Liposomal Formulations Enhance the Anti-Inflammatory Effect of Eicosapentaenoic Acid in HL60 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. EPA Liposome Preparation
2.2. Size and Zeta Potential Measurement
2.3. Calculation of the Loading Efficiency and Encapsulation Efficiency of EPA
2.4. Release Studies
2.5. EPA Measurement by HPLC
2.6. Stability Study
2.7. TEM Imaging
2.8. Cell Culture
2.9. Confocal Imaging
2.10. Cell Viability Assessment
2.11. Inhibition of Production of Reactive NO and ROS
2.12. Cytokine Measurement
2.13. Metabolite Measurement
2.14. Statistics
3. Results
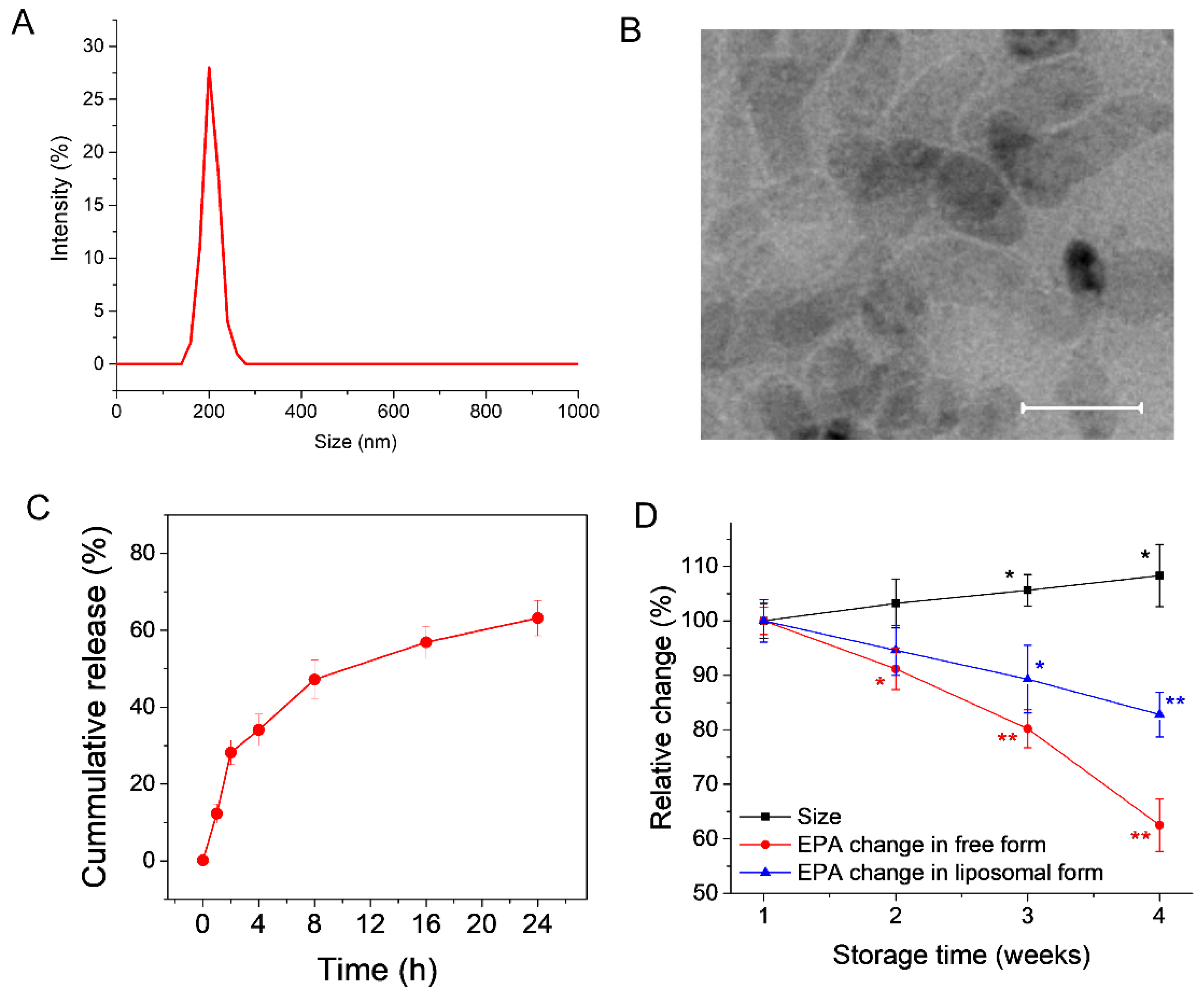
3.1. Preparation and Characterization of EPA-Liposomes
3.2. EPA-Liposomes Can Be Efficiently Internalized by HL60 Cells
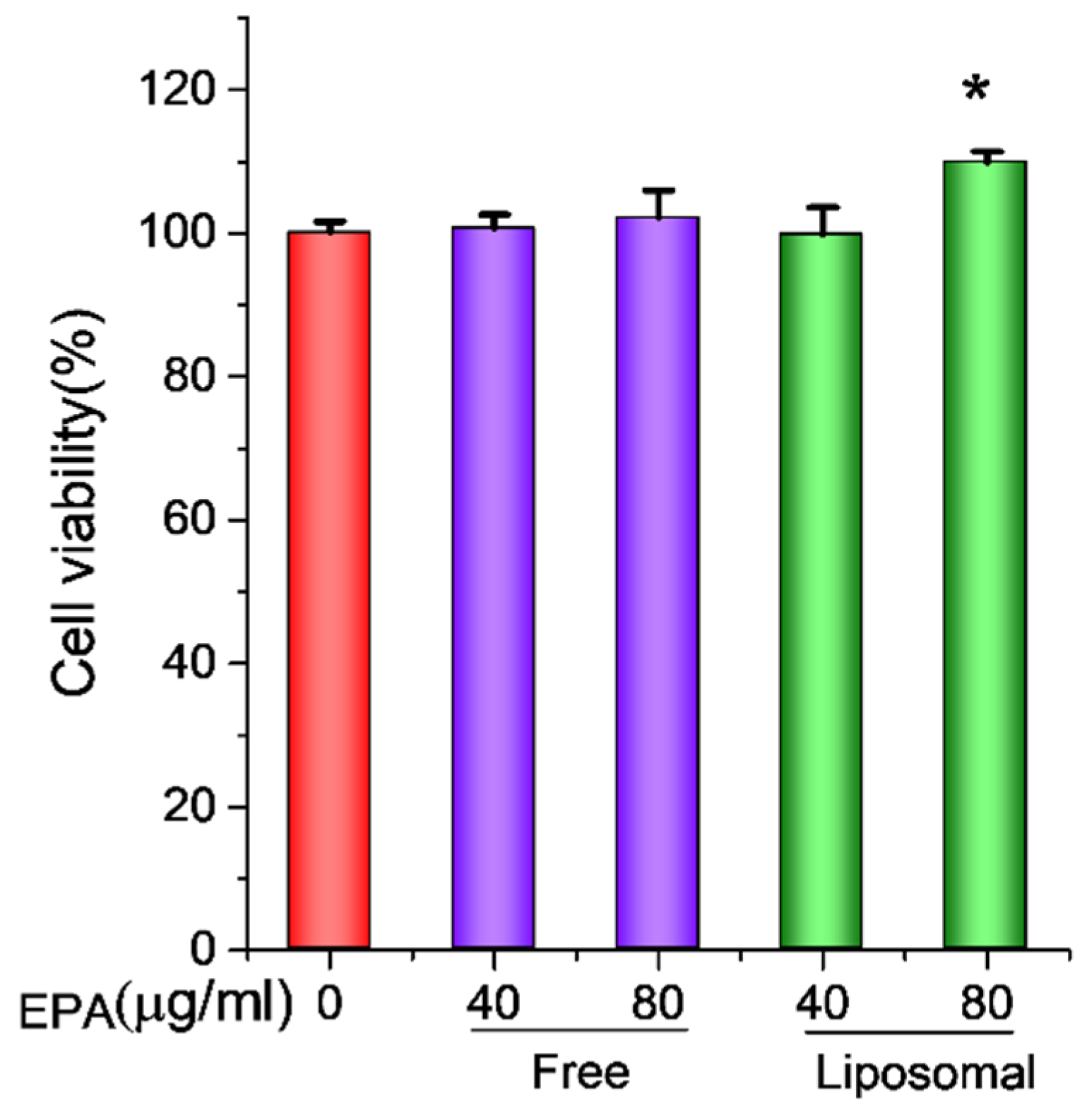
3.3. EPA-Liposomes Increased the Cell Viability of Activated HL60 Cells
3.4. EPA-Liposomes Inhibited Production of NO and ROS, and Cytokine Secretion in Activated HL60 Cells
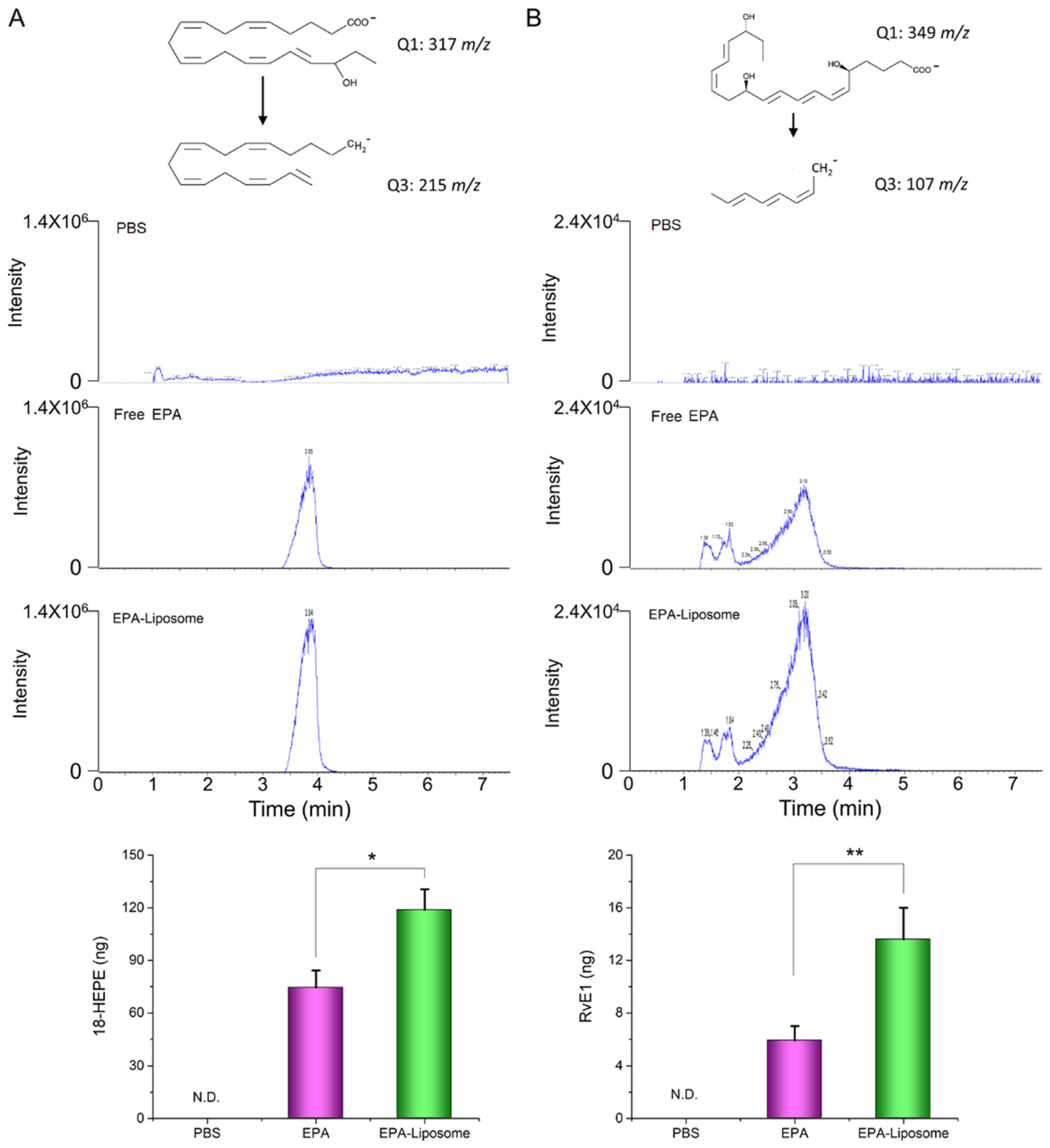
3.5. The Liposomal Formulation Increased the Conversion of EPA in Activated HL60 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Schacky, C.; Angerer, P.; Kothny, W.; Theisen, K.; Mudra, H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1999, 130, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Vas Dias, F.W.; Gibney, M.J.; Taylor, T.G. The effect of polyunsaturated fatty acids on the n-3 and n-6 series on platelet aggregation and platelet and aortic fatty acid composition in rabbits. Atherosclerosis 1982, 43, 245–257. [Google Scholar] [CrossRef]
- De Caterina, R.; Liao, J.K.; Libby, P. Fatty acid modulation of endothelial activation. Am. J. Clin. Nutr. 2000, 71, 213S–223S. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, S.; Dong, X.; Leanse, L.G.; Dai, T.; Wang, Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020, 3, 680. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Souza, E.; Schulte, F.; Chen, T.; Hardt, M.; Hasturk, H.; Van Dyke, T.E.; Holzhausen, M.; Kantarci, A. Maresin-1 and Resolvin E1 Promote Regenerative Properties of Periodontal Ligament Stem Cells Under Inflammatory Conditions. Front. Immunol. 2020, 11, 2509. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Colas, R.A.; Wheway, K.; Watkins, B.; Appleton, L.; Rees, J.; Gwilym, S.; Little, C.; Dalli, J.; Carr, A.J. Proresolving Mediators LXB4 and RvE1 Regulate Inflammation in Stromal Cells from Patients with Shoulder Tendon Tears. Am. J. Pathol. 2019, 189, 2258–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Dong, X.; Wang, Z. Generation, purification and engineering of extracellular vesicles and their biomedical applications. Methods 2020, 177, 114–125. [Google Scholar] [CrossRef]
- Wang, S.; Dong, X.; Gao, J.; Wang, Z. Targeting Inflammatory Vasculature by Extracellular Vesicles. AAPS J. 2018, 20, 37. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, S.H.; Wang, H.X.; Yang, Y.J.; Gao, J. Enhanced Anticancer Therapeutic Delivery to Tumor Microenvironment via Simultaneous Angiogenesis and Tumor Targeting. ChemNanoMat 2020, 6, 1346–1356. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Su, Y.; Wang, Z. Nanomedicine for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7600. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, H.; Ghorbani, M.; Jafari, S.M.; Sadeghi Mahoonak, A. Encapsulation of EPA and DHA concentrate from Kilka fish oil by milk proteins and evaluation of its oxidative stability. J. Food Sci. Technol. 2019, 56, 59–70. [Google Scholar] [CrossRef]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.F.; Pillai, P.S.; Recchiuti, A.; Yang, R.; Serhan, C.N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Investig. 2011, 121, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Lin, W.J.; Gao, J.; Shi, X.T.; Davaritouchaee, M.; Nielsen, A.E.; Mancini, R.J.; Wang, Z.J. pH-Responsive Nanoparticles Targeted to Lungs for Improved Therapy of Acute Lung Inflammation/Injury. ACS Appl. Mater. Inter. 2019, 11, 16380–16390. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.D.; Yen, W.J.; Yen, G.C. Oxidative stability of polyunsaturated fatty acids and soybean oil in an aqueous solution with emulsifiers. J. Am. Oil Chem. Soc. 1999, 76, 201–204. [Google Scholar] [CrossRef]
- Miyashita, K.; Nara, E.; Ota, T. Oxidative Stability of Polyunsaturated Fatty-Acids in an Aqueous-Solution. Biosci. Biotechnol. Biochem. 1993, 57, 1638–1640. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Gao, J.; Dong, X.; Su, Y.; Wang, Z. Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021, 123, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Wang, Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranipour, S.; Amini Kadijani, A.; Qujeq, D.; Shahrokh, S.; Haghazali, M.; Mirzaei, A.; Asadzadeh-Aghdaei, H. Inducible nitric oxide synthase as a potential blood-based biomarker in inflammatory bowel diseases. Gastroenterol. Hepatol. Bed Bench 2018, 11, S124–S128. [Google Scholar]
- Wang, H.X.; Liu, M.; Weng, S.Y.; Li, J.J.; Xie, C.; He, H.L.; Guan, W.; Yuan, Y.S.; Gao, J. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J. Gastroenterol. 2012, 18, 119–125. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef] [Green Version]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Siddiquee, A.; Patel, M.; Rajalingam, S.; Narke, D.; Kurade, M.; Ponnoth, D.S. Effect of omega-3 fatty acid supplementation on resolvin (RvE1)-mediated suppression of inflammation in a mouse model of asthma. Immunopharmacol. Immunotoxicol. 2019, 41, 250–257. [Google Scholar] [CrossRef]
- Ohira, T.; Arita, M.; Omori, K.; Recchiuti, A.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2010, 285, 3451–3461. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Faibish, D.; Fredman, G.; Herrera, B.S.; Chiang, N.; Serhan, C.N.; Van Dyke, T.E.; Gyurko, R. Resolvin E1 and chemokine-like receptor 1 mediate bone preservation. J. Immunol. 2013, 190, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, M.; Jordan, P.M.; Romp, E.; Czapka, A.; Rao, Z.; Kretzer, C.; Koeberle, A.; Garscha, U.; Pace, S.; Claesson, H.E.; et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019, 33, 6140–6153. [Google Scholar] [CrossRef] [PubMed]
- Kelley, W.J.; Fromen, C.A.; Lopez-Cazares, G.; Eniola-Adefeso, O. PEGylation of model drug carriers enhances phagocytosis by primary human neutrophils. Acta Biomater. 2018, 79, 283–293. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, P.; Gao, J.; Wang, Z. Liposomal Formulations Enhance the Anti-Inflammatory Effect of Eicosapentaenoic Acid in HL60 Cells. Pharmaceutics 2022, 14, 520. https://doi.org/10.3390/pharmaceutics14030520
Kaur P, Gao J, Wang Z. Liposomal Formulations Enhance the Anti-Inflammatory Effect of Eicosapentaenoic Acid in HL60 Cells. Pharmaceutics. 2022; 14(3):520. https://doi.org/10.3390/pharmaceutics14030520
Chicago/Turabian StyleKaur, Puneet, Jin Gao, and Zhenjia Wang. 2022. "Liposomal Formulations Enhance the Anti-Inflammatory Effect of Eicosapentaenoic Acid in HL60 Cells" Pharmaceutics 14, no. 3: 520. https://doi.org/10.3390/pharmaceutics14030520





