stent and affects DES performance. Moreover, ethanol treatment has been reported to influence the crystallinity of silk fibroin. Therefore, we investigated the sirolimus release with/without ethanol treatment [83]. As shown in Figure 11, without balloon expansion, silk fibroin exhibits a slow release regardless of ethanol treatment. However, with balloon expansion, a burst release of sirolimus is observed, and ethanol treatment of the silk fibroin suppresses the burst release at day 1. This is because ethanol treatment enriches the β-sheet structure and forms crystalline domains in the silk fibroin, which suppresses the burst release.
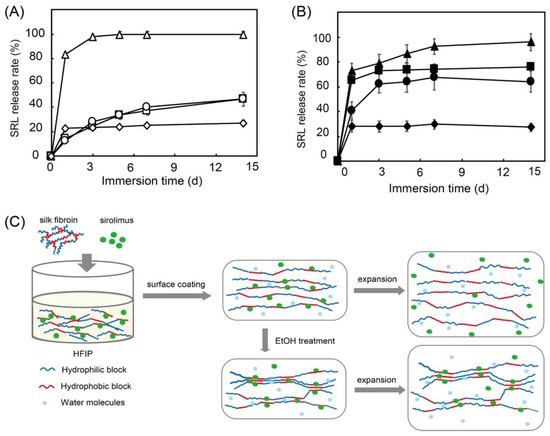
Figure 11.
Sirolimus release over 14 days from silk fibroin (squares), silk fibroin with ethanol treatment (circles), poly-D,L-lactic acid (diamonds), and poly-caprolactone (triangles) on Co-Cr stents before (A) and after (B) balloon expansion in 37 °C phosphate buffered saline. Hypothesized mechanism of sirolimus release from silk-fibroin-coated stents before and after balloon expansion (C). Adapted with permission from [82]; published by American Chemical Society, 2020.
Interestingly, synthetic biodegradable PDLLA and poly-caprolactone show no differences with or without balloon expansion. The acceleration of the sirolimus release from silk fibroin with balloon expansion indicates that plastic deformation of the silk fibroin layer loosens the interaction between silk fibroin and sirolimus, as shown in Figure 11C. A similar previous study by Lee et al. also demonstrated that sirolimus is slowly released from silk fibroin microneedle wraps owing to the interaction between the sirolimus and the silk fibroin [84]. In addition, the adhesion of human umbilical vein endothelial cells and platelets to silk fibroin was evaluated. It shows excellent endothelial cell adhesion and minimal platelet adhesion. Compared with collagen, we confirmed that silk fibroin not only exhibits excellent drug-release behavior, it also shows high biocompatibility and decreased platelet adhesion. This is preferential for cardiovascular applications. However, mechanical stress and environmental conditions, such as the pH and temperature of the medium, which significantly affect the drug-release behavior of silk fibroin, should be considered in relation to its application to DESs [85].
4. Conclusions and Future Prospects
The effectiveness of DES therapy is largely dependent on the drug, coating polymer, and coating method because these factors significantly influence drug-release behavior and the risks of thrombosis and restenosis. Biodegradable polymers are expected to overcome the long-term risks associated with durable polymer-coated DESs. As synthetic biodegradable polymers, PLLA, PDLLA, and PLGA are widely used in DESs owing to their controllable mechanical and chemical properties. However, striking a suitable balance between a long-lasting drug release, fast endothelialization, and suitable degradation remains difficult to attain for biodegradable DESs. The use of natural proteins for DESs is gaining acceptance owing to their excellent biocompatibilities, faster endothelialization, and lower risk of thrombosis. However, environmental conditions such as medium pH and temperature significantly affect the properties of proteins and affect their drug-release behavior. Moreover, bioresorbable metals such as magnesium alloy and zinc alloy with superior mechanical properties are also expected to offer revolutionary alternative scaffolds to traditional DESs. Although optimal DESs capable of efficient treatment are still required, the new generation of DESs has significantly improved the safety and efficacy of stent treatments. In the future, DESs with superior performance are expected.
Author Contributions
Conceptualization: W.X., M.S. and T.N.; Writing-original review: W.X.; Writing and editing review: M.S. and T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This review paper was supported from Financial Support System for English Manuscript Proofreading in Kumamoto University. We thank Jay Freeman from Edanz Group (https://jp.edanz.com/ac, accessed on 3 February 2022) for editing a draft of this manuscript.
Conflicts of Interest
M.S. is a president director of Charlie Lab Inc., the company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. Other authors declare no conflict of interest.
References
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 1987, 19, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Van Beusekom, H.M.M.; Whelan, D.M.; Hofma, S.H.; Krabbendam, S.C.; van Hinsbergh, W.W.M.; Verdouw, P.D.; van der Giessen, W.J. Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J. Am. Coll. Cardiol. 1998, 32, 1109–1117. [Google Scholar] [CrossRef]
- Fuke, S.; Maekawa, K.; Kawamoto, K.; Saito, H.; Sato, T.; Hioka, T.; Ohe, T. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ. J. 2007, 71, 220–225. [Google Scholar] [CrossRef]
- Chen, M.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 2006, 151, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Shlofmitz, E.; Lantorno, M.; Waksman, R. Restenosis of drug-eluting stents, A new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ. Cardiovasc. Interv. 2019, 12, e007023. [Google Scholar] [CrossRef]
- Tada, T.; Byrne, R.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Freixo, C.; Ferreira, V.; Martins, J.; Almeida, R.; Caldeira, D.; Rosa, M.; Costa, J.; Ferreira, J. Efficacy and safety of sirolimus in the treatment of vascular anomalies: A systematic review. J. Vasc. Surg. 2020, 71, 328. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Le, Y. Amorphous nanoparticulate formulation of sirolimus and its tablets. Pharmaceutics 2018, 10, 155. [Google Scholar] [CrossRef]
- Srdanovic, I. Factors influencing 1st and 2nd generation drug-eluting stent performance: Understanding the basic pharmaceutical drug-in-polymer formulation factors contributing to stent thrombosis do we really need to eliminate the polymer? J. Pharm. Pharm. Sci. 2021, 24, 435–461. [Google Scholar] [CrossRef]
- Galløe, A.M.; Kelbæk, H.; Thuesen, L.; Hansen, H.S.; Ravkilde, J.; Hansen, P.R.; Christiansen, E.H.; Abildgaard, U.; Stephansen, G.; Lassen, J.F.; et al. 10-Year clinical outcome after randomization to treatment by sirolimus- or paclitaxel-eluting coronary stents. J. Am. Coll. Cardiol. 2017, 69, 616–624. [Google Scholar] [CrossRef]
- Nakazawa, G.; Otsuka, F.; Nakano, M.; Vorpahl, M.; Yazdani, S.K.; Ladich, E.; Kolodgie, F.D.; Finn, A.V.; Virmani, R. The pathology of neoatherosclerosis in human coronary implants: Bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 2011, 57, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; de Winter, R.J.; Onuma, Y.; Serruys, P.W. The absorb bioresorbable vascular scaffold for the treatment of coronary artery disease. Expert Opin. Drug Deliv. 2016, 13, 1489–1499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kimura, T.; Morimoto, T.; Nakagawa, Y.; Kawai, K.; Miyazaki, S.; Muramatsu, T.; Shiode, N.; Namura, M.; Sone, T.; Oshima, S.; et al. Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation. Circulation 2012, 125, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Midei, M.; Newman, W.; Sanz, M.; Hermiller, J.B.; Williams, J.; Farhat, N.; Mahaffey, K.W.; Cutlip, D.E.; Fitzgerald, P.J.; et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease. JAMA 2008, 299, 1903–1913. [Google Scholar] [CrossRef]
- Von Birgelen, C.; Basalus, M.W.; Tandjung, K.; van Houwelingen, K.G.; Stoel, M.G.; Louwerenburg, J.W.; Linssen, G.C.; Saïd, S.A.; Kleijne, M.A.; Sen, H.; et al. A randomized controlled trial in second-generation zotarolimus-eluting resolute stents versus everolimus-eluting xience v stents in real-world patients: The twente trial. J. Am. Coll. Cardiol. 2012, 59, 1350–1361. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E.R. Stent Thrombogenicity Early in High-Risk Interventional Settings Is Driven by Stent Design and Deployment and Protected by Polymer-Drug Coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef]
- Paramasivam, G.; Devasia, T.; Ubaid, S.; Shetty, A.; Nayak, K.; Pai, U.; Rao, M.S. In-stent restenosis of drug-eluting stents: Clinical presentation and outcomes in a real-world scenario. Egypt Heart J. 2019, 71, 28. [Google Scholar] [CrossRef]
- Savonitto, S.; Caracciolo, M.; Cattaneo, M.; de Servi, S. Management of patients with recently implanted coronary stents on dual antiplatelet therapy who need to undergo major surgery. J. Thromb. Haemost. 2011, 9, 2133–2142. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, J.; Hwang, D.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; Kim, S.Y.; Park, K.-H.; Rha, S.-W.; et al. Durable polymer versus biodegradable polymer drug-eluting stents after percutaneous coronary intervention in patients with acute coronary syndrome. Circulation 2021, 143, 1081–1091. [Google Scholar] [CrossRef]
- Bedair, T.M.; EINaggar, M.A.; Joung, Y.K.; Han, D.K. Recent advances to accelerate re-endothelialization for vascular stents. J. Tissue. Eng. 2017, 8, 2041731417731546. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation behavior of polymers used as coating materials for drug delivery-A basic review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- MacKeigan, J.P.; Krueger, D.A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro-Oncology 2015, 17, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.O.; Thayssen, P.; Christiansen, E.H.; Maeng, M.; Ravkilde, J.; Hansen, K.N.; Hansen, H.S.; Krusell, L.; Kaltoft, A.; Tilsted, H.H.; et al. Safety and efficacy of everolimus-versus sirolimus-eluting stents: 5-year results from SORT OUT IV. J. Am. Coll. Cardiol. 2016, 23, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Seide, G. Biodegradable flame retardants for biodegradable polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef]
- Hawker, C.J.; Wooley, K.L. The Convergence of Synthetic Organic and Polymer Chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Beauson, J.; Schillani, G.; der Schueren, L.V.; Goutianos, S. The effect of processing conditions and polymer crystallinity on the mechanical properties of unidirectional self-reinforced PLA composites. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106668. [Google Scholar] [CrossRef]
- Pauck, R.G.; Reddy, B.D. Computational analysis of the radial mechanical performance of PLLA coronary artery stents. Med. Eng. Phys. 2015, 37, 7–12. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Furst, J.G.; Ellis, S.G.; Tuch, R.J.; Topol, E.J. Sustained local delivery of dexamethasone by a novel intravascular eluting stent to prevent restenosis in the porcine coronary injury model. J. Am. Coll. Cardiol. 1997, 29, 808–816. [Google Scholar] [CrossRef]
- Langer, R.; Anderson, D.G.; Farah, S. Physical and mechanical properties of PLA, and their functions in widespread applications-A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Han, Y.; Jing, Q.; Li, Y.; Yang, L.; Liu, H.; Shang, X.; Jiang, T.; Li, Z.; Zhang, H. Sustained clinical safety and efficacy of a biodegradable-polymer coated sirolimus-eluting stent in “real-world” practice: Three-year outcomes of the CREATE (multi-center registry of Excel biodegradable polymer drug eluting stents) study. Catheter. Cardiovasc. Interv. 2012, 79, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Toklu, B.; Patel, N.; Feit, F.; Stone, G.W. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 2018, 138, 2216–2226. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidome, T. Optimized polymer coating for magnesium alloy-based bioresorbable scaffolds for long-lasting drug release and corrosion resistance. Colloids. Surf. B 2018, 163, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Illner, S.; Kohse, S.; Michaelis, C.; Reske, T.; Eickner, T.; Schmitz, K.; Grabow, N. In vitro study of sirolimus release from nonwoven PLLA matrices. Curr. Direc. Biomed. Eng. 2018, 4, 591–594. [Google Scholar] [CrossRef]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T.; et al. Long-term (>10 years) clinical outcomes of first-in-man biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation 2012, 125, 2343. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, K.; Zen, K.; Yashige, M.; Ito, N.; Kadoya, Y.; Wakana, N.; Yanishi, K.; Matoba, S. Comparative analysis of the paclitaxel-eluting peripheral igaki-tamai stent and the drug-free Igaki-Tamai stent using optical coherence tomography and histological analysis in a porcine iliac artery model. Circ. J. 2020, 84, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, Q.; Wu, Y.; Hu, X.; Xie, J.; Ge, J. Short-term effects of fully bioabsorbable PLLA coronary stents in a porcine model. Polym. Bull. 2012, 68, 1171–1181. [Google Scholar] [CrossRef]
- Kaul, U. Fully Bioresorbable PLA-Based Sirolimus-Eluting MeRes 100 Scaffold (Meril Life Science); Asia PCR: Singapore, 2016; Available online: https://media.pcronline.com/diapos/AsiaPCR2016/45-20160121_1741_Room_336_Kaul_Upendra_1111_(2000)/Kaul_Upendra_20160121_1705_Room_336.pdf (accessed on 21 January 2016).
- Ang, H.Y.; Huang, Y.Y.; Lim, S.T.; Wong, P.; Joner, M.; Foin, N. Mechanical behavior of polymer-based vs. metallic-based bioresorbable stents. J. Tborac. Dis. 2017, 9, 923–934. [Google Scholar] [CrossRef]
- Kharlamov, A.N. Undiscovered pathology of transient scaffolding remains a driver of failures in clinical trials. World J. Cardiol. 2018, 10, 165–186. [Google Scholar] [CrossRef]
- Valenti, S.; Diaz, A.; Romanini, M.; del Valle, L.J.; Puiggali, J.; Tamarit, J.L.; Macovez, R. Amorphous binary dispersions of chloramphenicol in enantiomeric pure and racemic poly-lactic acid: Morphology, molecular relaxations, and controlled drug release. Int. J. Pharm. 2019, 568, 118565. [Google Scholar] [CrossRef]
- Kadota, K.; Muramatsu, T.; Iwabuchi, M.; Saito, S.; Hayashi, Y.; Ikari, Y.; Nanto, S.; Fujii, K.; Inoue, N.; Namiki, A.; et al. Randomized comparison of the nobori biolimus A9-eluting stent with the sirolimus-eluting stent in patients with stenosis in native coronary arteries. Catheter. Cardiovasc. Interv. 2012, 80, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Sakata, K.; Yokawa, J.; Nakanishi, C.; Murai, K.; Okada, H.; Shimojima, M.; Yoshida, S.; Yoshioka, K.; Takuwa, Y.; et al. Everolimus-eluting biodegradable abluminal coating stent versus durable conformal coating stent: Termination of the inflammatory response associated with neointimal healing in a porcine coronary model. J. Interven. Cardiol. 2020, 2020, 1956015. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Christiansen, E.; Raungaard, B.; Junker, A.; Christensen, M.K.; Kahlert, J.; Jakobsen, L.; Freeman, P.; Hansen, K.; Terkelsen, C.; et al. TCT-278 Five-Year Outcomes After Revascularization with the Biodegradable Polymer Ultrathin Strut Sirolimus-Eluting Orsiro Stent or the Biodegradable Polymer Biolimus-Eluting Nobori Stent in Patients with and Without Acute Coronary Syndromes: From the SORT OUT VII Trial. J. Am. Coll. Cardiol. 2021, 78, B114. [Google Scholar]
- Verma, B.; Patel, A.; Katyal, D.; Singh, V.R.; Singh, A.K.; Singh, A.; Kumar, M.; Nagarkoti, P. Real world experience of a biodegradable polymer sirolimus-eluting stent (Yukon choice PC elite) in patients with acute ST-segment elevation myocardial infarction undergoing primary angioplasty: A multicentric observational study (The elite India study). Open Access Maced. J. Med. Sci. 2019, 7, 1103–1109. [Google Scholar] [CrossRef]
- Li, C.; Guan, C.; Zhang, R.; Yang, Y.; Ma, C.; Li, H.; Chen, S.; Han, Y.; Xu, B.; Gao, R. Safety and efficacy of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent for the treatment of de novo coronary lesions: Final five-year results of the patient-level pooled analysis from the TARGET I and TARGET II trials. Cardiac. Cardiovascular. Interv. 2019, 93, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, B.; Smits, P.C.; Carrié, D.; Mehilli, J.; Van Boven, A.J.; Regar, E.; Sawaya, F.J.; Chamié, D.; Kraaijeveld, A.O.; Hovasse, T.; et al. Serial assessment of strut coverage of biodegradable polymer drug-eluting stent at 1, 2, and 3 months after stent implantation by optical frequency domain imaging. Circulation 2017, 10, e004801. [Google Scholar] [CrossRef]
- Xu, W.; Sato, K.; Koga, Y.; Sasaki, M.; Niidome, T. Corrosion resistance of HF-treated Mg alloy stent following balloon expansion and its improvement through biodegradable polymer coating. J. Coat. Technol. Res. 2020, 17, 1023–1032. [Google Scholar] [CrossRef]
- Colombo, A.; Chandrasekhar, J.; Aquino, M.; Ong, T.K.; Sartori, S.; Baber, U.; Lee, M.; Iniguez, A.; Hajek, P.; Borisov, B.; et al. Safety and efficacy of the COMBO bio-engineered stent in an all-comer PCI cohort: 1-Year final clinical outcomes from the MASCOT post-marketing registry. Int. J. Cardiol. 2019, 283, 67–72. [Google Scholar] [CrossRef]
- Li, F.; Gu, Y.; Hua, R.; Ni, Z.; Zhao, G. In vitro release study of sirolimus from a PDLLA matrix on a bioresorbable drug-eluting stent. J. Drug Deliv. Sci. Technol. 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, S.B.; Bedair, T.M.; Kim, M.H.; Park, B.J.; Joung, Y.K.; Han, D.K. The effect of solvents and hydrophilic additice on stable coating and controllable sirolimus release system for drug-eluting stent. Mater. Sci. Eng. C 2017, 78, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Han, Y.; Xu, B.; Jing, Q.; Lu, S.; Yang, L.; Xu, K.; Li, Y.; Li, J.; Guan, C.; Kirtane, A.J.; et al. A randomized comparison of novel biodegradable polymer- and durable polymer-coated cobalt-chromium sirolimus-eluting stents. J. Am. Coll. Cardiol. Intv. 2014, 7, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, L.; Yin, J.; Chen, J.; Qian, J.; Ge, L.; Ge, J. Twelve-month angiographic and clinical outcomes of the Xinsorb bioresorbable sirolimus-eluting scaffold and a metallic stent in patients with coronary artery disease. Int. J. Cardiol. 2019, 293, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Dubois, C. A novel platinum chromium everolimus-eluting stent for the treatment of coronary artery disease. Biologics 2013, 7, 149–159. [Google Scholar] [CrossRef]
- Sarno, G.; Lagerqvist, B.; Olivecrona, G.; Varenhorst, C.; Danielewicz, M.; Hambraeus, K.; Lindholm, D.; Ramunddal, T.; Witt, N.; James, S. Real-life clinical outcomes with everolimus eluting platinum chromium stent with an abluminal biodegradable polymer in patients from the Swedish coronary angiography and angioplasty registry (SCAAR). Catheter. Cardiovasc. Interv. 2017, 90, 881–887. [Google Scholar] [CrossRef]
- Tzafriri, A.R.; Garcia-Polite, F.; Li, X.; Keating, J.; Balaguer, J.M.; Zani, B.; Bailey, L.; Markham, P.; Kiorpes, T.C.; Carlyle, W.; et al. Defining drug and target protein distributions after stent-based drug release: Durable versus deployable coatings. J. Control. Rel. 2018, 274, 102–108. [Google Scholar] [CrossRef]
- Takahashi, K.; Serruys, P.W.; Kogame, N.; Buszman, P.; Lurz, P.; Jessurun, G.A.; Koch, K.T.; Troquay, R.P.; Hamer, B.; Ophuis, T.O.; et al. Final 3-year outcomes of MiStent biodegradable polymer crystalline sirolimus-eluting stent versus Xience permanent polymer everolimus-eluting stent. Circ. Cardiovasc. Interv. 2020, 13, e008737. [Google Scholar] [CrossRef]
- Xu, B.; Gao, R.; Yang, Y.; Cao, X.; Qin, L.; Li, Y.; Li, Z.; Li, X.; Lin, H.; Guo, Y.; et al. Biodegradable polymer-based sirolimus-eluting stents with differing elution and absorption kinetics. J. Am. Coll. Cardiol. 2016, 67, 2249–2258. [Google Scholar] [CrossRef]
- Jia, S.; Guan, C.; Yuan, J.; Cao, X.; Qin, L.; Li, Y.; Li, Z.; Nie, S.; Hou, S.; Zhang, M.; et al. Two-year safety evaluation of a biodegradable polymer sirolimus-eluting stent with increased drug elution and polymer absorption kinetics in complex patient and lesion cohort. Catheter. Cardiovasc. Interv. 2020, 95, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, D.; Mathisen, T.; Wistrand, A.F. Biocompatibility of resorbable polymers: A historical perspective and framework for the future. Biomacromolecules 2019, 20, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Braatz, R.D. A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion. J. Biomed. Mater. Res. A 2014, 103, 2269–2279. [Google Scholar] [CrossRef]
- Zheng, Q.; Chu, Z.; Li, X.; Kang, H.; Yang, X.; Fan, Y. The effect of fluid shear stress on the in vitro release kinetics of sirolimus from PLGA films. Polymers 2017, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. On the importance physical and mechanical properties of PLGA films during drug release. J. Drug Deliv. Sci. Technol. 2021, 63, 102446. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Kebdani, M.; Shirinbayan, M.; Champmartin, S.; Tcharkhtchi, A.; Kouidri, S.; Bakir, F. Development of a model based on physical mechanisms for the explanation of drug release: Application to diclofenac release from polyurethane films. Polymers 2021, 13, 1230. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 12, 1105. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein-polysaccharide composite materials: Fabrication and applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yan, J.; Qiu, F.; Song, X.; Fu, G.; Ji, J. Heparin/collagen multilayer as a thromboresistant and endothelial favorable coating for intravascular stent. J. Biomed. Mater. Res. A 2011, 96, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Liang, H.F.; Chiu, Y.L.; Chang, Y.; Wei, H.J.; Sung, H.W. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J. Control. Rel. 2005, 108, 178–189. [Google Scholar] [CrossRef]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef]
- An, B.; Lin, Y.S.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef]
- Jung, S.H.; Han, J.H.; Park, H.S.; Lee, J.J.; Yang, S.Y.; Kim, Y.H.; Heo, K.S.; Myung, C.S. Inhibition of collagen-induced platelet aggregation by the Secobutanolide Secolincomolide A from Lindera obtusiloba Blume. Front Pharmacol. 2017, 8, 560. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)—Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the structure of Bombyx mori silk fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar] [CrossRef]
- Asakura, T.; Tanaka, T.; Tanaka, R. Advanced silk fibroin biomaterials and application to small-diameter silk vascular grafts. ACS Biomater. Sci. Eng. 2019, 5, 5561–5577. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Asakura, T.; Sasaki, M.; Niidome, T. Silk fibroin as a coating polymer for sirolimus-eluting magnesium alloy stents. ACS Appl. Bio. Mater. 2020, 3, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Yokoyama, Y.; Hattori, S.; Kobayashi, H.; Tamada, Y. The outermost surface properties of silk fibroin films reflect ethanol-treatment conditions used in biomaterials preparation. Mater. Sci. Eng. C 2016, 58, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.H.; Kang, Y.; Park, S.; Lee, K.J.; Kim, J.H.; Youn, Y.N.; Ryu, W.H. Highly flexible and porous silk fibroin microneedle wraps for perivascular drug delivery. J. Control. Rel. 2021, 340, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Canup, B.S.B.; Tong, X.; Dai, F.; Xiao, B. Multi-responsive silk fibroin-based nanoparticles for drug delivery. Front Chem. 2020, 8, 585077. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).