Mixed Polymeric Micelles for Rapamycin Skin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymeric Micelles and Hydrogel Preparation
2.1.1. Preparation of Rapamycin Micelle Solution
2.1.2. Preparation of Polymer Micelle-Based Hydrogel Containing Rapamycin
2.2. HPLC for Rapamycin and Seco Rapamycin Determination in Micelles
2.3. Micelle Solution: Influence of the Polymeric Composition on Different Parameters Monitored
2.3.1. Design of Experiments to Assess the Influence of the Type of Polymer Used
2.3.2. DoE to Assess the Influence of the Polymer Amount (Drug-to-Polymer Ratio)
2.3.3. Morphology, Size, Polydispersity Index and Zeta Potential Determination
2.3.4. Drug Entrapment Efficiency (EE%) and Drug Loading (DL%) Determination
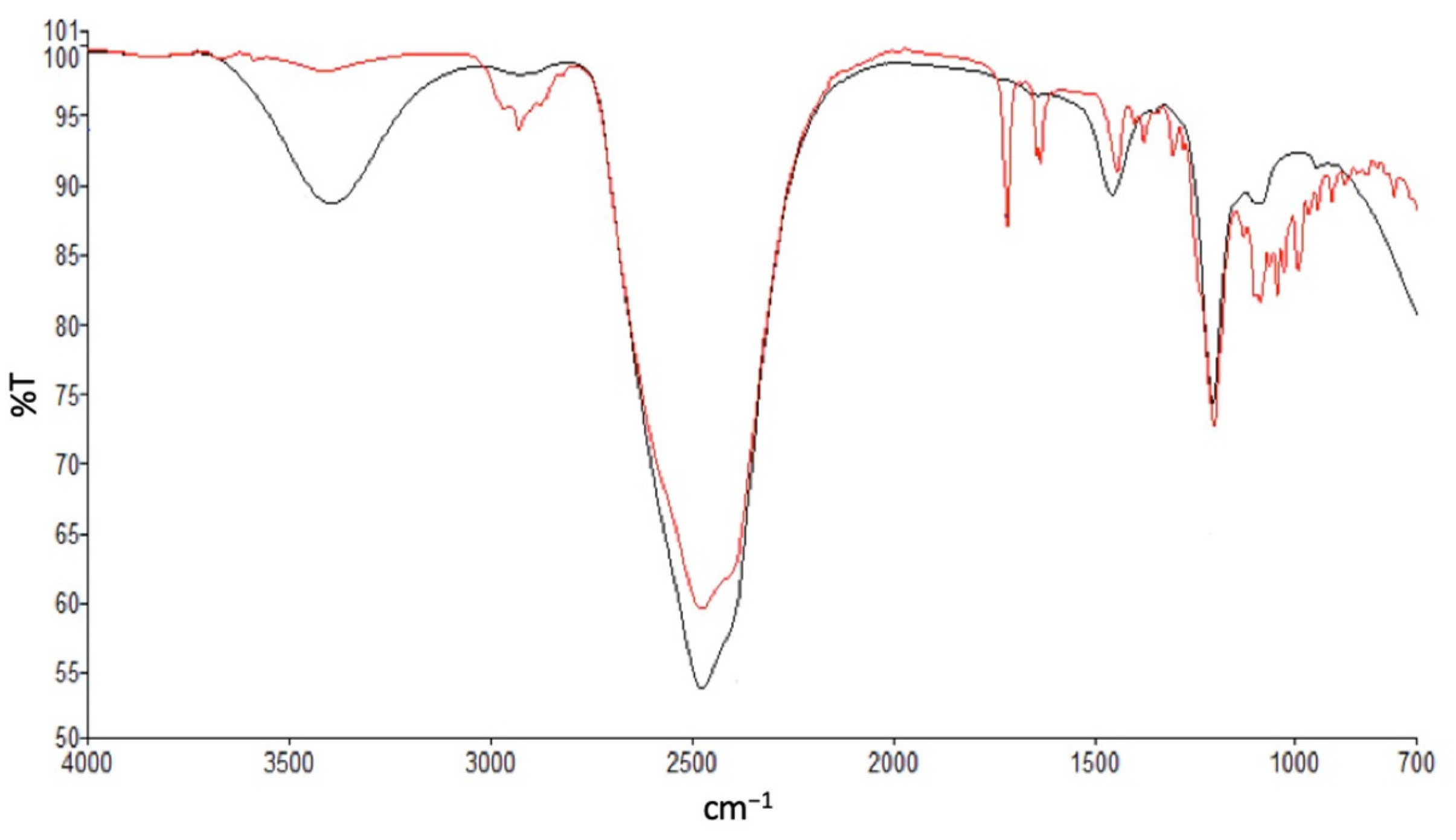
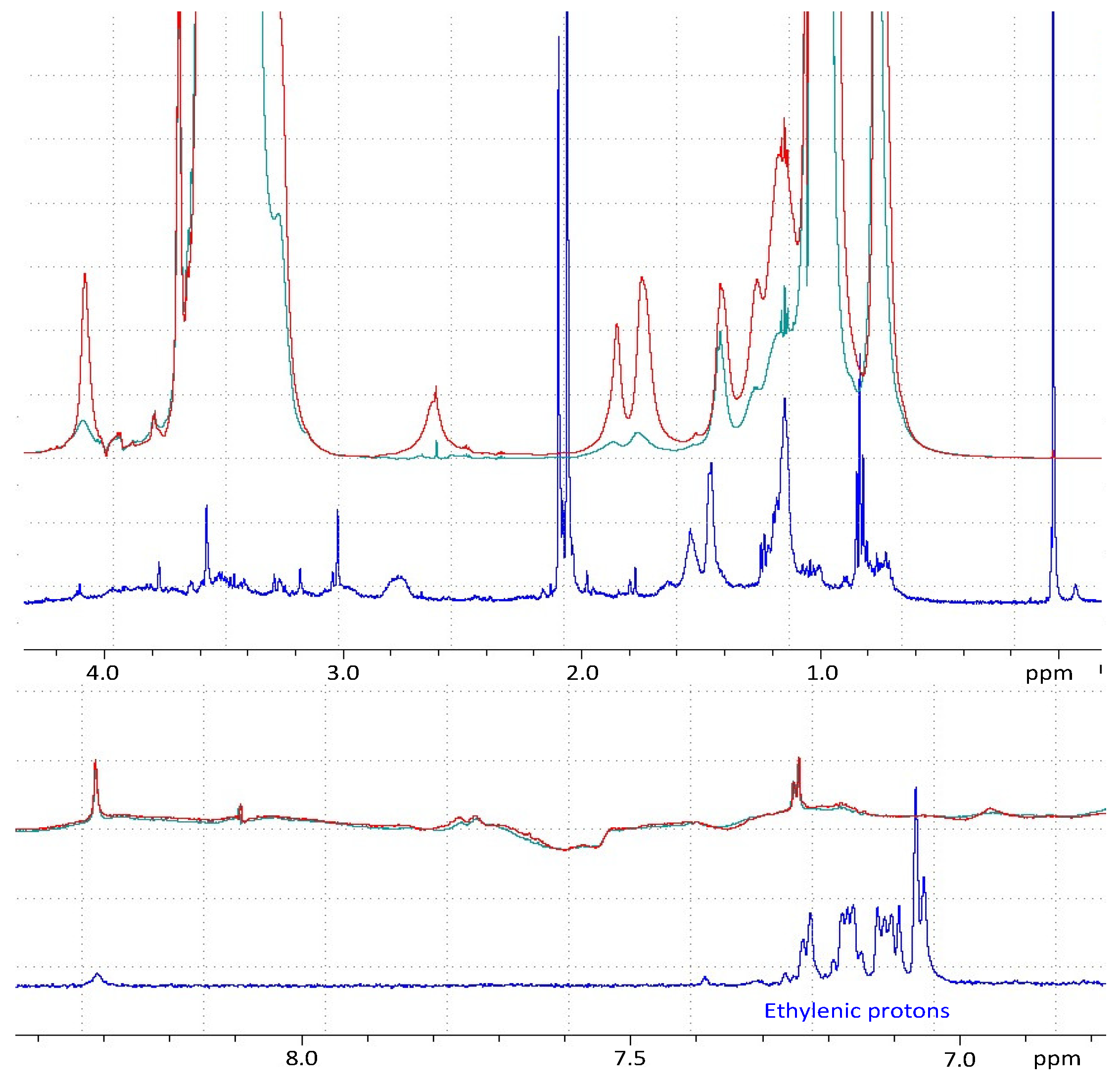
2.3.5. Study of Drug–Polymer Interactions/Inclusion by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Nuclear Magnetic Resonance (NMR)
Fourier Transform Infrared Spectroscopy (FTIR)
Nuclear Magnetic Resonance (NMR)
2.3.6. In Vitro Flux Determination
2.3.7. Ex Vivo Permeation Study on Micelle Hydrogel
2.4. Accelerated and Long-Term Stability Studies of Micelle Solution and Hydrogel
3. Results
3.1. Micelle Optimization and Characterization
3.1.1. Spectral Highlights of Rapamycin-Loading Micelles
3.1.2. Influence of Polymer Composition
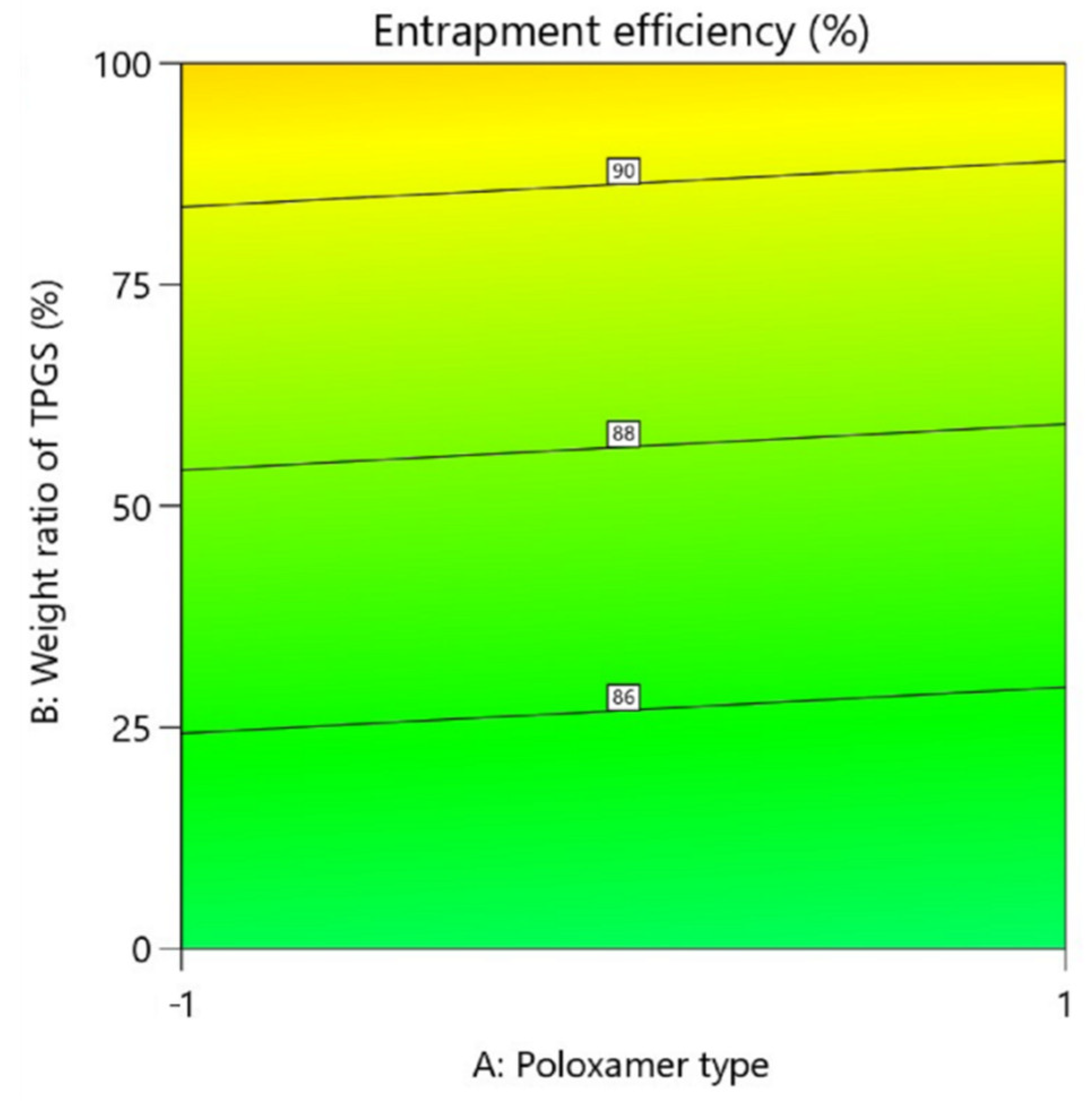
On the EE%
On the Micelle Size and Polydispersity Index
On the Zeta Potential
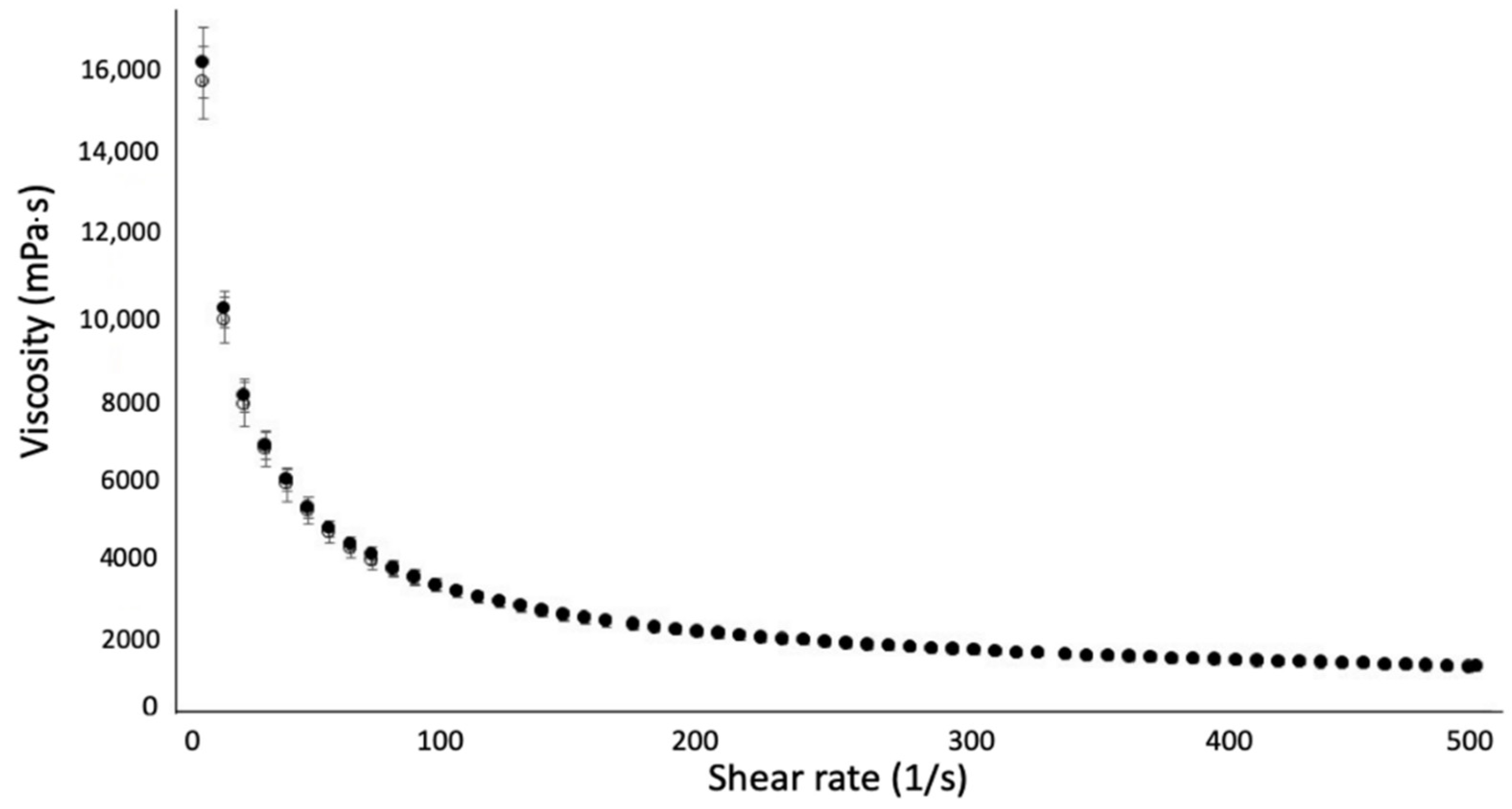
On Rapamycin In Vitro Permeation Using Franz Diffusion Cells
3.1.3. Influence of Polymer Concentration
3.2. Characterization of the Selected Micellar Hydrogel
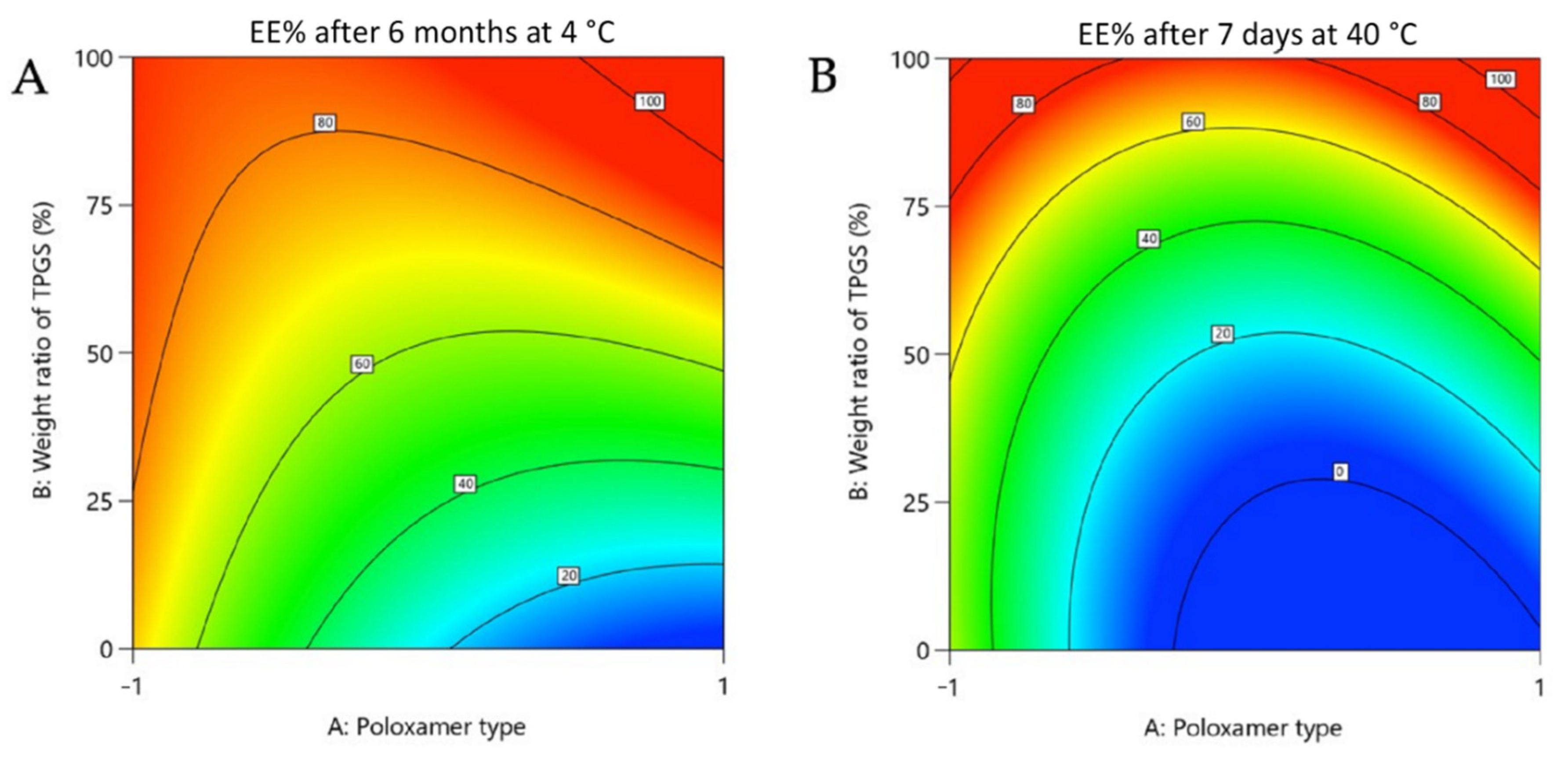
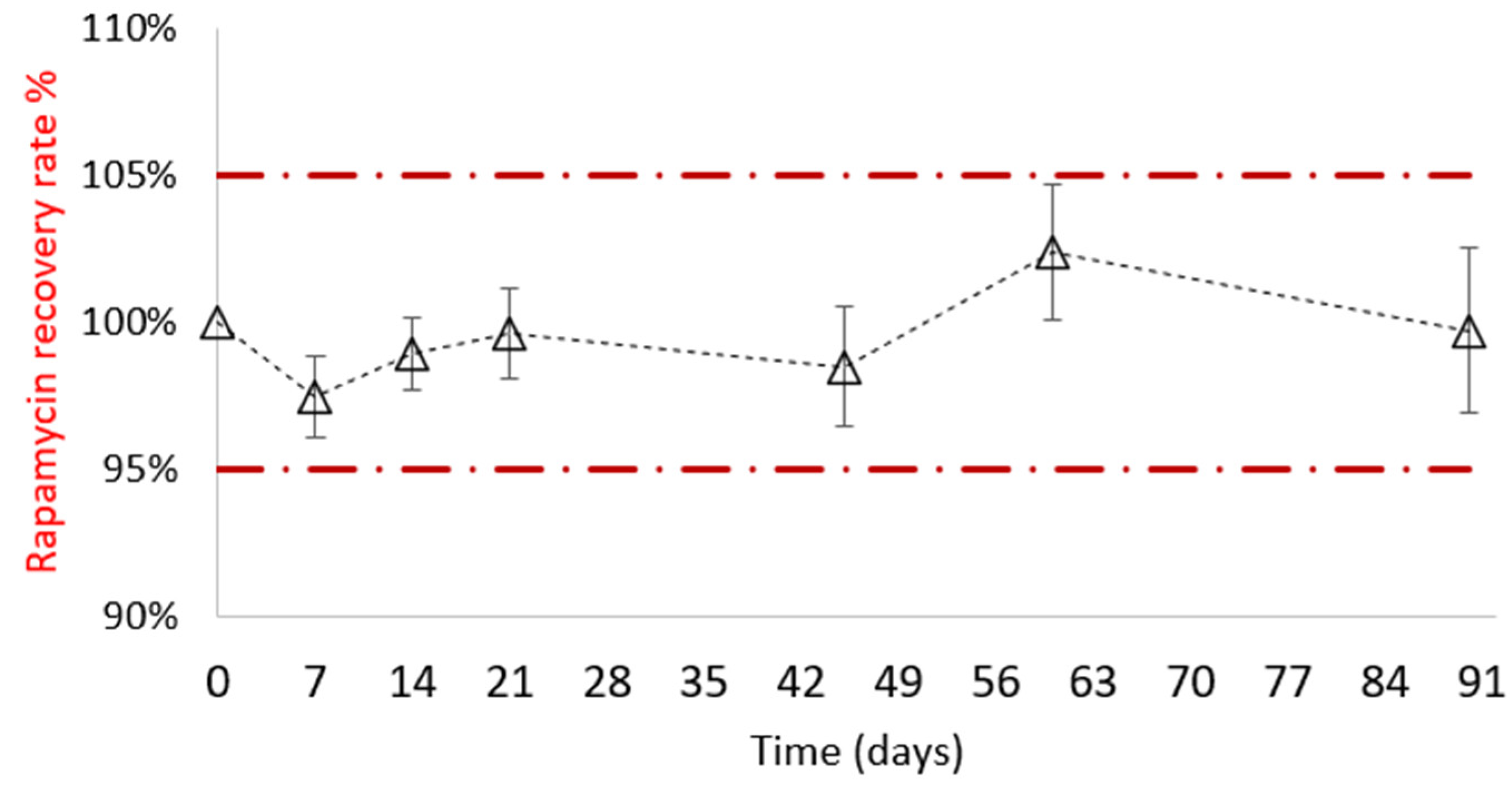
3.2.1. Physicochemical Stability
3.2.2. Ex Vivo Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orlova, K.A.; Crino, P.B. The tuberous sclerosis complex. Ann. N. Y. Acad. Sci. 2010, 1184, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Rosset, C.; Netto, C.B.O.; Ashton-Prolla, P. TSC1 and TSC2 gene mutations and their implications for treatment in tuberous sclerosis complex: A review. Genet. Mol. Biol. 2017, 40, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestri, R.; Neri, I.; Patrizi, A.; Angileri, L.; Ricci, L.; Magnano, M. Analysis of current data on the use of topical rapamycin in the treatment of facial angiofibromas in tuberous sclerosis complex. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 14–20. [Google Scholar] [CrossRef]
- McNamara, E.K.; Curtis, A.R.; Fleischer, A.B., Jr. Successful treatment of angiofibromata of tuberous sclerosis complex with rapamycin. J. Dermatol. Treat. 2012, 23, 46–48. [Google Scholar] [CrossRef]
- Salido-Vallejo, R.; Garnacho-Saucedo, G.; Moreno-Giménez, J. Opciones terapéuticas actuales para los angiofibromas faciales. Actas Dermo Sifilogr. 2014, 105, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Leducq, S.; Giraudeau, B.; Tavernier, E.; Maruani, A. Topical use of mammalian target of rapamycin inhibitors in dermatology: A systematic review with meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Haemel, A.K.; O’Brian, A.L.; Teng, J.M. Topical rapamycin: A novel approach to facial angiofibromas in tuberous sclerosis. Arch. Dermatol. 2010, 146, 715–718. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.I.; Lee, J.H.; Kim, D.Y.; Chung, K.Y.; Shin, J.U. Comparative effects of topical 0.2% sirolimus for angiofibromas in adults and pediatric patients with tuberous sclerosis complex. Dermatology 2018, 234, 13–22. [Google Scholar] [CrossRef]
- Wataya-Kaneda, M.; Nakamura, A.; Tanaka, M.; Hayashi, M.; Matsumoto, S.; Yamamoto, K.; Katayama, I. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: A randomized clinical trial. JAMA Dermatol. 2017, 153, 39–48. [Google Scholar] [CrossRef]
- Norrenberg, S.; Masconi, M.; Karamanou, M.; Meylan, P.; Golaz, R.; Christen-Zaech, S. Retrospective study of rapamycin or rapalog 0·1% cream for facial angiofibromas in tuberous sclerosis complex: Evaluation of treatment effectiveness and cost. Br. J. Dermatol. 2018, 179, 208–209. [Google Scholar] [CrossRef]
- Le Guyader, G.; Do, B.; Vieillard, V.; Andrieux, K.; Paul, M. Comparison of the in vitro and ex vivo permeation of existing topical formulations used in the treatment of facial angiofibroma and characterization of the variations observed. Pharmaceutics 2020, 12, 1060. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, G.; Vieillard, V.; Andrieux, K.; Rollo, M.; Thirion, O.; Wolkenstein, P.; Paul, M. Long-term stability of 0.1% rapamycin hydrophilic gel in the treatment of facial angiofibromas. Eur. J. Hosp. Pharm. Sci. Pract. 2018, 27, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Wataya-Kaneda, M.; Nakamura, A.; Matsumoto, S.; Katayama, I. First left-right comparative study of topical rapamycin vs. vehicle for facial angiofibromas in patients with tuberous sclerosis complex. Br. J. Dermatol. 2013, 169, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, S.; Jindal, N.; Jindal, A.; Bansal, R. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef]
- Quartier, J.; Lapteva, M.; Boulaguiem, Y.; Guerrier, S.; Kalia, Y.N. Polymeric micelle formulations for the cutaneous delivery of sirolimus: A new approach for the treatment of facial angiofibromas in tuberous sclerosis complex. Int. J. Pharm. 2021, 604, 120736. [Google Scholar] [CrossRef]
- Katare, O.P.; Raza, K.; Singh, B.; Dogra, S. Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 612–621. [Google Scholar] [CrossRef]
- Chavoshy, F.; Makhmalzade, B.S. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Dragicevic, N.; Maibach, H.I. (Eds.) Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-47861-5. [Google Scholar]
- Shenhar, R.; Norsten, T.B.; Rotello, V.M. Polymer-mediated nanoparticle assembly: Structural control and applications. Adv. Mater. 2005, 17, 657–669. [Google Scholar] [CrossRef]
- Gao, J.; Wu, P.; Fernandez, A.; Zhuang, J.; Thayumanavan, S. Cellular AND gates: Synergistic recognition to boost selective uptake of polymeric nanoassemblies. Angew. Chem. Int. Ed. 2020, 59, 10456–10460. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- ICH Expert Working Group. Q2(R1): Validation of analytical procedures: Text and methodology. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 15 May 2015; pp. 6–12. [Google Scholar]
- Hwang, T.-L.; Shaka, A. Water suppression that works. Excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. Ser. A 1995, 112, 275–279. [Google Scholar] [CrossRef]
- Chauhan, B.; Gupta, R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004, 39, 2115–2122. [Google Scholar] [CrossRef]
- Katti, D.S.; Seth, A. A one-step electrospray-based technique for modulating morphology and surface properties of poly(lactide-co-glycolide) microparticles using Pluronics®. Int. J. Nanomed. 2012, 7, 5129–5136. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Kim, J.-S.; Cho, W.K.; Hwang, S.-J. Enhanced solubility and oral absorption of sirolimus using D-α-tocopheryl polyethylene glycol succinate micelles. Artif. Cells Nanomed. Biotechnol. 2013, 41, 85–91. [Google Scholar] [CrossRef]
- Raval, A.; Parmar, A.; Raval, A.; Bahadur, P. Preparation and optimization of media using Pluronic® micelles for solubilization of sirolimus and release from the drug eluting stents. Colloids Surf. B Biointerfaces 2012, 93, 180–187. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; El-Zahaby, S.A.; Al-Mahallawi, A.M. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018, 25, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [Green Version]
- Thanitwatthanasak, S.; Sagis, L.M.; Chitprasert, P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: Mixture-design optimization, micellization, and solubilization behavior. J. Mol. Liq. 2019, 274, 223–238. [Google Scholar] [CrossRef]
- Pellosi, D.; Tessaro, A.L.; Moret, F.; Gaio, E.; Reddi, E.; Caetano, W.; Quaglia, F.; Hioka, N. Pluronic® mixed micelles as efficient nanocarriers for benzoporphyrin derivatives applied to photodynamic therapy in cancer cells. J. Photochem. Photobiol. A Chem. 2016, 314, 143–154. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.I.M.; Katas, H.; Sarisuta, N.; Witoonsaridsilp, W.; Benjakul, R. In vitro characterization of pluronic F127 and D-tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J. Nanomater. 2012, 2012, e916573. [Google Scholar] [CrossRef] [Green Version]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)poly(propylene oxide)poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Fares, A.R.; ElMeshad, A.N.; Kassem, M.A.A. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/F127 mixed polymeric micelles: Formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018, 25, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.D.; Saxena, V. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int. J. Nanomed. 2012, 7, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, D.; Wang, L.; Zhang, J.; Zhang, N. Docetaxel-loaded pluronic P123 polymeric micelles: In vitro and in vivo evaluation. Int. J. Mol. Sci. 2011, 12, 1684–1696. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.-W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [Green Version]
- Lauterbach, A.; Müller-Goymann, C.C. Comparison of rheological properties, follicular penetration, drug release, and permeation behavior of a novel topical drug delivery system and a conventional cream. Eur. J. Pharm. Biopharm. 2014, 88, 614–624. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Meinke, M.; Lange-Asschenfeldt, B.; Antoniou, C.; Mak, W.C.; Renneberg, R.; Sterry, W.; Patzelt, A. Drug delivery with topically applied nanoparticles: Science fiction or reality? Skin Pharmacol. Physiol. 2013, 26, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Nelemans, L.C.; Gurevich, L. Drug delivery with polymeric nanocarriers—Cellular uptake mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]






| Formulae (Fn) | Factors (Independent Variables) | |
|---|---|---|
| A: Poloxamer Type (−1: P123, 0: No Poloxamer, 1: F127) | B: Weight Ratio of TPGS to Total Copolymer (% w/w) | |
| F1 | −1 | 0 |
| F2 | −1 | 30 |
| F3 | −1 | 50 |
| F4 | −1 | 70 |
| F5 | 0 | 100 |
| F6 | 1 | 0 |
| F7 | 1 | 30 |
| F8 | 1 | 50 |
| F9 | 1 | 70 |
| Responses (Yn, Dependent Variables) | Constraints | |
| Characterization | Y1: Entrapment efficiency (%) | Maximize |
| Y2: Micellar size (nm) | Minimize | |
| Y3: Polydispersity index | Minimize | |
| Y4: Zeta potential (mV) | None | |
| Rapamycin EE% (chemical stability) | Y5: 4 °C for 6 months | Maximize |
| Y6: 40 °C for 7 days | Maximize | |
| In vitro study | Y7: Percutaneous flux (µg∙cm−² h−1) | Maximize |
| Formulae | 1: EE (%) a | 2: Size (nm) a | 3: PDI a | 4: ZP (mV) a | 5: Stability a | 6: In Vitro Flux (µg∙cm−2 h−1) a | |
|---|---|---|---|---|---|---|---|
| EE (%) 4 °C— 6 Months | EE (%) 40 °C—7 Days | ||||||
| F1 | 83.2 ± 2.1 | 17.7 ± 0.5 | 0.030 ± 0.013 | −1.2 ± 0.9 | 78.7 ± 1.5 | 53.1 ± 0.9 | 2.07 ± 0.13 |
| F2 | 89.3 ± 2.2 | 16.0 ± 0.4 | 0.021 ± 0.005 | −1.4 ± 2.0 | 75.1 ± 2.4 | 53.5 ± 0.9 | 1.85 ± 0.04 |
| F3 | 89.1 ± 2.7 | 12.6 ± 0.3 | 0.026 ± 0.013 | −1.2 ± 0.8 | 81.0 ± 2.5 | 65.4 ± 1.6 | 1.83 ± 0.13 |
| F4 | 87.6 ± 2.8 | 10.9 ± 0.2 | 0.034 ± 0.019 | −2.2 ± 1.0 | 82.5 ± 2.5 | 73.1 ± 1.2 | 1.65 ± 0.29 |
| F5 | 88.8 ± 2.3 | 10.7 ± 0.1 | 0.020 ± 0.011 | −2.5 ± 1.5 | 84.3 ± 2.9 | 77.1 ± 2.7 | 0.85 ± 0.11 |
| F6 | 82.1 ± 2.5 | 29.7 ± 2.6 | 0.158 ± 0.004 | −1.5 ± 1.0 | 3.1 ± 1.6 | 0.5 ± 0.3 | 0.85 ± 0.04 |
| F7 | 88.7 ± 2.4 | 14.9 ± 0.9 | 0.126 ± 0.015 | −0.4 ± 0.5 | 28.0 ± 0.9 | 11.0 ± 0.3 | 0.96 ± 0.11 |
| F8 | 88.6 ± 2.7 | 12.5 ± 0.6 | 0.083 ± 0.029 | −0.8 ± 0.4 | 75.8 ± 2.4 | 50.2 ± 2.6 | 0.89 ± 0.07 |
| F9 | 88.2 ± 2.4 | 11.7 ± 0.3 | 0.049 ± 0.009 | −0.7 ± 0.6 | 78.3 ± 3.2 | 65.8 ± 2.3 | 0.86 ± 0.14 |
| Fit Statistics | |||||||
| Adjusted R² | 0.1643 | 0.8974 | 0.9150 | 0.0731 | 0.9134 | 0.9567 | 0.9028 |
| Predicted R² | 0.0195 | 0.8660 | 0.9026 | –0.3066 | 0.9000 | 0.9501 | 0.8685 |
| Adequate precision | 5.0306 | 20.2157 | 25.5917 | 0.0910 | 2.7519 | 2.1166 | 17.9242 |
| Formula | Polymers (TPGS + P123; TPGS/P123 50/50) Concentration (mg∙mL−1) | EE (%) | DL (%) | Size (nm) | PDI |
|---|---|---|---|---|---|
| F3–25 | 25 | 89.1 ± 2.7 | 3.4 ± 0.1 | 12.6 ± 0.3 | 0.026 ± 0.013 |
| F3–10 | 10 | 89.1 ± 1.1 | 8.1 ± 0.1 | 13.9 ± 0.2 | 0.016 ± 0.090 |
| F3–5 | 5 | 88.1 ± 1.9 | 14.7 ± 0.3 | 14.5 ± 0.2 | 0.015 ± 0.008 |
| F3–2.5 | 2.5 | 88.4 ± 2.2 | 25.3 ± 0.6 | 84.7 ± 4.3 | 0.156 ± 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Guyader, G.; Do, B.; Rietveld, I.B.; Coric, P.; Bouaziz, S.; Guigner, J.-M.; Secretan, P.-H.; Andrieux, K.; Paul, M. Mixed Polymeric Micelles for Rapamycin Skin Delivery. Pharmaceutics 2022, 14, 569. https://doi.org/10.3390/pharmaceutics14030569
Le Guyader G, Do B, Rietveld IB, Coric P, Bouaziz S, Guigner J-M, Secretan P-H, Andrieux K, Paul M. Mixed Polymeric Micelles for Rapamycin Skin Delivery. Pharmaceutics. 2022; 14(3):569. https://doi.org/10.3390/pharmaceutics14030569
Chicago/Turabian StyleLe Guyader, Guillaume, Bernard Do, Ivo B. Rietveld, Pascale Coric, Serge Bouaziz, Jean-Michel Guigner, Philippe-Henri Secretan, Karine Andrieux, and Muriel Paul. 2022. "Mixed Polymeric Micelles for Rapamycin Skin Delivery" Pharmaceutics 14, no. 3: 569. https://doi.org/10.3390/pharmaceutics14030569
APA StyleLe Guyader, G., Do, B., Rietveld, I. B., Coric, P., Bouaziz, S., Guigner, J.-M., Secretan, P.-H., Andrieux, K., & Paul, M. (2022). Mixed Polymeric Micelles for Rapamycin Skin Delivery. Pharmaceutics, 14(3), 569. https://doi.org/10.3390/pharmaceutics14030569







