Pleiotropic Long-Term Effects of Atorvastatin on Posttraumatic Joint Contracture in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
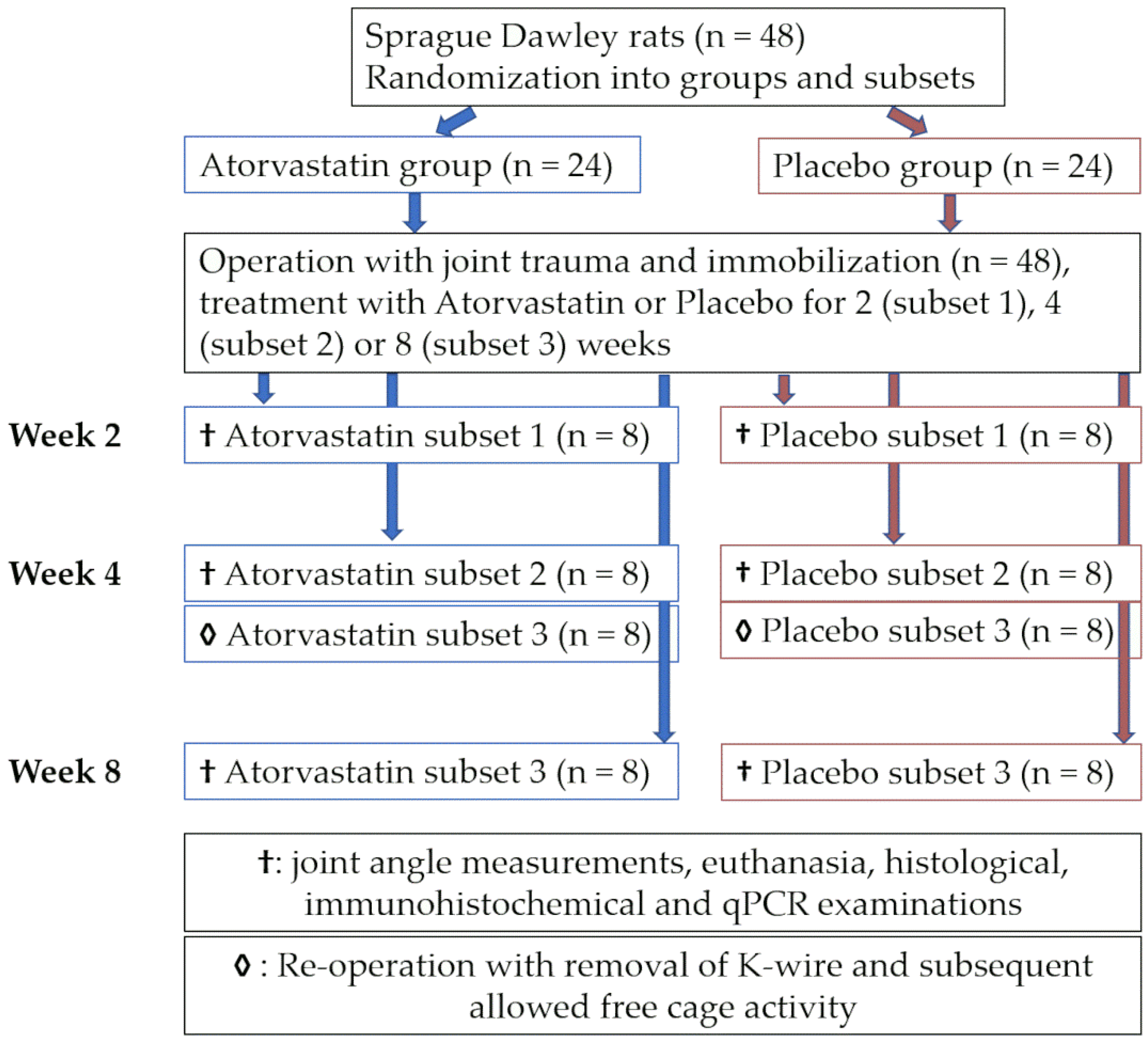
2.1. Animals and Study Design
2.2. PTJS Model and Surgical Procedure
2.3. Knee Joint Angle Measurements
2.4. Tissue Preparation
2.5. Tissue Morphometrics and Histology
2.6. Tissue Preparation and Quantitative PCR
2.7. Statistical Analysis
3. Results
3.1. Perioperative Weight Development and Complications
3.2. Development of Posttraumatic Joint Contracture
3.2.1. Overall Joint Contracture
3.2.2. Myogenic Component of Joint Contracture
3.2.3. Arthrogenic Component of Joint Contracture
3.3. Histological Changes in the Joint Capsule
3.3.1. Joint Capsule Length and Diameter
3.3.2. Numerical Proportion of Effector Cells and Collagen Deposition
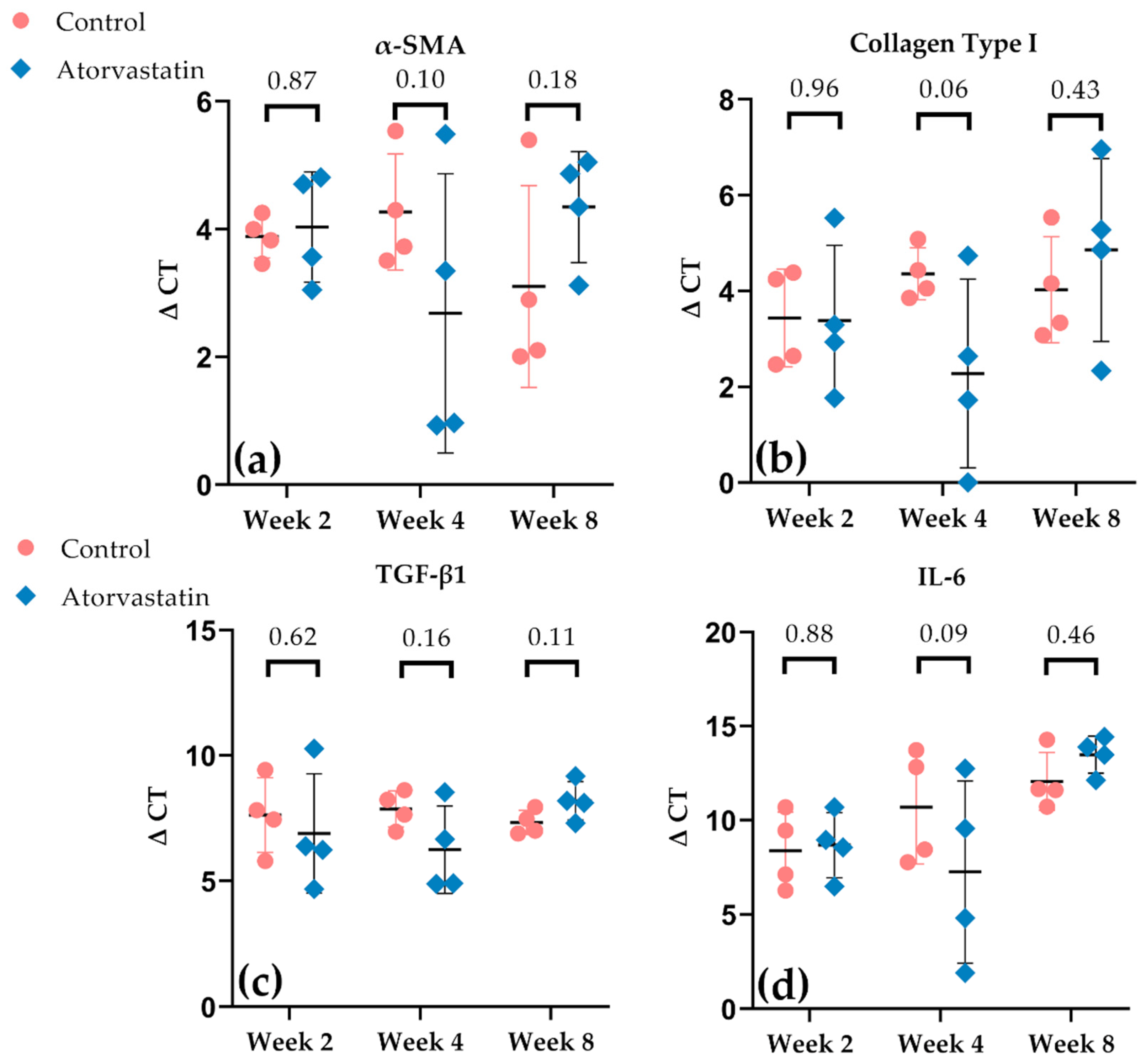
3.4. Alterations in Gene Expression in the Joint Capsule
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-smooth muscle actin |
| ΔCt | Delta cycle threshold |
| ANOVA | Analysis of variance |
| BSA | Bovine serum albumin |
| cDNA | Complementary deoxyribonucleic acid |
| CO2 | Carbon dioxide |
| COL-1A1 | Alpha-1 type I collagen |
| DAB | 3,3′-Diaminobenzidine |
| ECM | Extracellular matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| EtOH | Ethanol alcohol |
| FPD | Fibroproliferative disorder |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| H&E | Hemotoxylin and Eosin |
| HMGCR | 3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| HRP | Horseradish peroxidase |
| IL | Interleukin |
| K-wire | Kirschner wire |
| NK-1 | Neurokinin 1 |
| n.s. | Not significant |
| PBS | Phosphate-buffered saline |
| PJTS | Posttraumatic joint stiffness |
| qPCR | Quantitative polymerase chain reaction |
| Ras | Rat sarcoma |
| RNA | Ribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| ROM | Range of motion |
| SD | Standard deviation |
| TGF-ß1 | Transforming growth factor beta 1 |
| TRIS | Tris(hydroxymethyl)aminomethane |
References
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Trudel, G.; Laneuville, O. Noninflammatory Joint Contractures Arising from Immobility: Animal Models to Future Treatments. BioMed Res. Int. 2015, 2015, 848290. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.O.; Nazarian, A.; Rodriguez, E.K. Clinical Management of Arthrofibrosis: State of the Art and Therapeutic Outlook. JBJS Rev. 2020, 8, e1900223. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell 2003, 14, 2508–2519. [Google Scholar] [CrossRef]
- Sandbo, N.; Dulin, N. Actin cytoskeleton in myofibroblast differentiation: Ultrastructure defining form and driving function. Transl. Res. 2011, 158, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmouliere, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef]
- Hinz, B.; Suki, B. Does Breathing Amplify Fibrosis? Am. J. Respir. Crit. Care Med. 2016, 194, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Abdel, M.P.; Morrey, M.E.; Barlow, J.D.; Kreofsky, C.R.; An, K.N.; Steinmann, S.P.; Morrey, B.F.; Sanchez-Sotelo, J. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J. Orthop. Res. 2012, 30, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, K.A.; Zhang, M.; van Snellenberg, W.; King, G.J.; Hart, D.A. Myofibroblast numbers are elevated in human elbow capsules after trauma. Clin. Orthop. Relat. Res. 2004, 419, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kis, K.; Liu, X.; Hagood, J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev. Mol. Med. 2011, 13, e27. [Google Scholar] [CrossRef] [PubMed]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W.; Humphreys, B.D.; Bellusci, S. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem. Cell 2017, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef] [PubMed]
- Usher, K.M.; Zhu, S.; Mavropalias, G.; Carrino, J.A.; Zhao, J.; Xu, J. Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res. 2019, 7, 9. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.P.; McTavish, D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs 1997, 53, 828–847. [Google Scholar] [CrossRef]
- Tulbah, A.S. The potential of Atorvastatin for chronic lung diseases therapy. Saudi. Pharm. J. 2020, 28, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Ghorbanihaghjo, A.; Argani, H. The balance between induction and inhibition of mevalonate pathway regulates cancer suppression by statins: A review of molecular mechanisms. Chem. Biol. Interact. 2017, 273, 273–285. [Google Scholar] [CrossRef]
- Kavalipati, N.; Shah, J.; Ramakrishan, A.; Vasnawala, H. Pleiotropic effects of statins. Indian J. Endocrinol. Metab. 2015, 19, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Klosel, J.; Schierwagen, R.; Korner, C.; Granzow, M.; Huss, S.; Mazar, I.G.; Weber, S.; van den Ven, P.F.; Pieper-Furst, U.; et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab. Investig. 2012, 92, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Kayalar, O.; Atahan, E.; Oztay, F. Anti-fibrotic effect of Atorvastatin on the lung fibroblasts and myofibroblasts. Eur. Respir. J. 2018, 52, PA991. [Google Scholar] [CrossRef]
- Baranowski, A.; Schlemmer, L.; Forster, K.; Mattyasovszky, S.G.; Ritz, U.; Wagner, D.; Rommens, P.M.; Hofmann, A. A novel rat model of stable posttraumatic joint stiffness of the knee. J. Orthop. Surg. Res. 2018, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Hennenberg, M.; Laleman, W.; Shelest, N.; Biecker, E.; Schepke, M.; Nevens, F.; Sauerbruch, T.; Heller, J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology 2007, 46, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Schlemmer, L.; Forster, K.; Slotina, E.; Mickan, T.; Truffel, S.; Klein, A.; Mattyasovszky, S.G.; Hofmann, A.; Ritz, U.; et al. Effects of losartan and atorvastatin on the development of early posttraumatic joint stiffness in a rat model. Drug Des. Devel. Ther. 2019, 13, 2603–2618. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hino, K.; Kutsuna, T.; Watamori, K.; Tsuda, T.; Miura, H. Efficacy of posterior capsular release for flexion contracture in posterior-stabilized total knee arthroplasty. J. Exp. Orthop. 2021, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Lobenhoffer, H.P.; Bosch, U.; Gerich, T.G. Role of posterior capsulotomy for the treatment of extension deficits of the knee. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 237–241. [Google Scholar] [CrossRef]
- Trudel, G.; Jabi, M.; Uhthoff, H.K. Localized and adaptive synoviocyte proliferation characteristics in rat knee joint contractures secondary to immobility. Arch. Phys. Med. Rehabil. 2003, 84, 1350–1356. [Google Scholar] [CrossRef]
- Onoda, Y.; Hagiwara, Y.; Ando, A.; Watanabe, T.; Chimoto, E.; Suda, H.; Yabe, Y.; Saijo, Y.; Itoi, E. Joint haemorrhage partly accelerated immobilization-induced synovial adhesions and capsular shortening in rats. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Dahners, L.E. An examination of the mechanism of ligament contracture. Clin. Orthop. Relat. Res. 1988, 227, 286–291. [Google Scholar]
- Dunham, C.L.; Castile, R.M.; Chamberlain, A.M.; Lake, S.P. The Role of Periarticular Soft Tissues in Persistent Motion Loss in a Rat Model of Posttraumatic Elbow Contracture. J. Bone Jt. Surg. Am. 2019, 101, e17. [Google Scholar] [CrossRef] [PubMed]
- Trudel, G.; Uhthoff, H.K. Contractures secondary to immobility: Is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch. Phys. Med. Rehabil. 2000, 81, 6–13. [Google Scholar] [CrossRef]
- Trudel, G.; Uhthoff, H.K.; Goudreau, L.; Laneuville, O. Quantitative analysis of the reversibility of knee flexion contractures with time: An experimental study using the rat model. BMC Musculoskelet Disord. 2014, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Trudel, G.; Laneuville, O.; Coletta, E.; Goudreau, L.; Uhthoff, H.K. Quantitative and temporal differential recovery of articular and muscular limitations of knee joint contractures; results in a rat model. J. Appl. Physiol. 2014, 117, 730–737. [Google Scholar] [CrossRef]
- Abdel-Sattar, A.R.; Abo-Saif, A.A.; Aboyoussef, A.M. Nicorandil and atorvastatin attenuate carbon tetrachloride-induced liver fibrosis in rats. Immunopharmacol. Immunotoxicol. 2020, 42, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Guo, B.; Xue, L.; Wang, L. Atorvastatin Prevents Myocardial Fibrosis in Spontaneous Hypertension via Interleukin-6 (IL-6)/Signal Transducer and Activator of Transcription 3 (STAT3)/Endothelin-1 (ET-1) Pathway. Med. Sci. Monit. 2019, 25, 318–323. [Google Scholar] [CrossRef]
- Fitzgerald, J.P.; Chou, S.Y.; Franco, I.; Mooppan, U.M.; Kim, H.; Saini, R.; Gulmi, F.A. Atorvastatin ameliorates tubulointerstitial fibrosis and protects renal function in chronic partial ureteral obstruction cases. J. Urol. 2009, 182, 1860–1868. [Google Scholar] [CrossRef]
- Khodayar, M.J.; Kiani, M.; Hemmati, A.A.; Rezaie, A.; Zerafatfard, M.R.; Rashidi Nooshabadi, M.R.; Goudarzi, M. The preventive effect of atorvastatin on paraquat-induced pulmonary fibrosis in the rats. Adv. Pharm. Bull. 2014, 4, 345–349. [Google Scholar] [CrossRef]
- Sun, Y.; Li, F.; Fan, C. Effect of pERK2 on extracellular matrix turnover of the fibrotic joint capsule in a post-traumatic joint contracture model. Exp. Ther. Med. 2016, 11, 547–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, D.; Mi, J.; Pan, X.; Li, F.; Rui, Y. Suppression of TGF-beta activity with remobilization attenuates immobilization-induced joint contracture in rats. Injury 2021, 52, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, J.; Kaneguchi, A.; Tanaka, R.; Kito, N.; Moriyama, H. Cyclooxygenase-2 inhibitor celecoxib attenuates joint contracture following immobilization in rat knees. BMC Musculoskelet. Disord. 2016, 17, 446. [Google Scholar] [CrossRef] [PubMed]
- Steplewski, A.; Fertala, J.; Beredjiklian, P.K.; Abboud, J.A.; Wang, M.L.Y.; Namdari, S.; Barlow, J.; Rivlin, M.; Arnold, W.V.; Kostas, J.; et al. Blocking collagen fibril formation in injured knees reduces flexion contracture in a rabbit model. J. Orthop. Res. 2017, 35, 1038–1046. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Wu, J.X.; Liu, W.B.; Sun, J.K. Reduction of adhesion formation after knee surgery in a rat model by botulinum toxin A. Biosci. Rep. 2017, 37, BSR20160460. [Google Scholar] [CrossRef]
- Aaboud, M.; Aad, G.; Abbott, B.; Abdinov, O.; Abeloos, B.; Abhayasinghe, D.K.; Abidi, S.H.; AbouZeid, O.S.; Abraham, N.L.; Abramowicz, H.; et al. Combination of the Searches for Pair-Produced Vectorlike Partners of the Third-Generation Quarks at sqrt[s]=13 TeV with the ATLAS Detector. Phys. Rev. Lett. 2018, 121, 211801. [Google Scholar] [CrossRef]
- Efird, W.; Kellam, P.; Yeazell, S.; Weinhold, P.; Dahners, L.E. An evaluation of prophylactic treatments to prevent post traumatic joint stiffness. J. Orthop. Res. 2014, 32, 1520–1524. [Google Scholar] [CrossRef]
- Li, F.; He, B.; Liu, S.; Fan, C. Celecoxib effectively inhibits the formation of joint adhesions. Exp. Ther. Med. 2013, 6, 1507–1511. [Google Scholar] [CrossRef]
- Morrey, M.E.; Abdel, M.P.; Riester, S.M.; Dudakovic, A.; van Wijnen, A.J.; Morrey, B.F.; Sanchez-Sotelo, J. Molecular landscape of arthrofibrosis: Microarray and bioinformatic analysis of the temporal expression of 380 genes during contracture genesis. Gene 2017, 610, 15–23. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Robbins, S.L.S.L.; Cotran, R.S. 1932–2000 Robbins pathologic basis of disease. In Robbins and Cotran Pathologic Basis of Disease, 7th ed.; Vinay, K., Abul, K., Abbas, N.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Monument, M.J.; Hart, D.A.; Salo, P.T.; Befus, A.D.; Hildebrand, K.A. Posttraumatic elbow contractures: Targeting neuroinflammatory fibrogenic mechanisms. J. Orthop. Sci. 2013, 18, 869–877. [Google Scholar] [CrossRef]
- Hildebrand, K.A.; Zhang, M.; Germscheid, N.M.; Wang, C.; Hart, D.A. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop. 2008, 79, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Morrey, M.E.; Sanchez-Sotelo, J.; Lewallen, E.A.; An, K.N.; Grill, D.E.; Steinmann, S.P.; Yao, J.J.; Salib, C.G.; Trousdale, W.H.; Reina, N.; et al. Intra-articular injection of a substance P inhibitor affects gene expression in a joint contracture model. J. Cell Biochem. 2018, 119, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.R.; Dagneaux, L.; Limberg, A.K.; Bettencourt, J.W.; Bayram, B.; Bolon, B.; Berry, D.J.; Morrey, M.E.; Sanchez-Sotelo, J.; van Wijnen, A.J.; et al. Biomechanical, histological, and molecular characterization of a new posttraumatic model of arthrofibrosis in rats. J. Orthop. Res. 2022, 40, 323–337. [Google Scholar] [CrossRef] [PubMed]







| Gene | Sequence | Primer |
|---|---|---|
| GAPDH | Forward | AACGACCCCTTCATTGACCT |
| Reverse | CCCCATTTGATGTTAGCGGG | |
| TGF-β1 | Forward | CCCTACATTTGGAGCCTGGA |
| Reverse | CGCACGATCATGTTGGACAA | |
| IL-6 | Forward | CCACCCACAACAGACCAGTA |
| Reverse | ACTCCAGAAGACCAGAGCAG | |
| α-SMA | Forward | CATCATGCGTCTGGACTTGG |
| Reverse | CCAGGGAAGAAGAGGAAGCA | |
| Collagen type I | Forward | CCCCAAATGCTGCCTTTTCT |
| Reverse | CTGGGTAGGGAAGTAGGCTG |
| Time Since Operation | Capsular Length, Superior (in mm) | Capsular Length, Inferior (in mm) | Capsular Diameter (in mm) | |||
|---|---|---|---|---|---|---|
| Atorvastatin | Control | Atorvastatin | Control | Atorvastatin | Control | |
| Week 2 | 2.0 ± 0.9 | 1.7 ± 1.6 | 0.9 ± 0.9 | 1.1 ± 0.4 | 0.7 ± 0.1 | 0.8 ± 0.6 |
| Week 4 | 2.2 ± 0.2 | 2.2 ± 0.7 | 1.6 ± 0.8 | 1.9 ± 1.3 | 1.3 ± 0.3 | 1.0 ± 0.2 |
| Week 8 | 3.0 ± 1.0 | 2.7 ± 0.6 | 2.5 ± 1.9 | 4.1 ± 2.1 | 1.3 ± 0.2 | 1.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegner, E.; Slotina, E.; Mickan, T.; Truffel, S.; Arand, C.; Wagner, D.; Ritz, U.; Rommens, P.M.; Gercek, E.; Drees, P.; et al. Pleiotropic Long-Term Effects of Atorvastatin on Posttraumatic Joint Contracture in a Rat Model. Pharmaceutics 2022, 14, 523. https://doi.org/10.3390/pharmaceutics14030523
Wegner E, Slotina E, Mickan T, Truffel S, Arand C, Wagner D, Ritz U, Rommens PM, Gercek E, Drees P, et al. Pleiotropic Long-Term Effects of Atorvastatin on Posttraumatic Joint Contracture in a Rat Model. Pharmaceutics. 2022; 14(3):523. https://doi.org/10.3390/pharmaceutics14030523
Chicago/Turabian StyleWegner, Erik, Ekaterina Slotina, Tim Mickan, Sebastian Truffel, Charlotte Arand, Daniel Wagner, Ulrike Ritz, Pol M. Rommens, Erol Gercek, Philipp Drees, and et al. 2022. "Pleiotropic Long-Term Effects of Atorvastatin on Posttraumatic Joint Contracture in a Rat Model" Pharmaceutics 14, no. 3: 523. https://doi.org/10.3390/pharmaceutics14030523
APA StyleWegner, E., Slotina, E., Mickan, T., Truffel, S., Arand, C., Wagner, D., Ritz, U., Rommens, P. M., Gercek, E., Drees, P., & Baranowski, A. (2022). Pleiotropic Long-Term Effects of Atorvastatin on Posttraumatic Joint Contracture in a Rat Model. Pharmaceutics, 14(3), 523. https://doi.org/10.3390/pharmaceutics14030523






