The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Differentiation of Epithelial Cells
2.4. USMB Treatment of Epithelial Barriers
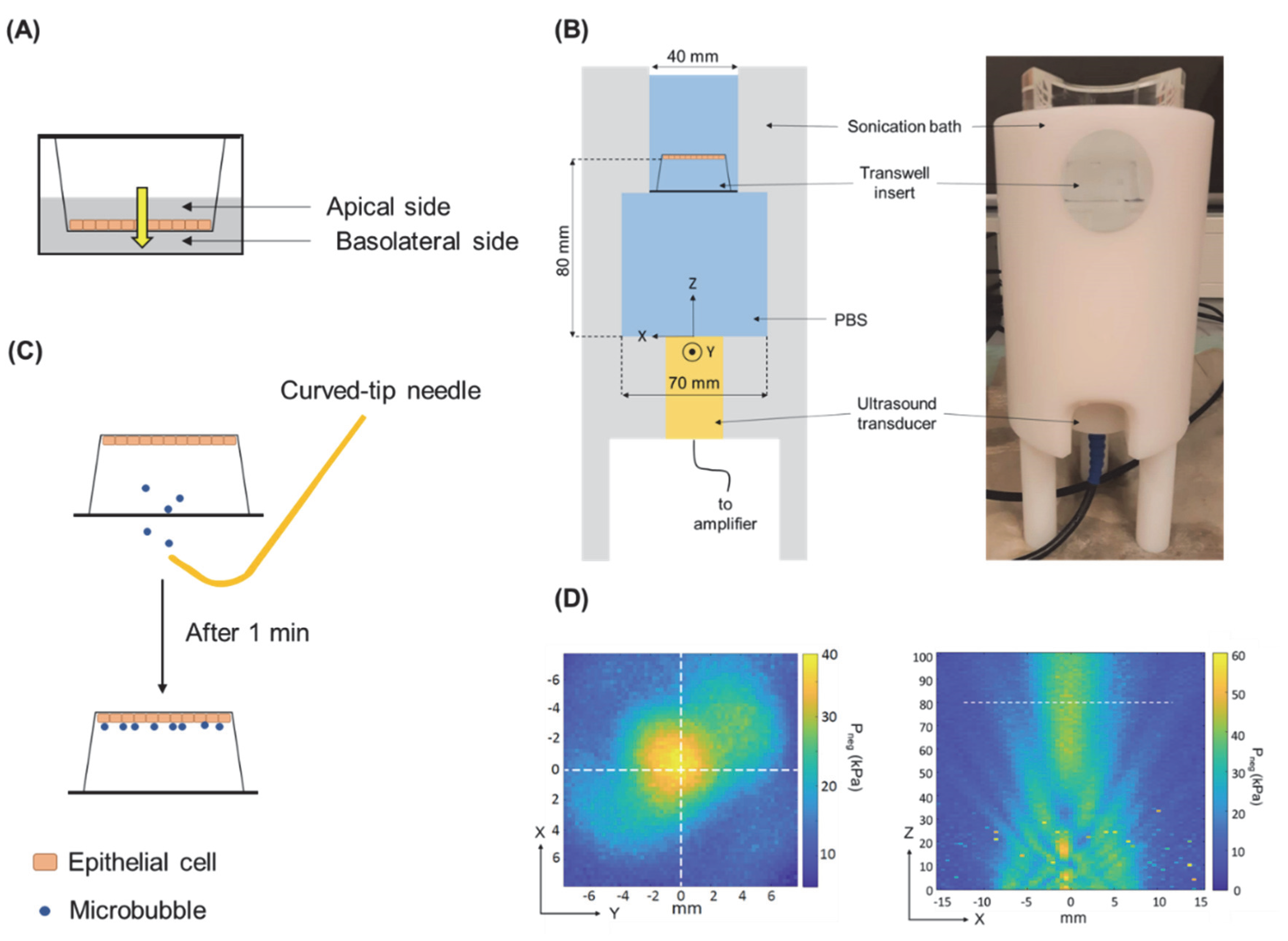
2.5. Permeability Experiments with an Epithelial Barrier and Model Drugs
2.6. Intracellular Accumulation Study
2.7. USMB-Induced Permeability of Anti-CXCR4 Nanobody across an Epithelial Barrier
2.8. Statistical Analysis
3. Results
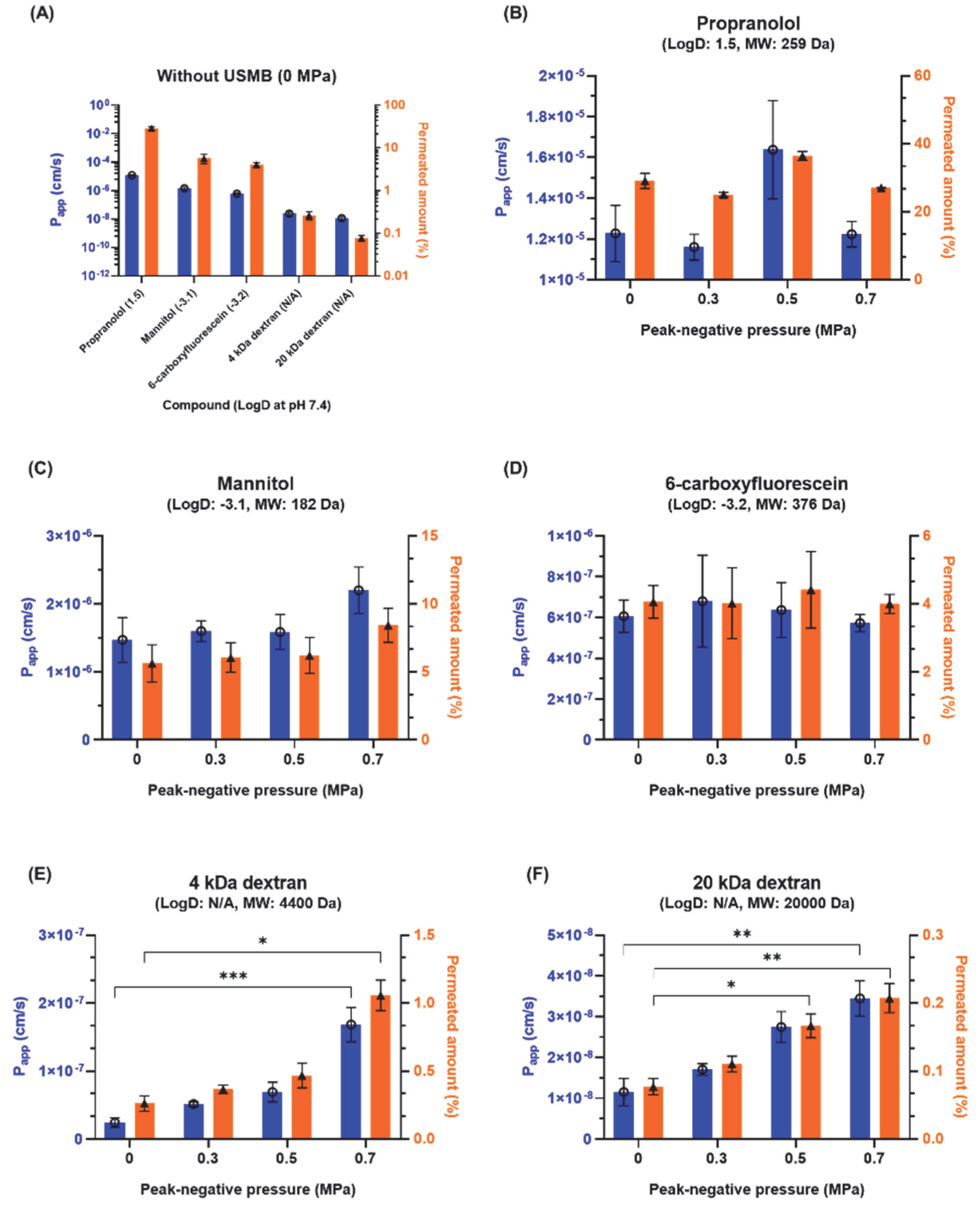
3.1. Effect of USMB on Molecular Permeability across an Epithelial Barrier
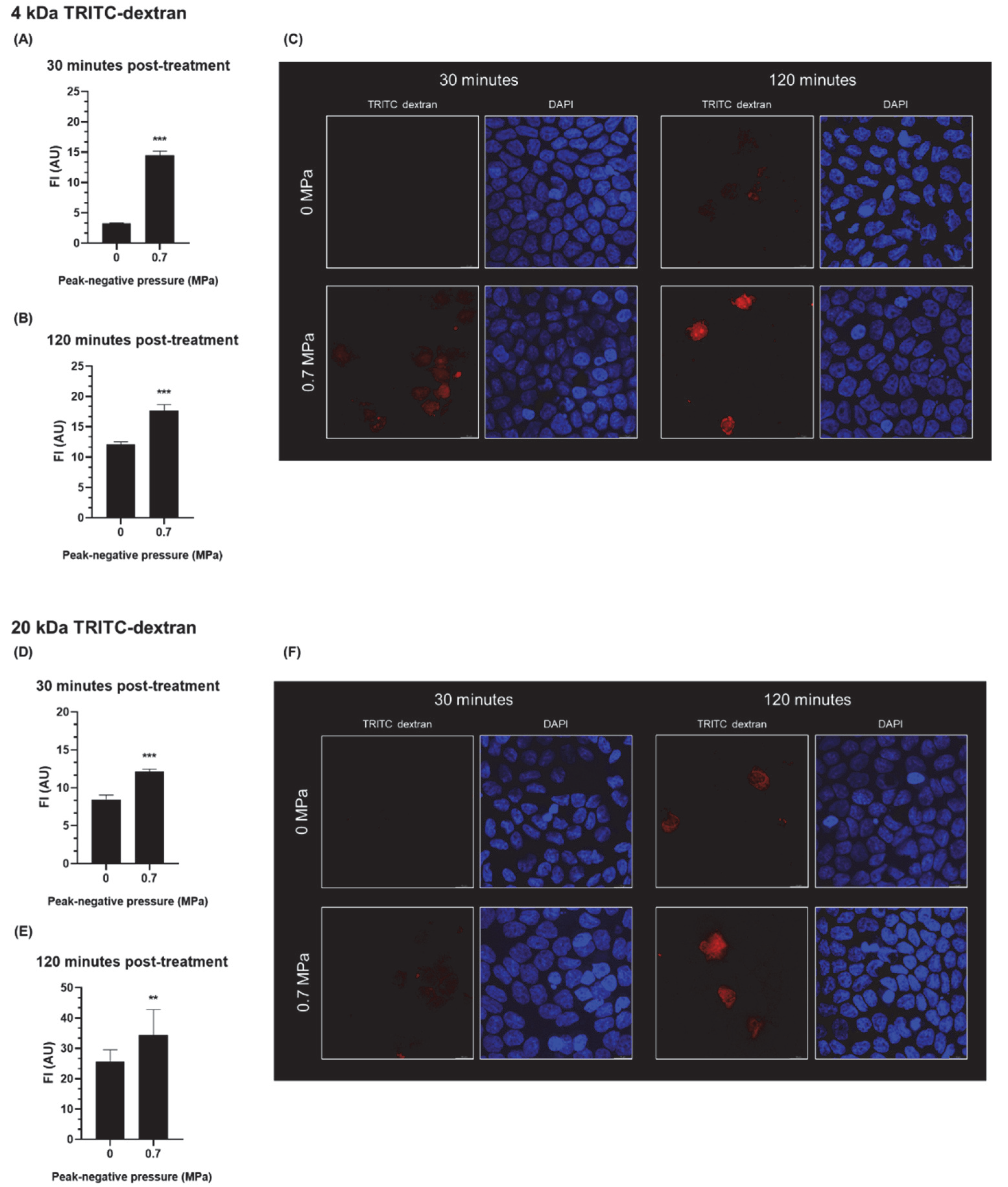
3.2. The Effect of USMB on the Intracellular Accumulation of Fluorescent Dextrans in Epithelial Cells
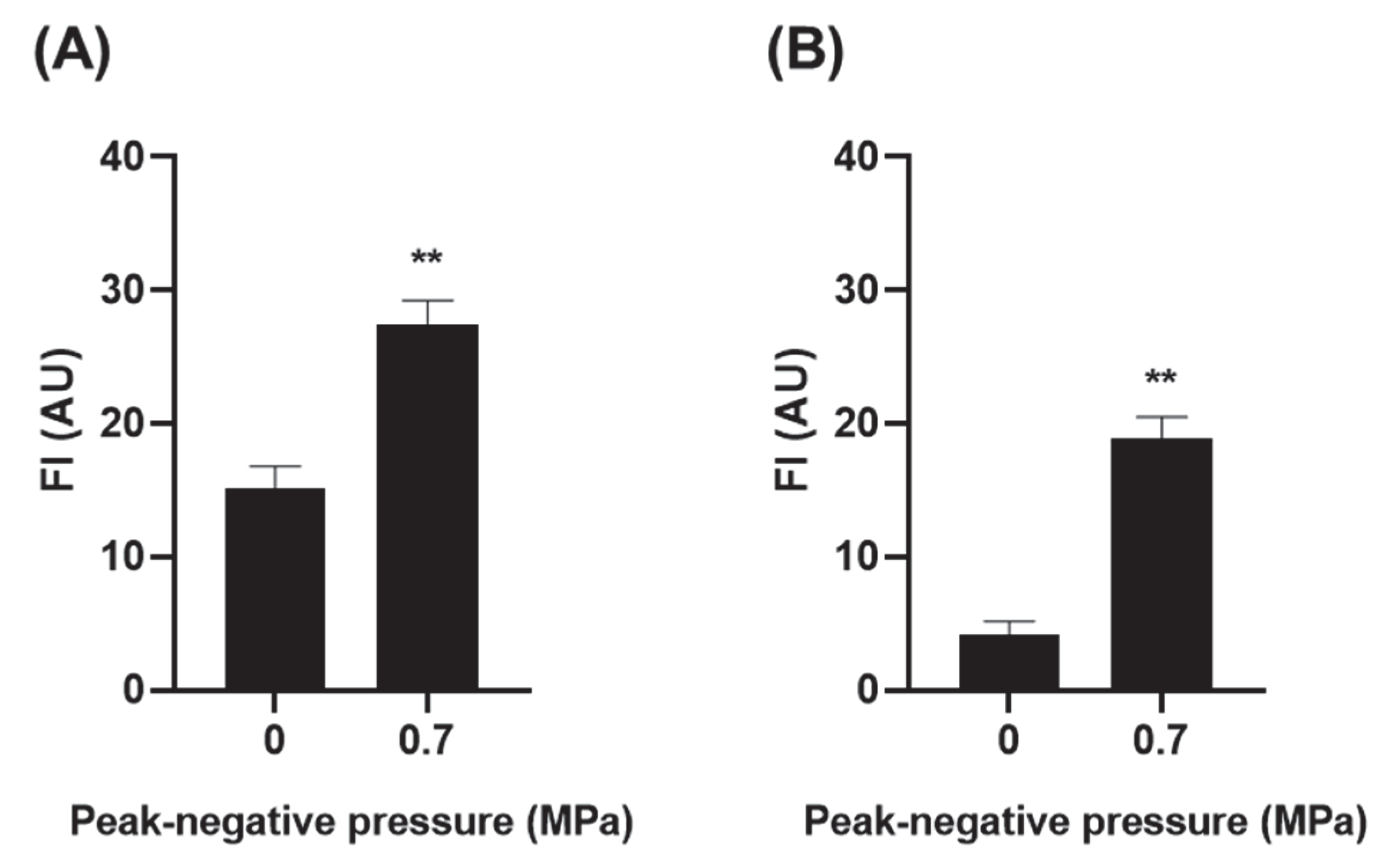
3.3. Effect of USMB on the Permeability of Anti-CXCR4 Nanobody across an Epithelial Barrier
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.R. Molecular Basis of Epithelial Barrier Regulation. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Vaz, J. The Blood-Retinal Barrier in the Management of Retinal Disease: EURETINA Award Lecture. Ophthalmologica 2017, 237, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef] [Green Version]
- González-Mariscal, L.; Nava, P.; Hernández, S. Critical Role of Tight Junctions in Drug Delivery across Epithelial and Endothelial Cell Layers. J. Membr. Biol. 2005, 207, 55–68. [Google Scholar] [CrossRef]
- McMahon, D.; O’Reilly, M.A.; Hynynen, K. Therapeutic Agent Delivery Across the Blood–Brain Barrier Using Focused Ultrasound. Annu. Rev. Biomed. Eng. 2021, 23, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Rousou, C.; Schuurmans, C.C.L.; Urtti, A.; Mastrobattista, E.; Storm, G.; Moonen, C.; Kaarniranta, K.; Deckers, R. Ultrasound and Microbubbles for the Treatment of Ocular Diseases: From Preclinical Research towards Clinical Application. Pharmaceutics 2021, 13, 1782. [Google Scholar] [CrossRef]
- Averkiou, M.A.; Bruce, M.F.; Powers, J.E.; Sheeran, P.S.; Burns, P.N. Imaging Methods for Ultrasound Contrast Agents. Ultrasound Med. Biol. 2020, 46, 498–517. [Google Scholar] [CrossRef] [Green Version]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.C.; Lentacker, I. Opening Doors with Ultrasound and Microbubbles: Beating Biological Barriers to Promote Drug Delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Qin, P.; Han, T.; Yu, A.C.H.; Xu, L. Mechanistic Understanding the Bioeffects of Ultrasound-Driven Microbubbles to Enhance Macromolecule Delivery. J. Control. Release 2018, 272, 169–181. [Google Scholar] [CrossRef]
- Hirokawa, T.; Karshafian, R.; Pavlin, C.J.; Burns, P.N. Insonation of the Eye in the Presence of Microbubbles: Preliminary Study of the Duration and Degree of Vascular Bioeffects-Work in Progress. J. Ultrasound Med. 2007, 26, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Akula, J.D.; McDannold, N.J. Targeted and Reversible Blood-Retinal Barrier Disruption via Focused Ultrasound and Microbubbles. PLoS ONE 2012, 7, e42754. [Google Scholar] [CrossRef] [PubMed]
- Touahri, Y.; Dixit, R.; Kofoed, R.H.; Miloska, K.; Park, E.; Raeisossadati, R.; Markham-Coultes, K.; David, L.A.; Rijal, H.; Zhao, J.; et al. Focused Ultrasound as a Novel Strategy for Noninvasive Gene Delivery to Retinal Müller Glia. Theranostics 2020, 10, 2982–2999. [Google Scholar] [CrossRef]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; Pople, C.B.; Davidson, B.; et al. MR-Guided Focused Ultrasound Enhances Delivery of Trastuzumab to Her2-Positive Brain Metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Shih, C.-P.; Chen, H.-C.; Chou, Y.-L.; Sytwu, H.-K.; Fang, M.-C.; Lin, Y.-Y.; Kuo, C.-Y.; Su, H.-H.; Hung, C.-L.; et al. Ultrasound Microbubble–Facilitated Inner Ear Delivery of Gold Nanoparticles Involves Transient Disruption of the Tight Junction Barrier in the Round Window Membrane. Front. Pharmacol. 2021, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Zhang, W.; Kohane, D.S. Drug Delivery across Barriers to the Middle and Inner Ear. Adv. Funct. Mater. 2020, 31, 2008701. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bian, Q.; Xu, Y.; Xu, D.; Gao, J. Recent Advances in Mechanical Force-Assisted Transdermal Delivery of Macromolecular Drugs. Int. J. Pharm. 2021, 602, 120598. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ryu, H.; Kim, H.S.; Kim, Y.; Choi, K.-S.; Park, H.; Seo, J. Sonophoresis Using Ultrasound Contrast Agents for Transdermal Drug Delivery: An In Vivo Experimental Study. Ultrasound Med. Biol. 2012, 38, 642–650. [Google Scholar] [CrossRef]
- Park, D.; Yoon, J.; Park, J.; Jung, B.; Park, H.; Seo, J. Transdermal Drug Delivery Aided by an Ultrasound Contrast Agent: An In Vitro Experimental Study. Open Biomed. Eng. J. 2010, 4, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Liao, A.-H.; Ho, H.-C.; Lin, Y.-C.; Chen, H.-K.; Wang, C.-H. Effects of Microbubble Size on Ultrasound-Induced Transdermal Delivery of High-Molecular-Weight Drugs. PLoS ONE 2015, 10, e0138500. [Google Scholar] [CrossRef]
- Van Hout, A.; Klarenbeek, A.; Bobkov, V.; Doijen, J.; Arimont, M.; Zhao, C.; Heukers, R.; Rimkunas, R.; de Graaf, C.; Verrips, T.; et al. CXCR4-Targeting Nanobodies Differentially Inhibit CXCR4 Function and HIV Entry. Biochem. Pharmacol. 2018, 158, 402–412. [Google Scholar] [CrossRef]
- Jahnichen, S.; Blanchetot, C.; Maussang, D.; Gonzalez-Pajuelo, M.; Chow, K.Y.; Bosch, L.; De Vrieze, S.; Serruys, B.; Ulrichts, H.; Vandevelde, W.; et al. CXCR4 Nanobodies (VHH-Based Single Variable Domains) Potently Inhibit Chemotaxis and HIV-1 Replication and Mobilize Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 20565–20570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellinen, L.; Pirskanen, L.; Tengvall-Unadike, U.; Urtti, A.; Reinisalo, M. Reinisalo Retinal Pigment Epithelial Cell Line with Fast Differentiation and Improved Barrier Properties. Pharmaceutics 2019, 11, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellinen, L.; Hongisto, H.; Ramsay, E.; Kaarniranta, K.; Vellonen, K.-S.; Skottman, H.; Ruponen, M. Drug Flux Across RPE Cell Models: The Hunt for An Appropriate Outer Blood–Retinal Barrier Model for Use in Early Drug Discovery. Pharmaceutics 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Van der Meer, S.M.; Versluis, M.; Lohse, D.; Chin, C.T.; Bouakaz, A.; Jong, N.d. The Resonance Frequency of SonoVue as Observed by High-Speed Optical Imaging; IEEE: Piscataway, NJ, USA, 2004; Volume 1, pp. 343–345. [Google Scholar]
- Schneider, M. Characteristics of SonoVueTM. Echocardiography 1999, 16, 743–746. [Google Scholar] [CrossRef]
- De Maar, J.S.; Rousou, C.; van Elburg, B.; Vos, H.J.; Lajoinie, G.P.R.; Bos, C.; Moonen, C.T.W.; Deckers, R. Ultrasound-Mediated Drug Delivery With a Clinical Ultrasound System: In Vitro Evaluation. Front. Pharmacol. 2021, 12, 768436. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, L.; Ranta, V.-P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Investig. Opthalmol. Vis. Sci. 2005, 46, 641. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Karlsson, J. Correlation between Oral Drug Absorption in Humans and Apparent Drug Permeability Coefficients in Human Intestinal Epithelial (Caco-2) Cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Grimes, P.A. Carboxyfluorescein: A Probe of the Blood-Ocular Barriers With Lower Membrane Permeability Than Fluorescein. Arch. Ophthalmol. 1982, 100, 635. [Google Scholar] [CrossRef]
- Balla, M.M.S.; Vemuganti, G.K.; Kannabiran, C.; Honavar, S.G.; Murthy, R. Phenotypic Characterization of Retinoblastoma for the Presence of Putative Cancer Stem-like Cell Markers by Flow Cytometry. Investig. Opthalmol. Vis. Sci. 2009, 50, 1506. [Google Scholar] [CrossRef]
- Frost, T.S.; Jiang, L.; Lynch, R.M.; Zohar, Y. Permeability of Epithelial/Endothelial Barriers in Transwells and Microfluidic Bilayer Devices. Micromachines 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fihn, B.; Sjöqvist, A.; Jodal, M. Permeability of the Rat Small Intestinal Epithelium along the Villus-Crypt Axis: Effects of Glucose Transport. Gastroenterology 2000, 119, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Linnankoski, J.; Mäkelä, J.; Palmgren, J.; Mauriala, T.; Vedin, C.; Ungell, A.; Lazorova, L.; Artursson, P.; Urtti, A.; Yliperttula, M. Paracellular Porosity and Pore Size of the Human Intestinal Epithelium in Tissue and Cell Culture Models. J. Pharm. Sci. 2010, 99, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Venturoli, D.; Rippe, B. Ficoll and Dextran vs. Globular Proteins as Probes for Testing Glomerular Permselectivity: Effects of Molecular Size, Shape, Charge, and Deformability. Am. J. Physiol.-Ren. Physiol. 2005, 288, F605–F613. [Google Scholar] [CrossRef]
- Fix, S.M.; Koppolu, B.P.; Novell, A.; Hopkins, J.; Kierski, T.M.; Zaharoff, D.A.; Dayton, P.A.; Papadopoulou, V. Ultrasound-Stimulated Phase-Change Contrast Agents for Transepithelial Delivery of Macromolecules, Toward Gastrointestinal Drug Delivery. Ultrasound Med. Biol. 2019, 45, 1762–1776. [Google Scholar] [CrossRef]
- Lelu, S.; Afadzi, M.; Berg, S.; Aslund, A.K.O.; Torp, S.H.; Sattler, W.; Davies, C.D.L. Primary Porcine Brain Endothelial Cells as In Vitro Model to Study Effects of Ultrasound and Microbubbles on Blood–Brain Barrier Function. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Lammertink, B.; Deckers, R.; Storm, G.; Moonen, C.; Bos, C. Duration of Ultrasound-Mediated Enhanced Plasma Membrane Permeability. Int. J. Pharm. 2015, 482, 92–98. [Google Scholar] [CrossRef]
- De Cock, I.; Zagato, E.; Braeckmans, K.; Luan, Y.; de Jong, N.; De Smedt, S.C.; Lentacker, I. Ultrasound and Microbubble Mediated Drug Delivery: Acoustic Pressure as Determinant for Uptake via Membrane Pores or Endocytosis. J. Control. Release 2015, 197, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Afadzi, M.; Strand, S.P.; Nilssen, E.A.; Masoy, S.-E.; Johansen, T.F.; Hansen, R.; Angelsen, B.A.; de L Davies, C. Mechanisms of the Ultrasound-Mediated Intracellular Delivery of Liposomes and Dextrans. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 21–33. [Google Scholar] [CrossRef]
- Meijering, B.D.M.; Juffermans, L.J.M.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.G.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and Microbubble-Targeted Delivery of Macromolecules Is Regulated by Induction of Endocytosis and Pore Formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef]
- Hicks, G.R.; Raikhel, N.V. Protein Import into the Nucleus: An Integrated View. Annu. Rev. Cell Dev. Biol. 1995, 11, 155–188. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive Localized Delivery of Herceptin to the Mouse Brain by MRI-Guided Focused Ultrasound-Induced Blood-Brain Barrier Disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef] [Green Version]
- Raymond, S.B.; Treat, L.H.; Dewey, J.D.; McDannold, N.J.; Hynynen, K.; Bacskai, B.J. Ultrasound Enhanced Delivery of Molecular Imaging and Therapeutic Agents in Alzheimer’s Disease Mouse Models. PLoS ONE 2008, 3, e2175. [Google Scholar] [CrossRef]
- Punjabi, M.; Xu, L.; Ochoa-Espinosa, A.; Kosareva, A.; Wolff, T.; Murtaja, A.; Broisat, A.; Devoogdt, N.; Kaufmann, B.A. Ultrasound Molecular Imaging of Atherosclerosis With Nanobodies: Translatable Microbubble Targeting Murine and Human VCAM (Vascular Cell Adhesion Molecule) 1. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, L.; Guo, Y.; Tu, Z.; Li, L.; Tong, H.; Xu, Y.; Li, R.; Fang, K. Ultrasonic Nanobubbles Carrying Anti-PSMA Nanobody: Construction and Application in Prostate Cancer-Targeted Imaging. PLoS ONE 2015, 10, e0127419. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, M.; Li, Z.; Xu, D.; Zhu, L.; Guo, Y.; Liu, Q.; Lan, W.; Jiang, J.; Wang, L. Anti-G250 Nanobody-Functionalized Nanobubbles Targeting Renal Cell Carcinoma Cells for Ultrasound Molecular Imaging. Nanotechnology 2020, 31, 205101. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yamamoto, N. Role of CXCR4 in HIV Infection and Its Potential as a Therapeutic Target. Future Microbiol. 2010, 5, 1025–1039. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J.; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 1969. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Liu, C.-J.; Redd, R.A.; Perez, R.P.; Baz, R.; Zavidij, O.; Sklavenitis-Pistofidis, R.; Richardson, P.G.; Anderson, K.C.; Laubach, J.; et al. A Phase Ib/II Trial of the First-in-Class Anti-CXCR4 Antibody Ulocuplumab in Combination with Lenalidomide or Bortezomib Plus Dexamethasone in Relapsed Multiple Myeloma. Clin. Cancer Res. 2020, 26, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, I.; Hornburger, M.C.; Mayer, B.A.; Beyerle, A.; Wegener, J.; Fürst, R. Pitfalls in Assessing Microvascular Endothelial Barrier Function: Impedance-Based Devices versus the Classic Macromolecular Tracer Assay. Sci. Rep. 2016, 6, 23671. [Google Scholar] [CrossRef] [Green Version]
- Bichsel, C.A.; Hall, S.R.R.; Schmid, R.A.; Guenat, O.T.; Geiser, T. Primary Human Lung Pericytes Support and Stabilize In Vitro Perfusable Microvessels. Tissue Eng. Part A 2015, 21, 2166–2176. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, C.G.M.; Brandt, M.M.; Poulis, N.; Anten, J.; van der Moolen, M.; Kramer, L.; Homburg, E.F.G.A.; Louzao-Martinez, L.; Pei, J.; Krebber, M.M.; et al. A New Microfluidic Model That Allows Monitoring of Complex Vascular Structures and Cell Interactions in a 3D Biological Matrix. Lab. Chip 2020, 20, 1827–1844. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Jolesz, F.A.; McDannold, N.J. The Kinetics of Blood Brain Barrier Permeability and Targeted Doxorubicin Delivery into Brain Induced by Focused Ultrasound. J. Control. Release 2012, 162, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.B.; Sheeran, P.S.; Averkiou, M.A. Cavitation Therapy Monitoring of Commercial Microbubbles With a Clinical Scanner. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 1144–1154. [Google Scholar] [CrossRef]
| Compound | Hydrophilic/Lipophilic (LogD) | MW (Da) | Label |
|---|---|---|---|
| Propranolol | Lipophilic (1.5 [28]) | 259 | Radioactive |
| Mannitol | Hydrophilic (−3.1 [28]) | 182 | Radioactive |
| 6-carboxyfluorescein | Hydrophilic (−3.2 [29]) | 376 | Fluorescent |
| 4 kDa dextran | Hydrophilic (N/A) | 4400 | Fluorescent (TRITC) |
| 20 kDa dextran | Hydrophilic (N/A) | 20,000 | Fluorescent (TRITC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousou, C.; de Maar, J.; Qiu, B.; van der Wurff-Jacobs, K.; Ruponen, M.; Urtti, A.; Oliveira, S.; Moonen, C.; Storm, G.; Mastrobattista, E.; et al. The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers. Pharmaceutics 2022, 14, 494. https://doi.org/10.3390/pharmaceutics14030494
Rousou C, de Maar J, Qiu B, van der Wurff-Jacobs K, Ruponen M, Urtti A, Oliveira S, Moonen C, Storm G, Mastrobattista E, et al. The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers. Pharmaceutics. 2022; 14(3):494. https://doi.org/10.3390/pharmaceutics14030494
Chicago/Turabian StyleRousou, Charis, Josanne de Maar, Boning Qiu, Kim van der Wurff-Jacobs, Marika Ruponen, Arto Urtti, Sabrina Oliveira, Chrit Moonen, Gert Storm, Enrico Mastrobattista, and et al. 2022. "The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers" Pharmaceutics 14, no. 3: 494. https://doi.org/10.3390/pharmaceutics14030494
APA StyleRousou, C., de Maar, J., Qiu, B., van der Wurff-Jacobs, K., Ruponen, M., Urtti, A., Oliveira, S., Moonen, C., Storm, G., Mastrobattista, E., & Deckers, R. (2022). The Effect of Microbubble-Assisted Ultrasound on Molecular Permeability across Cell Barriers. Pharmaceutics, 14(3), 494. https://doi.org/10.3390/pharmaceutics14030494







