Experimental Evaluation of Dry Powder Inhalers during Inhalation and Exhalation Using a Model of the Human Respiratory System (xPULM™)
Abstract
:1. Introduction
2. Materials and Methods
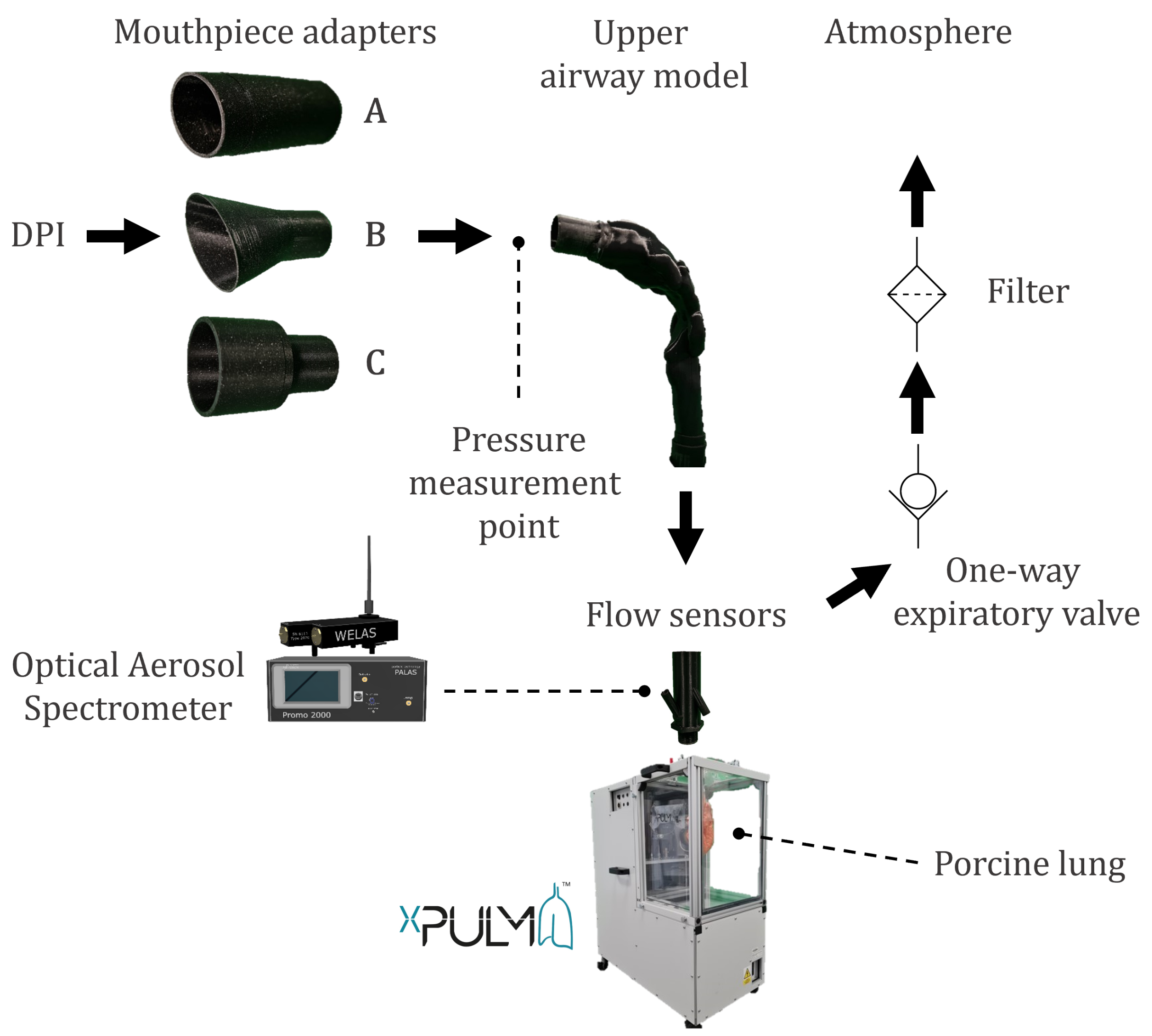
2.1. Measurement Setup and Procedure
2.2. Model of the Human Respiratory System
2.2.1. Representation of the Upper Respiratory Tract
2.2.2. Representation of the Lower Respiratory Tract
2.3. Dry Powder Inhalers
2.4. Data Processing and Statistics
3. Results and Discussion
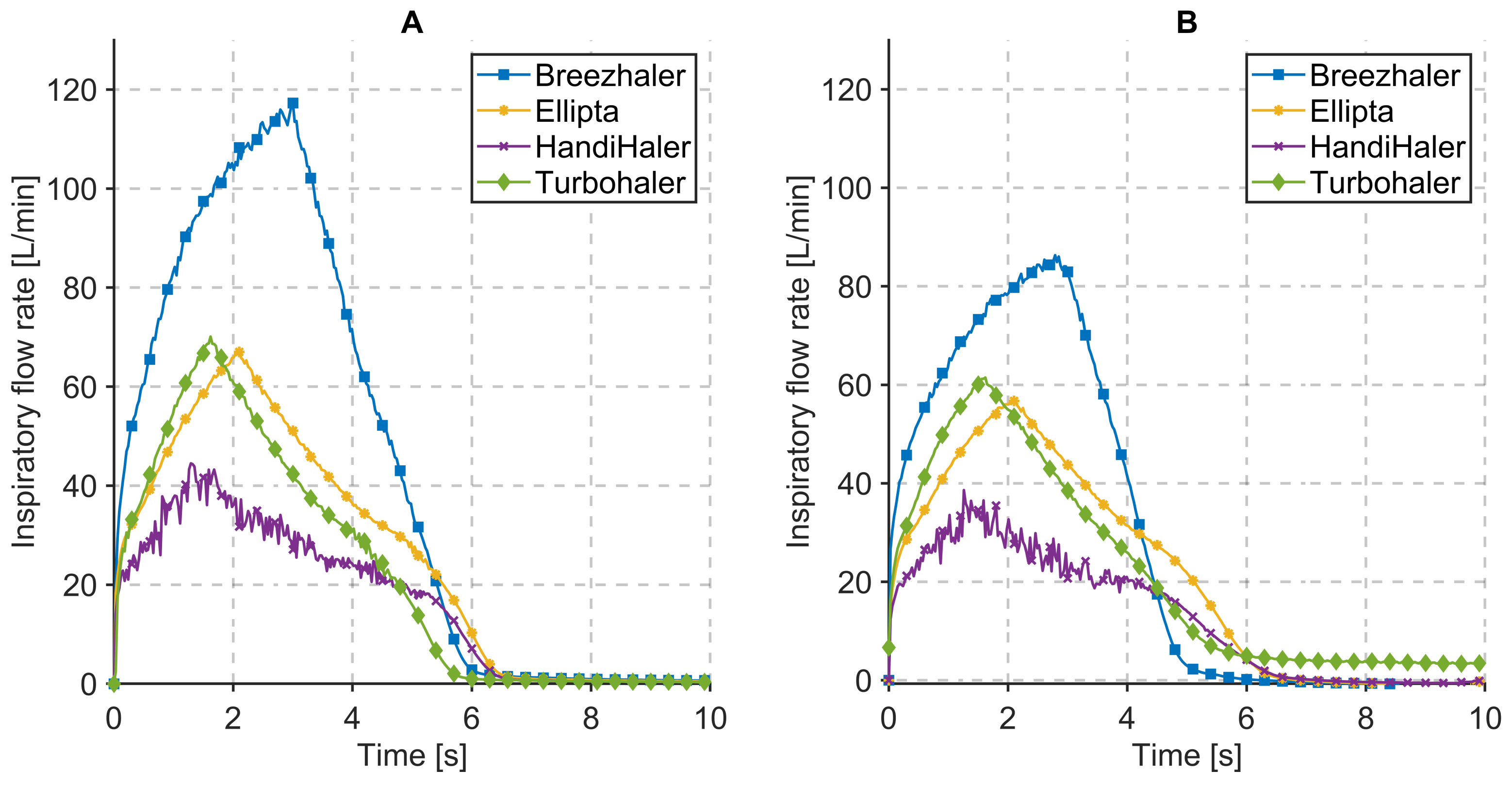
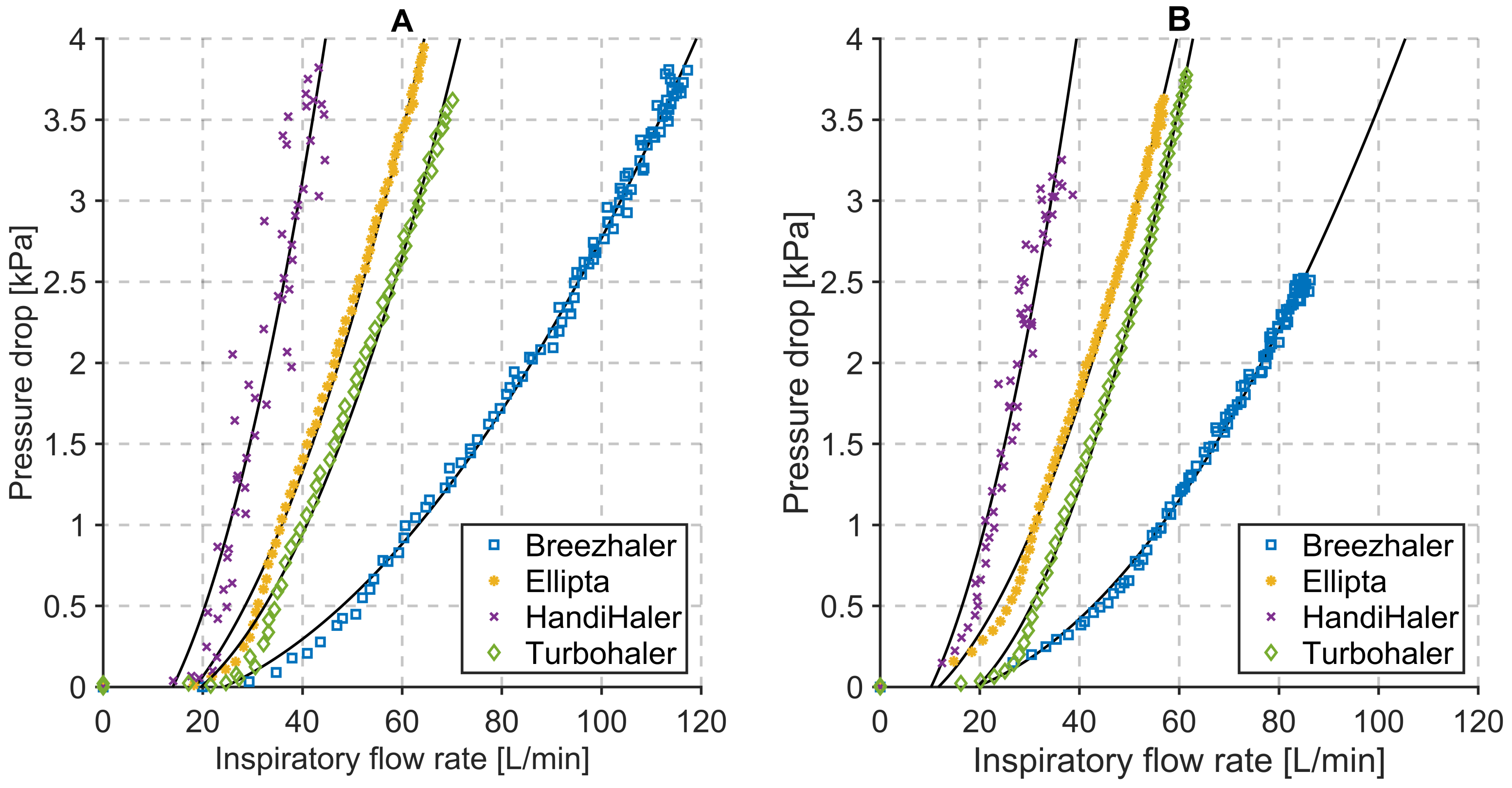
3.1. Inspiratory Flow Rate and Pressure Drop Measurements
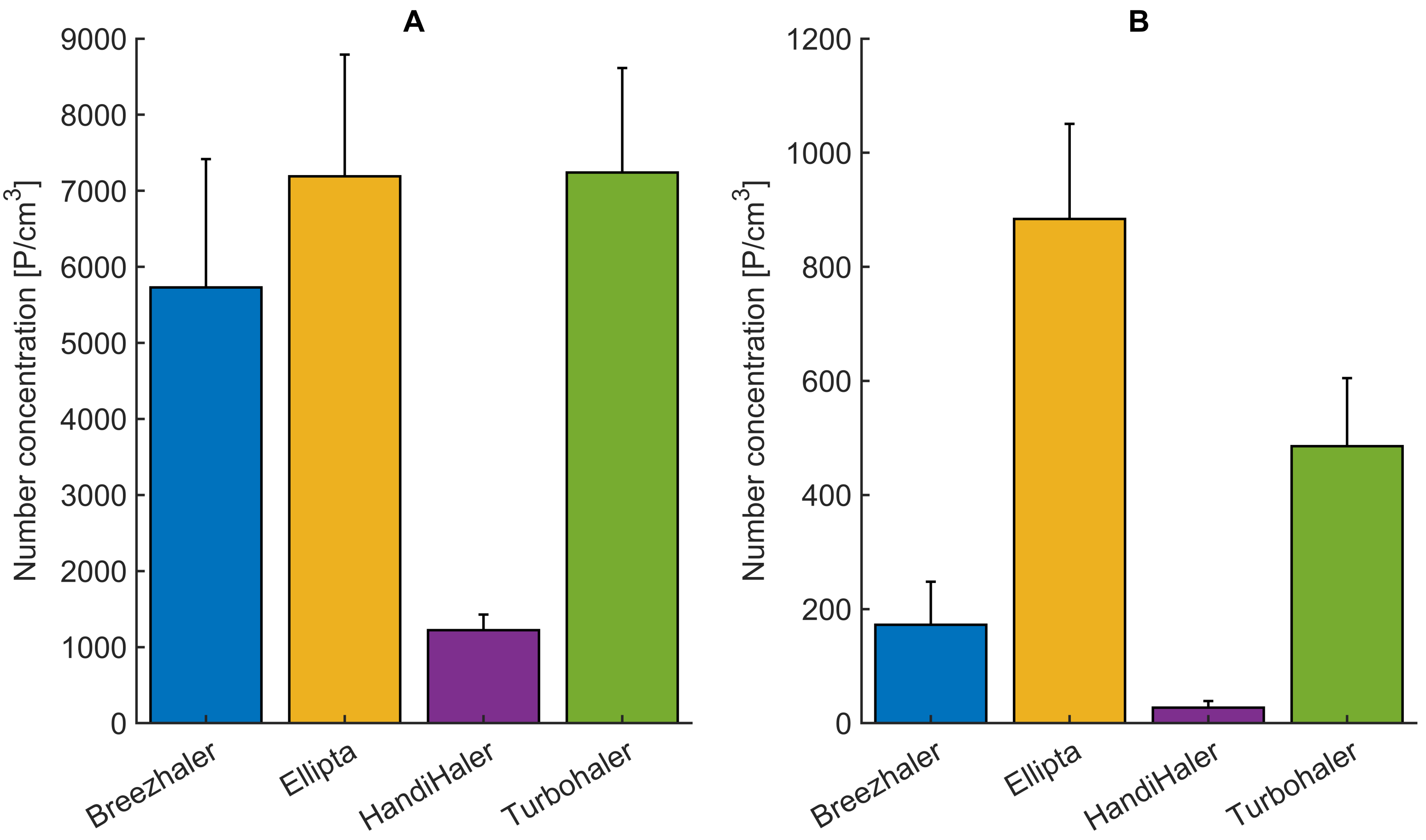
3.2. Influence of the Mechanical UAM and the Primed Porcine Lung
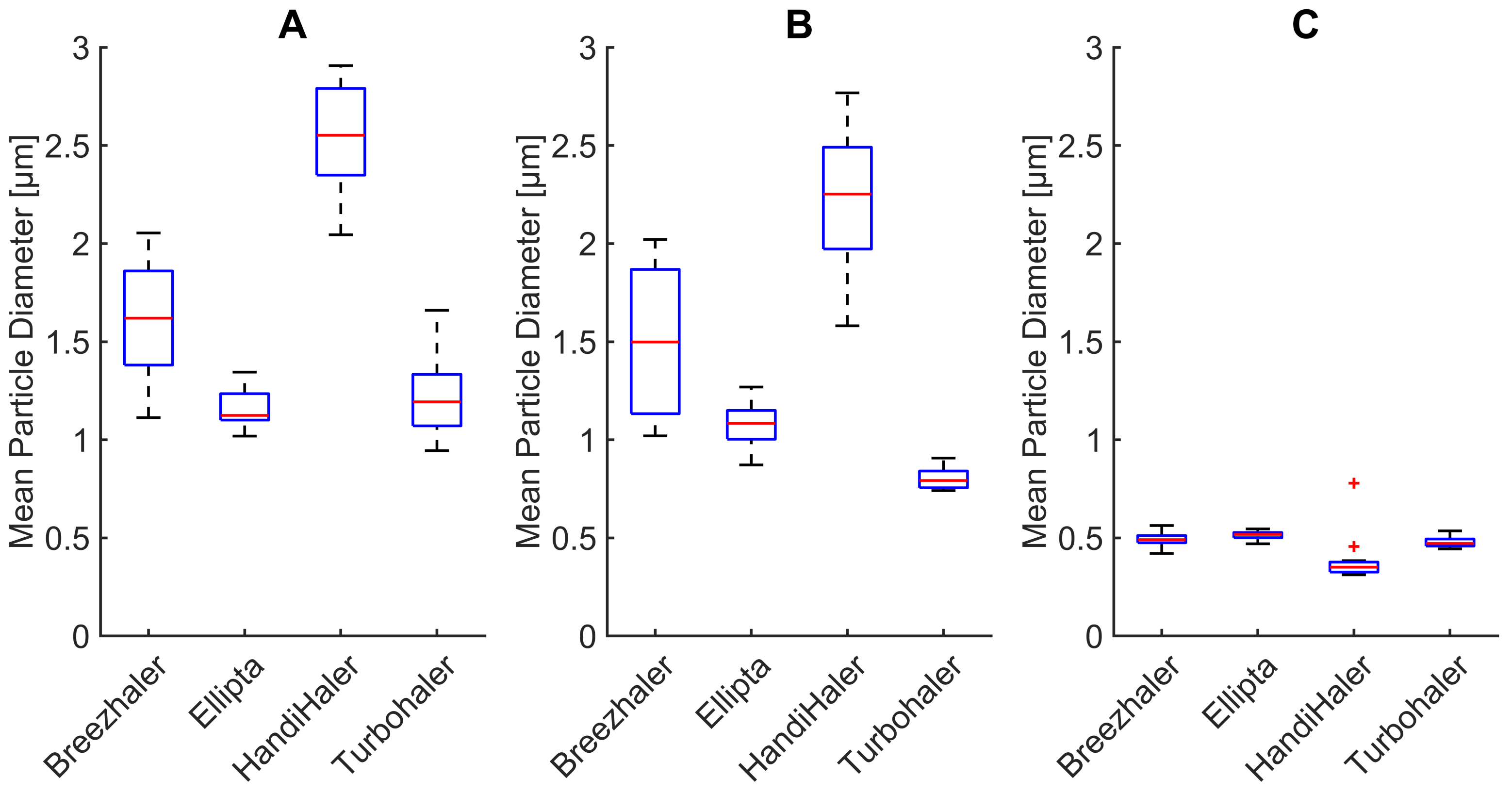
3.3. Changes in Mean Particle Diameter
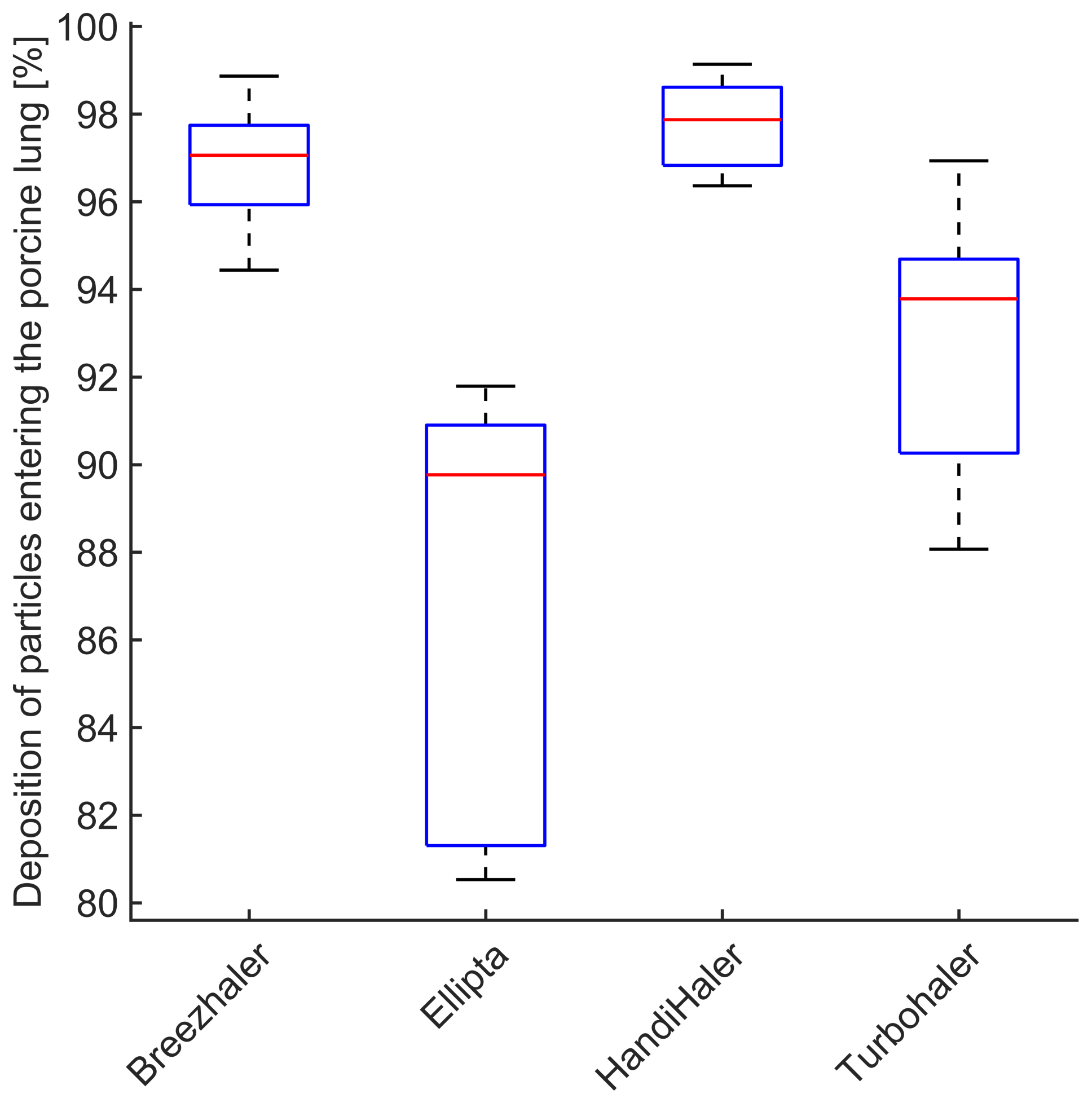
3.4. Deposition of Particles in the Porcine Lung
4. Summary and Conclusions
- Integration of a mechanical UAM, as a part of the xPULM™, increases the resistance of the overall system. This affects inhalatory flow and pressure characteristics of DPIs with lower inner resistance more than DPIs with high inner resistance, where the change is negligible.
- Handling and placement of a capsule into single-dose DPIs influences aerosol production during inhalation drug therapy. Slight changes in capsule placement may influence the amount of delivered drug. Correct handling of the inhaler should be emphasized alongside acceptable inhalation maneuvers (as defined by the device manufacturer) to ensure the desired result.
- Mean particle diameter is reduced by the filtration properties of the mechanical UAM, affecting mostly larger particles (>3 μm). Such models, when based on CT examinations, are reliably representing the function of the human upper respiratory tract.
- The majority of particles entering the porcine lung deposit within minimum deposition is reached for the particle size of ( μm). The primed porcine lung is therefore a suitable lung equivalent and representation of the human lung.
- Sampling of the airstream during inhalation and exhalation and its subsequent evaluation using optical aerosol spectrometry techniques is a viable alternative to impactors for evaluating pharmaceutical aerosols.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forum of International Respiratory Societies. The Global Impact of Respiratory Disease; European Respiratory Society: Sheffield, UK, 2017. [Google Scholar]
- Eurostat. Respiratory Diseases Statistics—Statistics Explained. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Respiratory_diseases_statistics&oldid=497079 (accessed on 18 December 2021).
- Sorino, C.; Negri, S.; Spanevello, A.; Visca, D.; Scichilone, N. Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur. J. Intern. Med. 2020, 75, 15–18. [Google Scholar] [CrossRef]
- Stein, S.W.; Thiel, C.G. The History of Therapeutic Aerosols: A Chronological Review. J. Aerosol Med. Pulm. Drug Deliv. 2017, 30, 20–41. [Google Scholar] [CrossRef]
- Wintemute, K.; Miller, F. Dry powder inhalers are environmentally preferable to metered-dose inhalers. CMAJ 2020, 192, E846. [Google Scholar] [CrossRef]
- Rau, J.L. Practical problems with aerosol therapy in COPD. Respir. Care 2006, 51, 158–172. [Google Scholar]
- Holmes, M.S.; Seheult, J.N.; O’Connell, P.; D’Arcy, S.; Ehrhardt, C.; Healy, A.M.; Costello, R.W.; Reilly, R.B. An Acoustic-Based Method to Detect and Quantify the Effect of Exhalation into a Dry Powder Inhaler. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 247–253. [Google Scholar] [CrossRef]
- Geller, D.E. Comparing Clinical Features of the Nebulizer, Metered-Dose Inhaler, and Dry Powder Inhaler. Respir. Care 2005, 50, 1313–1322. [Google Scholar]
- Clark, A.R.; Weers, J.G.; Dhand, R. The Confusing World of Dry Powder Inhalers: It Is All about Inspiratory Pressures, Not Inspiratory Flow Rates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Atkins, S.; MacIntyre, H.; Amato, M.T. Dry powder inhalers: An overview—Discussion. Respir. Care 2005, 50, 1312. [Google Scholar]
- Mahler, D.A.; Waterman, L.A.; Gifford, A.H. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the diskus® dry powder inhaler. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 174–179. [Google Scholar] [CrossRef]
- Grant, A.C.; Walker, R.; Hamilton, M.; Garrill, K. The ELLIPTA® dry powder inhaler: Design, functionality, in vitro dosing performance and critical task compliance by patients and caregivers. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Mahler, D.A. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2017, 14, 1103–1107. [Google Scholar] [CrossRef]
- Duarte, A.G.; Tung, L.; Zhang, W.; Hsu, E.S.; Kuo, Y.F.; Sharma, G. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr. Pulm. Dis. 2019, 6, 246–255. [Google Scholar] [CrossRef]
- Chen, S.Y.; Huang, C.K.; Peng, H.C.; Yu, C.J.; Chien, J.Y. Inappropriate Peak Inspiratory Flow Rate with Dry Powder Inhaler in Chronic Obstructive Pulmonary Disease. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Taki, M.; Marriott, C.; Zeng, X.M.; Martin, G.P. Aerodynamic deposition of combination dry powder inhaler formulations in vitro: A comparison of three impactors. Int. J. Pharm. 2010, 388, 40–51. [Google Scholar] [CrossRef]
- Versteeg, H.K.; Roberts, D.L.; Chambers, F.; Cooper, A.; Copley, M.; Mitchell, J.P.; Mohammed, H. A cross-industry assessment of the flow rate-elapsed time profiles of test equipment typically used for dry-powder inhaler (DPI) testing: Part 2– analysis of transient air flow in the testing of DPIs with compendial cascade impactors. Aerosol Sci. Technol. 2020, 54, 1448–1470. [Google Scholar] [CrossRef]
- Greguletz, R.; Andersson, P.U.; Cooper, A.; Chambers, F.; Copley, M.A.; Daniels, G.; Hamilton, M.; Hammond, M.; Mohammed, H.; Roberts, D.L.; et al. A cross-industry assessment of the flow rate-time profiles of test equipment typically used for dry-powder inhaler (DPI) testing: Part 1–compendial apparatuses. Aerosol Sci. Technol. 2020, 54, 1424–1447. [Google Scholar] [CrossRef]
- Wei, X.; Hindle, M.; Kaviratna, A.; Huynh, B.K.; Delvadia, R.R.; Sandell, D.; Byron, P.R. In vitro tests for aerosol deposition. VI: Realistic testing with different mouth-throat models and in vitro - In vivo correlations for a dry powder inhaler, metered dose inhaler, and soft mist inhaler. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 358–371. [Google Scholar] [CrossRef]
- Ravi Kannan, R.; Przekwas, A.J.; Singh, N.; Delvadia, R.; Tian, G.; Walenga, R. Pharmaceutical aerosols deposition patterns from a Dry Powder Inhaler: Euler Lagrangian prediction and validation. Med. Eng. Phys. 2017, 42, 35–47. [Google Scholar] [CrossRef]
- Kopsch, T.; Murnane, D.; Symons, D. Computational modelling and experimental validation of drug entrainment in a dry powder inhaler. Int. J. Pharm. 2018, 553, 37–46. [Google Scholar] [CrossRef]
- Chalvatzaki, E.; Chatoutsidou, S.E.; Lazaridis, M. Simulations of the deposition of pharmaceutical aerosols in the human respiratory tract by dry powder inhalers (DPIs). J. Drug Deliv. Sci. Technol. 2020, 59, 101915. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N. Analytical Methods and Typical Atmospheric Concentrations for Gases and Particles. Chem. Up. Low. Atmos. 2000, 11, 547–656. [Google Scholar] [CrossRef]
- Kulkarni, V. Handbook of Non-Invasive Drug Delivery Systems: Science and Technology; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Bonam, M.; Christopher, D.; Cipolla, D.; Donovan, B.; Goodwin, D.; Holmes, S.; Lyapustina, S.; Mitchell, J.; Nichols, S.; Pettersson, G.; et al. Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS PharmSciTech 2008, 9, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Darquenne, C. Deposition Mechanisms. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 181–185. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle Transport and Deposition: Basic Physics of Particle Kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, C.; Fuzzi, S.; Kochanovskij, A.A. (Eds.) Atmospheric Aerosols: Life Cycles and Effects on Air Quality and Climate; Wiley Series in Atmospheric Physics and Remote Sensing; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled Therapy in Respiratory Disease: The Complex Interplay of Pulmonary Kinetic Processes. Can. Respir. J. 2018, 2018, 2732017. [Google Scholar] [CrossRef] [Green Version]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Boer, A.H.D.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Characterization of inhalation aerosols: A critical evaluation of cascade impactor analysis and laser diffraction technique. Int. J. Pharm. 2002, 249, 219–231. [Google Scholar] [CrossRef]
- Judge, E.P.; Hughes, J.M.L.; Egan, J.J.; Maguire, M.; Molloy, E.L.; O’Dea, S. Anatomy and Bronchoscopy of the Porcine Lung. A Model for Translational Respiratory Medicine. Am. J. Respir. Cell Mol. Biol. 2014, 51, 334–343. [Google Scholar] [CrossRef]
- Rogers, C.S.; Abraham, W.M.; Brogden, K.A.; Engelhardt, J.F.; Fisher, J.T.; McCray, P.B.; McLennan, G.; Meyerholz, D.K.; Namati, E.; Ostedgaard, L.S.; et al. The porcine lung as a potential model for cystic fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L240–L263. [Google Scholar] [CrossRef] [Green Version]
- The United States Pharmacopeial Convention. USP35 NF30, 2012: <601> Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers; United States Pharmacopeial: Rockville, MD, USA, 2011. [Google Scholar]
- Pasteka, R.; Santos da Costa, J.P.; Barros, N.; Kolar, R.; Forjan, M. Patient–Ventilator Interaction Testing Using the Electromechanical Lung Simulator xPULM™ during V/A-C and PSV Ventilation Mode. Appl. Sci. 2021, 11, 3745. [Google Scholar] [CrossRef]
- Pasteka, R.; Forjan, M.; Sauermann, S.; Drauschke, A. Electro-mechanical Lung Simulator Using Polymer and Organic Human Lung Equivalents for Realistic Breathing Simulation. Sci. Rep. 2019, 9, 19778. [Google Scholar] [CrossRef] [PubMed]
- Ahookhosh, K.; Pourmehran, O.; Aminfar, H.; Mohammadpourfard, M.; Sarafraz, M.M.; Hamishehkar, H. Development of human respiratory airway models: A review. Eur. J. Pharm. Sci. 2020, 145, 105233. [Google Scholar] [CrossRef] [PubMed]
- Lizal, F.; Jedelsky, J.; Morgan, K.; Bauer, K.; Llop, J.; Cossio, U.; Kassinos, S.; Verbanck, S.; Ruiz-Cabello, J.; Santos, A.; et al. Experimental methods for flow and aerosol measurements in human airways and their replicas. Eur. J. Pharm. Sci. 2018, 113, 95–131. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; 238p. [Google Scholar]
- Horváth, A.; Balásházy, I.; Tomisa, G.; Farkas, Á. Significance of breath-hold time in dry powder aerosol drug therapy of COPD patients. Eur. J. Pharm. Sci. 2017, 104, 145–149. [Google Scholar] [CrossRef]
- Buttini, F.; Brambilla, G.; Copelli, D.; Sisti, V.; Balducci, A.G.; Bettini, R.; Pasquali, I. Effect of Flow Rate on In Vitro Aerodynamic Performance of NEXThaler® in Comparison with Diskus® and Turbohaler® Dry Powder Inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Sahin-Yilmaz, A.; Naclerio, R.M. Anatomy and physiology of the upper airway. Proc. Am. Thorac. Soc. 2011, 8, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J. Particle size and pathogenicity in the respiratory tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Lippmann, M.; Yeates, D.B.; Albert, R.E. Deposition, retention, and clearance of inhaled particles. Br. J. Ind. Med. 1980, 37, 337–362. [Google Scholar] [CrossRef] [Green Version]
- Darquenne, C. Aerosol deposition in health and disease. J. Aerosol Med. Pulm. Drug Deliv. 2012, 25, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Usmani, O.S.; Biddiscombe, M.F.; Nightingale, J.A.; Underwood, S.R.; Barnes, P.J. Effects of bronchodilator particle size in asthmatic patients using monodisperse aerosols. J. Appl. Physiol. 2003, 95, 2106–2112. [Google Scholar] [CrossRef] [Green Version]
- Usmani, O.S.; Biddiscombe, M.F.; Barnes, P.J. Regional lung deposition and bronchodilator response as a function of β2-agonist particle size. Am. J. Respir. Crit. Care Med. 2005, 172, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Malik, S.; Hirst, P.; Pitcairn, G.; Heide, A.; Pabst, J.; Dinkelaker, A.; Fleischer, W. Lung deposition of salbutamol in healthy human subjects from the MAGhaler dry powder inhaler. Respir. Med. 2002, 96, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virchow, J.C.; Poli, G.; Herpich, C.; Kietzig, C.; Ehlich, H.; Braeutigam, D.; Sommerer, K.; Häussermann, S.; Mariotti, F. Lung Deposition of the Dry Powder Fixed Combination Beclometasone Dipropionate Plus Formoterol Fumarate Using NEXThaler® Device in Healthy Subjects, Asthmatic Patients, and COPD Patients. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.L.; Carroll, W.; Izquierdo Alonso, J.L.; Keller, C.; Lavorini, F.; Lehtimäki, L. Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes. Adv. Ther. 2019, 36, 2547–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, S.P.; Busse, W.W. REVIEW: Evolution of dry powder inhaler design, formulation, and performance. Respir. Med. 2002, 96, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadelah, M.; Abdalla, G.; Chrystyn, H.; Larhrib, H. Gaining an insight into the importance of each inhalation manoeuvre parameter using altered patients’ inhalation profiles. J. Drug Deliv. Sci. Technol. 2021, 61, 102181. [Google Scholar] [CrossRef]
- Hira, D.; Okuda, T.; Mizutani, A.; Tomida, N.; Okamoto, H. In Vitro Evaluation of Optimal Inhalation Flow Patterns for Commercial Dry Powder Inhalers and Pressurized Metered Dose Inhalers With Human Inhalation Flow Pattern Simulator. J. Pharm. Sci. 2018, 107, 1731–1735. [Google Scholar] [CrossRef]
- Hamilton, M.; Leggett, R.; Pang, C.; Charles, S.; Gillett, B.; Prime, D. In vitro dosing performance of the ELLIPTA® dry powder inhaler using asthma and COPD patient inhalation profiles replicated with the electronic lung (eLung™). J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 498–506. [Google Scholar] [CrossRef] [Green Version]
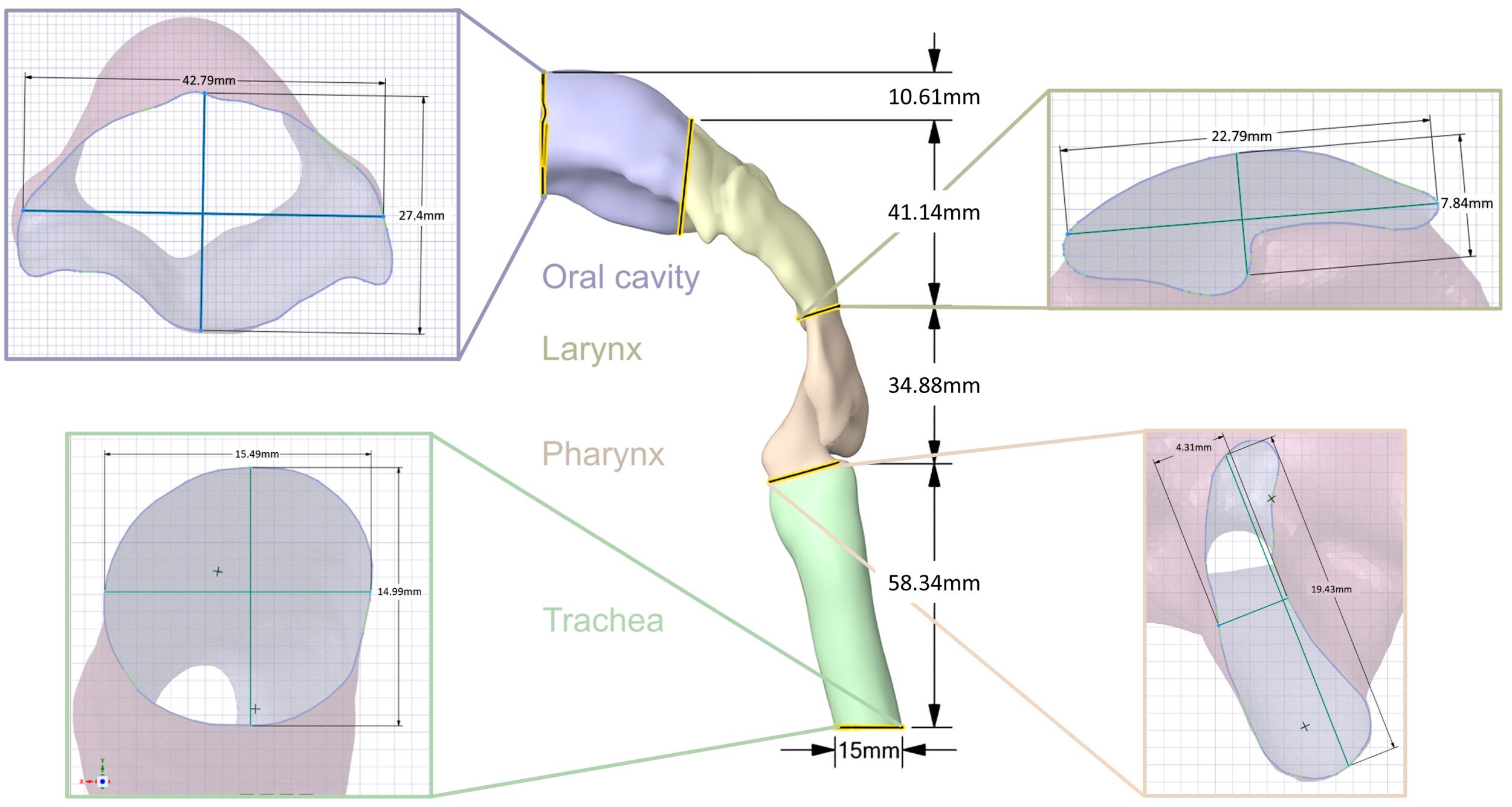
| Section | Volume [mm] | Lower SA [mm] | Diameter Y [mm] | Dimeter X [mm] | Upper SA [mm] | Diameter Y [mm] | Diameter X [mm] |
|---|---|---|---|---|---|---|---|
| Trachea (green) | 10,657.02 | 188.39 | 15.49 | 14.99 | 86.60 | 18 | 4.93 |
| Pharynx (orange) | 7311.52 | 119.06 | 19.43 | 4.31 | 86.60 | 4.86 | 24.51 |
| Larynx (yellow) | 15,902.39 | 119.11 | 7.84 | 22.79 | 777.57 | 24.93 | 44.34 |
| Oral cavity (blue) | 22,265.60 | 777.60 | 7.84 | 22.79 | 529.90 | 7.84 | 22.79 |
| Device | Active Substance | Resistance [kPa1/2/L/min] | Metered Dose [] | Lactose [mg] | Dose Type |
|---|---|---|---|---|---|
| Seebri® Breezhaler® | Glycopyrronium | 0.0216 | 44 | 23.6 | multidose, predispensed |
| Anoro® Ellipta® | Fluticasone furoate and vilanterol | 0.0286 | 55/22 | 25 | multidose, predispensed |
| Spiriva® HandiHaler® | Tiotropium bromide | 0.0504 | 18 | 5.5 | single-dose, hard capsules |
| Symbicort® Turbohaler® | Budesonide and formoterol | 0.0313 | 200/6 | 0.73 | multidose, predispensed |
| Dry Powder Inhalers | Characterization Parameters | Respiration Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Device | Vinh [L] | PDROP [kPa] | PIF [L/min] | Tinh [s] | System Resistance [kPa1/2/L/min] | Vinh [L] | PDROP [kPa] | PIF [L/min] | Tinh [s] | System Resistance [kPa1/2/L/min] |
| Seebri® Breezhaler® | 6.98 | 3.81 | 117.28 | 3.00 | 0.0166 | 4.55 | 2.52 | 86.31 | 3.00 | 0.0184 * |
| Anoro® Ellipta® | 4.06 | 4.27 | 67.01 | 2.10 | 0.0308 | 3.38 | 3.63 | 56.94 | 2.10 | 0.0335 * |
| Spiriva® HandiHaler® | 2.60 | 3.82 | 44.50 | 1.40 | 0.0439 | 2.05 | 3.25 | 38.62 | 1.40 | 0.0467 * |
| Symbicort® Turbohaler® | 3.52 | 3.83 | 70.11 | 1.60 | 0.0279 | 3.40 | 3.89 | 61.48 | 1.60 | 0.0320 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasteka, R.; Schöllbauer, L.A.; Santos da Costa, J.P.; Kolar, R.; Forjan, M. Experimental Evaluation of Dry Powder Inhalers during Inhalation and Exhalation Using a Model of the Human Respiratory System (xPULM™). Pharmaceutics 2022, 14, 500. https://doi.org/10.3390/pharmaceutics14030500
Pasteka R, Schöllbauer LA, Santos da Costa JP, Kolar R, Forjan M. Experimental Evaluation of Dry Powder Inhalers during Inhalation and Exhalation Using a Model of the Human Respiratory System (xPULM™). Pharmaceutics. 2022; 14(3):500. https://doi.org/10.3390/pharmaceutics14030500
Chicago/Turabian StylePasteka, Richard, Lara Alina Schöllbauer, Joao Pedro Santos da Costa, Radim Kolar, and Mathias Forjan. 2022. "Experimental Evaluation of Dry Powder Inhalers during Inhalation and Exhalation Using a Model of the Human Respiratory System (xPULM™)" Pharmaceutics 14, no. 3: 500. https://doi.org/10.3390/pharmaceutics14030500







