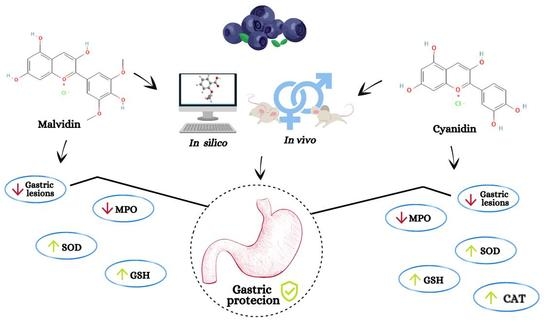
Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vivo Analysis
2.2.1. Animals
2.2.2. Groups and Doses Used
2.2.3. Bilateral Ovariectomy
2.2.4. Hormonal Supplementation
2.2.5. Gastric Ulcer Induced by Absolute Ethanol
2.2.6. Quantification of Ulcerative Lesions
2.2.7. Determination of Myeloperoxidase (MPO) Activity
2.2.8. Determination of Reduced Glutathione (GSH) Level
2.2.9. Determination of Superoxide Dismutase (SOD) Activity
2.2.10. Determination of Catalase (CAT) Activity
2.3. In Silico Analysis
2.3.1. Simulation of the Pharmacokinetic Profile Using the SwissADME Server
2.3.2. Simulation of Pharmacokinetic Property Prediction Using the pkCSM Server
2.3.3. Simulation of Toxic Substructure Using eMolTox Server
2.3.4. Simulation of the Probable Biological Targets Using the SwissTargetPrediction Server
2.3.5. Simulation of Molecular Docking Using the Achilles Blind Docking Server
2.4. Statistical Analysis
3. Results
3.1. In Vivo
3.1.1. Absolute Ethanol-Induced Gastric Lesions
3.1.2. Malvidin and Cyanidin Showed Anti-Inflammatory Activity in Stomach Samples of Ethanol-Treated Mice
3.1.3. Anthocyanidins Showed Antioxidant Activity in Stomach Samples of Ethanol-Treated Mice
SOD Activity Analysis
CAT Activity Analysis
Analysis of GSH Level
3.2. In Silico
3.2.1. Pharmacokinetic Profile of Malvidin and Cyanidin Chloride
3.2.2. Investigation of Molecular Interactions of Malvidin and Cyanidin on Various Biological Targets
3.2.3. Superoxide Dismutase as a Biological Target
3.2.4. Catalase as a Biological Target
3.2.5. Reduced Glutathione as a Biological Target
3.2.6. Myeloperoxidase as a Biological Target
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Serafim, C.; Araruna, M.E.; Junior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A review of the role of flavonoids in peptic ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.L.; Emilio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; Rocha, L.; Batista, L.M.; Hiruma-Lima, C.A. Systematic analysis of monoterpenes: Advances and challenges in the treatment of peptic ulcer diseases. Biomolecules 2020, 10, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galura, G.M.; Chavez, L.O.; Robles, A.; McCallum, R. Gastroduodenal injury: Role of protective factors. Curr. Gastroenterol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Garcia Rodriguez, L.A.; Masso-Gonzalez, E.L.; Hernandez-Diaz, S. Uncomplicated peptic ulcer in the UK: Trends from 1997 to 2005. Aliment. Pharmacol. Ther. 2009, 30, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- Pace, S.; Rossi, A.; Krauth, V.; Dehm, F.; Troisi, F.; Bilancia, R.; Weinigel, C.; Rummler, S.; Werz, O.; Sautebin, L. Sex differences in prostaglandin biosynthesis in neutrophils during acute inflammation. Sci. Rep. 2017, 7, 3759. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.L.; Rodrigues, V.P.; Ohara, R.; Bueno, G.; Nunes, V.V.A.; Dos Santos, R.C.; Camargo, A.C.L.; Justulin Junior, L.A.; de Andrade, S.F.; Steimbach, V.M.B.; et al. Sex-specific effects of Eugenia punicifolia extract on gastric ulcer healing in rats. World J. Gastroenterol. 2018, 24, 4369–4383. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Chan, F.K.; McColl, K.E. Peptic ulcer disease. Lancet 2009, 374, 1449–1461. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chan, E.W.; Wong, A.Y.S.; Chen, L.; Wong, I.C.K.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiriac, S.A.; Ciobica, A.; Boiculese, L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Silvan, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombo, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Fukuda, T.; Arakawa, T.; Shimizu, Y.; Ohtani, K.; Higuchi, K.; Kobayashi, K. Effects of lansoprazole on ethanol-induced injury and PG synthetic activity in rat gastric mucosa. J. Clin. Gastroenterol. 1995, 20 (Suppl. S2), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Delgobo, M.; Agnes, J.P.; Goncalves, R.M.; Dos Santos, V.W.; Parisotto, E.B.; Zamoner, A.; Zanotto-Filho, A. N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J. Nutr. Biochem. 2019, 67, 190–200. [Google Scholar] [CrossRef]
- Gaertner, D.J. Anesthesia and analgesia for laboratory rodents. Anesth. Analg. Lab. Anim. 2008, 60, 240–297. [Google Scholar]
- Matsumoto, Y.K.; Kasai, M.; Tomihara, K. The enhancement effect of estradiol on contextual fear conditioning in female mice. PLoS ONE 2018, 13, e0197441. [Google Scholar] [CrossRef] [PubMed]
- Yasrebi, A.; Rivera, J.A.; Krumm, E.A.; Yang, J.A.; Roepke, T.A. Activation of estrogen response element-independent ERalpha signaling protects female mice from diet-induced obesity. Endocrinology 2017, 158, 319–334. [Google Scholar] [CrossRef]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Hanchar, A.J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 1979, 77, 433–443. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.-L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems; Favier, A.E., Cadet, J., Kalyanaraman, B., Fontecave, M., Pierre, J.L., Eds.; Birkhäuser Basel: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Winterbourn, C.C.; Hawkins, R.E.; Brian, M.; Carrell, R.W. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337–341. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Ji, C.; Svensson, F.; Zoufir, A.; Bender, A. eMolTox: Prediction of molecular toxicity with confidence. Bioinformatics 2018, 34, 2508–2509. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Davies, M.; Nowotka, M.; Papadatos, G.; Dedman, N.; Gaulton, A.; Atkinson, F.; Bellis, L.; Overington, J.P. ChEMBL web services: Streamlining access to drug discovery data and utilities. Nucleic Acids Res. 2015, 43, W612–W620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrian-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Jupp, S.; Malone, J.; Bolleman, J.; Brandizi, M.; Davies, M.; Garcia, L.; Gaulton, A.; Gehant, S.; Laibe, C.; Redaschi, N.; et al. The EBI RDF platform: Linked open data for the life sciences. Bioinformatics 2014, 30, 1338–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Linares, I.; Perez-Sanchez, H.; Cecilia, J.M.; Garcia, J.M. High-throughput parallel blind virtual screening using BINDSURF. BMC Bioinform. 2012, 13 (Suppl. S14), S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Hasanzadeh, M.; Jabbari, F.; Farkhondeh, T.; Samini, M. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn. Mag. 2016, 12, S436–S440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.P.; Bajpai, A.; Chandra, S. A comprehensive review on the screening models for the pharmacological assessment of antiulcer drugs. Curr. Clin. Pharmacol. 2019, 14, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, S.; Brzozowski, T.; Konturek, S.J. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J. Physiol. Pharmacol. 2002, 53, 39–50. [Google Scholar] [PubMed]
- Bafna, P.A.; Balaraman, R. Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J. Ethnopharmacol. 2004, 90, 123–127. [Google Scholar] [CrossRef]
- Al-Howiriny, T.; Alsheikh, A.; Alqasoumi, S.; Al-Yahya, M.; ElTahir, K.; Rafatullah, S. Protective effect of origanum majorana L. ’marjoram’ on various models of gastric mucosal injury in rats. Am. J. Chin. Med. 2009, 37, 531–545. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A.; Jones, M.K. The mechanisms of gastric mucosal injury: Focus on microvascular endothelium as a key target. Curr. Med. Chem. 2012, 19, 4–15. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Czimmer, J.; Debreceni, A.; Szolcsanyi, J.; Mozsik, G. Gastric mucosal integrity: Gastric mucosal blood flow and microcirculation. An overview. J. Physiol. Paris 2001, 95, 105–127. [Google Scholar] [CrossRef]
- Siegmund, E.; Weber, H.; Kasper, M.; Jonas, L. Role of PGE2 in the development of pancreatic injury induced by chronic alcohol feeding in rats. Pancreatology 2003, 3, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M.; et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Tuo, B.G.; Wu, W.M.; Gao, Y.; Xu, Q.Q.; Zhao, K. Prevalence of peptic ulcer in dyspeptic patients and the influence of age, sex, and Helicobacter pylori infection. Dig. Dis. Sci. 2008, 53, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Kekki, M.; Sipponen, P.; Siurala, M.; Laszewicz, W. Peptic ulcer and chronic gastritis: Their relation to age and sex, and to location of ulcer and gastritis. Gastroenterol. Clin. Biol. 1990, 14, 217–223. [Google Scholar]
- Smith, A.; Contreras, C.; Ko, K.H.; Chow, J.; Dong, X.; Tuo, B.; Zhang, H.H.; Chen, D.B.; Dong, H. Gender-specific protection of estrogen against gastric acid-induced duodenal injury: Stimulation of duodenal mucosal bicarbonate secretion. Endocrinology 2008, 149, 4554–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houdeau, E. Chapter 8-Sex differences in gastrointestinal physiology and diseases: From endogenous sex hormones to environmental endocrine disruptor agents. In Sex Differences in Physiology; Neigh, G.N., Mitzelfelt, M.M., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 125–143. [Google Scholar]
- La Casa, C.; Villegas, I.; Alarcon de la Lastra, C.; Motilva, V.; Martin Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Takeuchi, K.; Ueshima, K.; Hironaka, Y.; Fujioka, Y.; Matsumoto, J.; Okabe, S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion 1991, 49, 175–184. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ma, L. Inflammatory mediators in gastrointestinal defense and injury. Exp. Biol. Med. 2001, 226, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liao, H.; Liu, Y.; Zheng, Y.; Wu, X.; Su, Z.; Zhang, X.; Lai, Z.; Lai, X.; Lin, Z.X.; et al. Protective effects of pogostone from Pogostemonis Herba against ethanol-induced gastric ulcer in rats. Fitoterapia 2015, 100, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhao, X.; Qian, Y.; Sun, P.; Zhu, K.; Li, J.; Sun, B. Lactobacillus fermentum Suo attenuates HCl/Ethanol induced gastric injury in mice through its antioxidant effects. Nutrients 2016, 8, 155. [Google Scholar] [CrossRef]
- Glavin, G.B.; Szabo, S. Experimental gastric mucosal injury: Laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J. 1992, 6, 825–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Abdulla, M.A.; Hajrezaie, M.; Bader, A.; Shahzad, N.; Al-Ghamdi, S.S.; Gushash, A.S.; Hasanpourghadi, M. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug Des. Devel. Ther. 2016, 10, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Meister, A. Metabolism and function of glutathione: An overview. Biochem. Soc. Trans. 1982, 10, 78–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.T.; Li, J.; Chen, Y.; Yang, C.M.; Tang, H.L.; Wang, J.C. Effects of glutathione on plasma heat shock protein 70 of acute gastric mucosal injury in rats exposed to positive acceleration. Zhonghua Yi Xue Za Zhi 2013, 93, 3708–3710. [Google Scholar] [PubMed]
- Speir, E.; Yu, Z.X.; Takeda, K.; Ferrans, V.J.; Cannon, R.O., 3rd. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation 2000, 102, 2990–2996. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Inamori, M.; Imajyo, K.; Chiba, H.; Nonaka, T.; Shiba, T.; Sakaguchi, T.; Atsukawa, K.; Takahashi, H.; Hoshino, E.; et al. Gender differences of low-dose aspirin-associated gastroduodenal ulcer in Japanese patients. World J. Gastroenterol. 2010, 16, 1896–1900. [Google Scholar] [CrossRef]
- Boeckxstaens, G.; El-Serag, H.B.; Smout, A.J.; Kahrilas, P.J. Symptomatic reflux disease: The present, the past and the future. Gut 2014, 63, 1185–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, K.; Shimosegawa, T. Involvement of luminal nitric oxide in the pathogenesis of the gastroesophageal reflux disease spectrum. J. Gastroenterol. Hepatol. 2014, 29, 898–905. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Lindh, A.; Carlstrom, K.; Eklund, J.; Wilking, N. Serum steroids and prolactin during and after major surgical trauma. Acta Anaesthesiol. Scand. 1992, 36, 119–124. [Google Scholar] [CrossRef]
- Ng, K.Y.; Yong, J.; Chakraborty, T.R. Estrous cycle in ob/ob and ovariectomized female mice and its relation with estrogen and leptin. Physiol. Behav. 2010, 99, 125–130. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A-4I. [Google Scholar] [CrossRef] [Green Version]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [Green Version]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarricueta, M.L.; Fagundes, F.L.; Pereira, Q.C.; Pantaleão, S.Q.; Santos, R.d.C.d. Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach. Pharmaceutics 2022, 14, 565. https://doi.org/10.3390/pharmaceutics14030565
Zarricueta ML, Fagundes FL, Pereira QC, Pantaleão SQ, Santos RdCd. Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach. Pharmaceutics. 2022; 14(3):565. https://doi.org/10.3390/pharmaceutics14030565
Chicago/Turabian StyleZarricueta, Melina Luzzi, Felipe Leonardo Fagundes, Quélita Cristina Pereira, Simone Queiroz Pantaleão, and Raquel de Cássia dos Santos. 2022. "Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach" Pharmaceutics 14, no. 3: 565. https://doi.org/10.3390/pharmaceutics14030565
APA StyleZarricueta, M. L., Fagundes, F. L., Pereira, Q. C., Pantaleão, S. Q., & Santos, R. d. C. d. (2022). Relationship between Hormonal Modulation and Gastroprotective Activity of Malvidin and Cyanidin Chloride: In Vivo and In Silico Approach. Pharmaceutics, 14(3), 565. https://doi.org/10.3390/pharmaceutics14030565