Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery
Abstract
1. Introduction
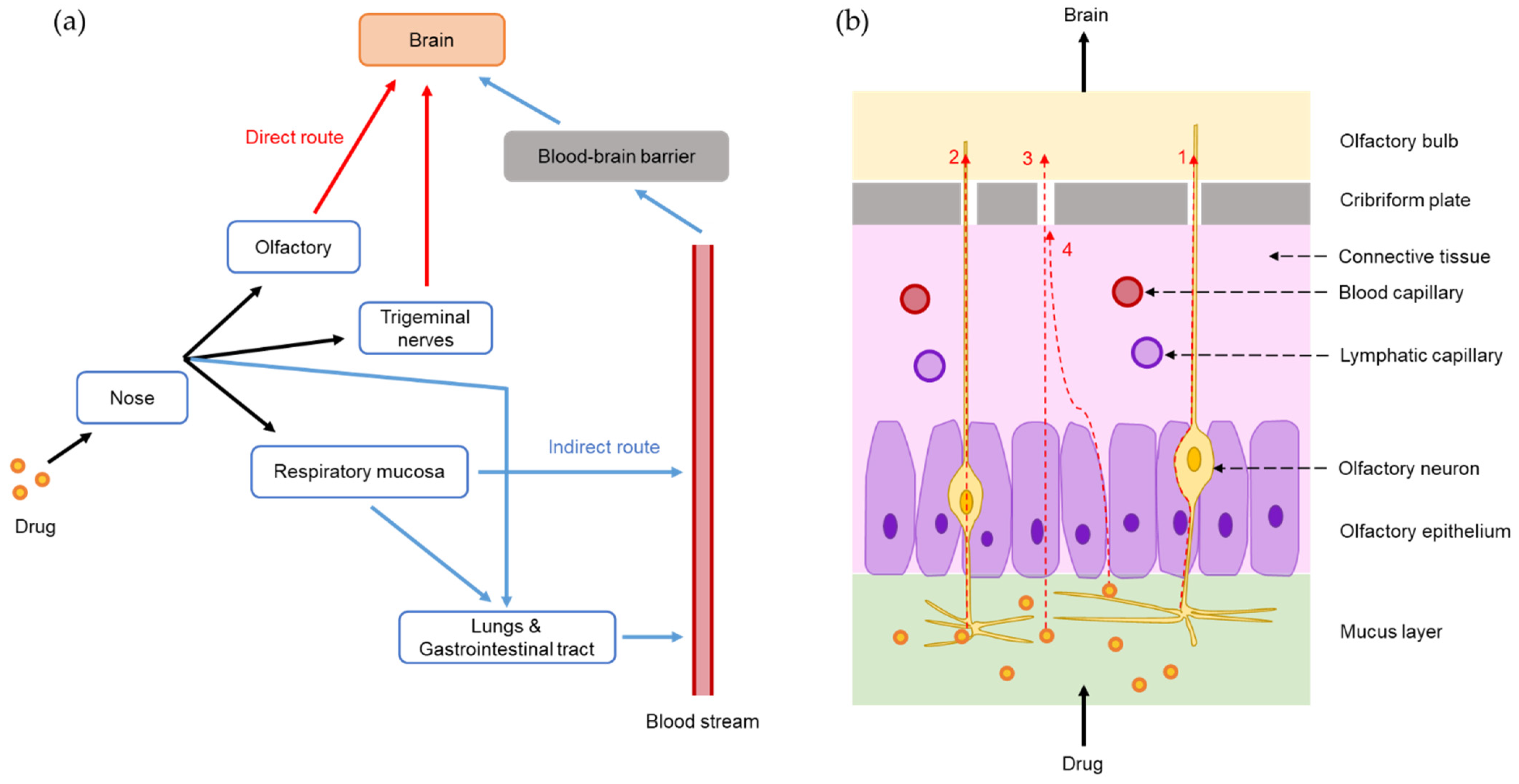
2. Nose-to-Brain Delivery Pathways and Feasibilities of SLNs and NLCs for Nose-to-Brain Drug Delivery
2.1. Nose-to-Brain Delivery Pathways
2.2. Feasibilities of SLNs and NLCs for Nose-to-Brain Drug Delivery
3. In Vivo Evaluation of Intranasal Formulations for Nose-to-Brain Delivery
3.1. PK and Biodistribution Studies
3.2. Pharmacodynamic Studies
3.3. Toxicity Studies
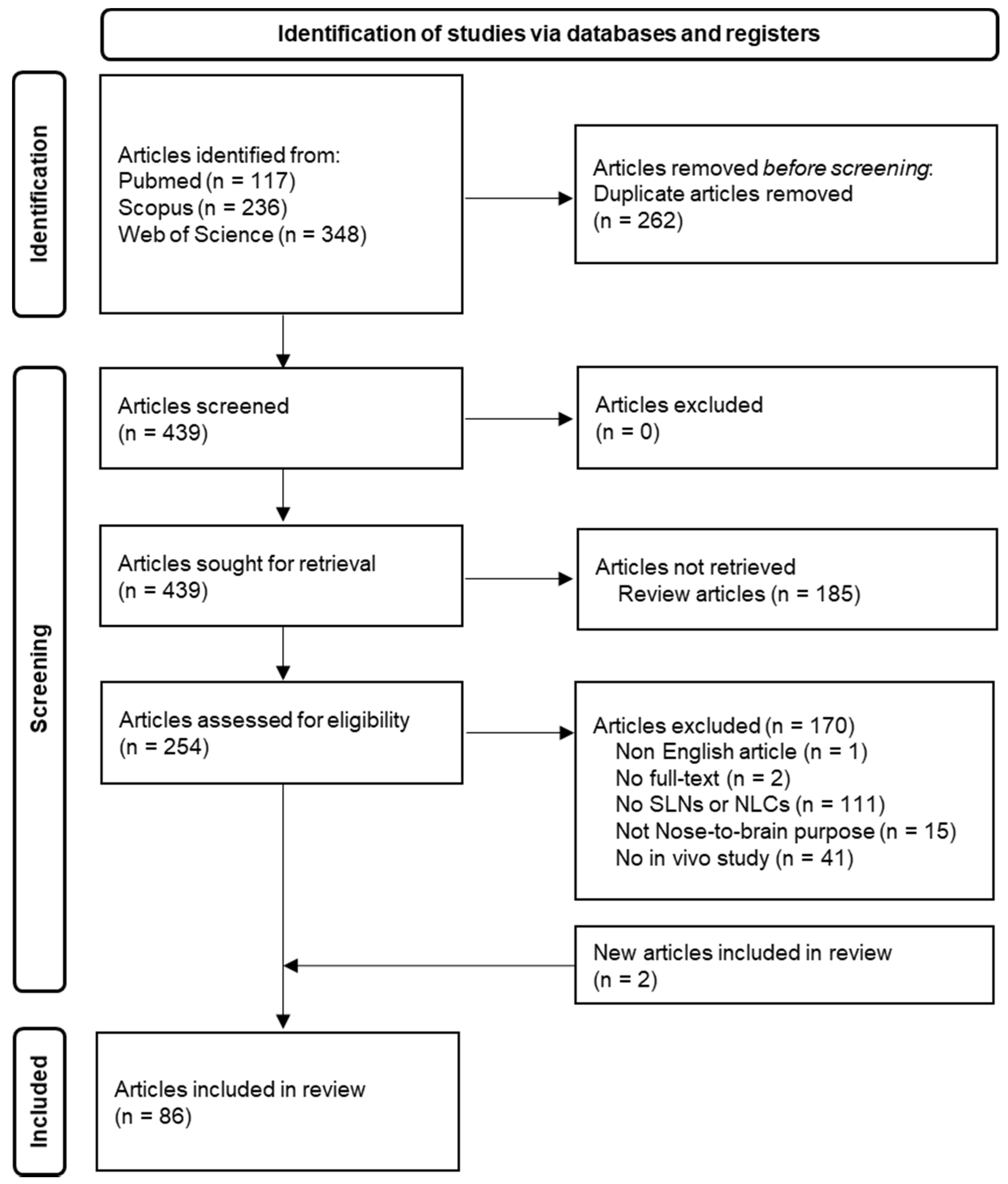
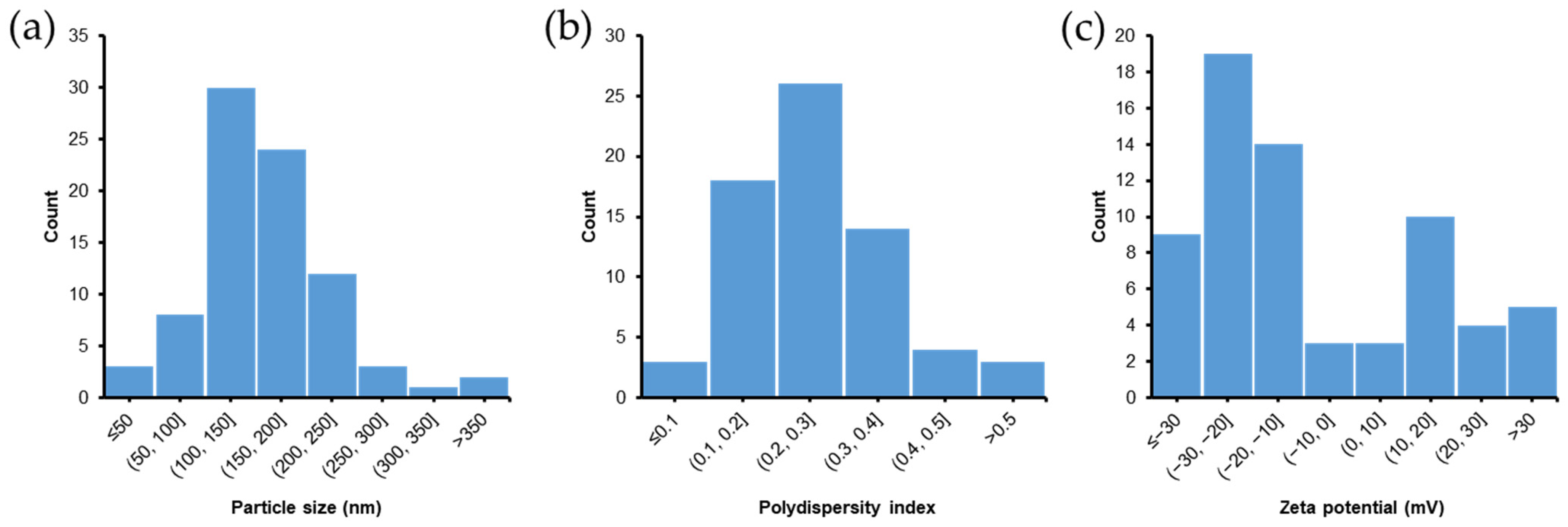
4. Nose-to-Brain Delivery of SLN and NLC-Based Formulations: Summary of a Literature Search for In Vivo Studies
5. In Vivo Evaluations of SLN and NLC-Based Formulations for Nose-to-Brain Delivery
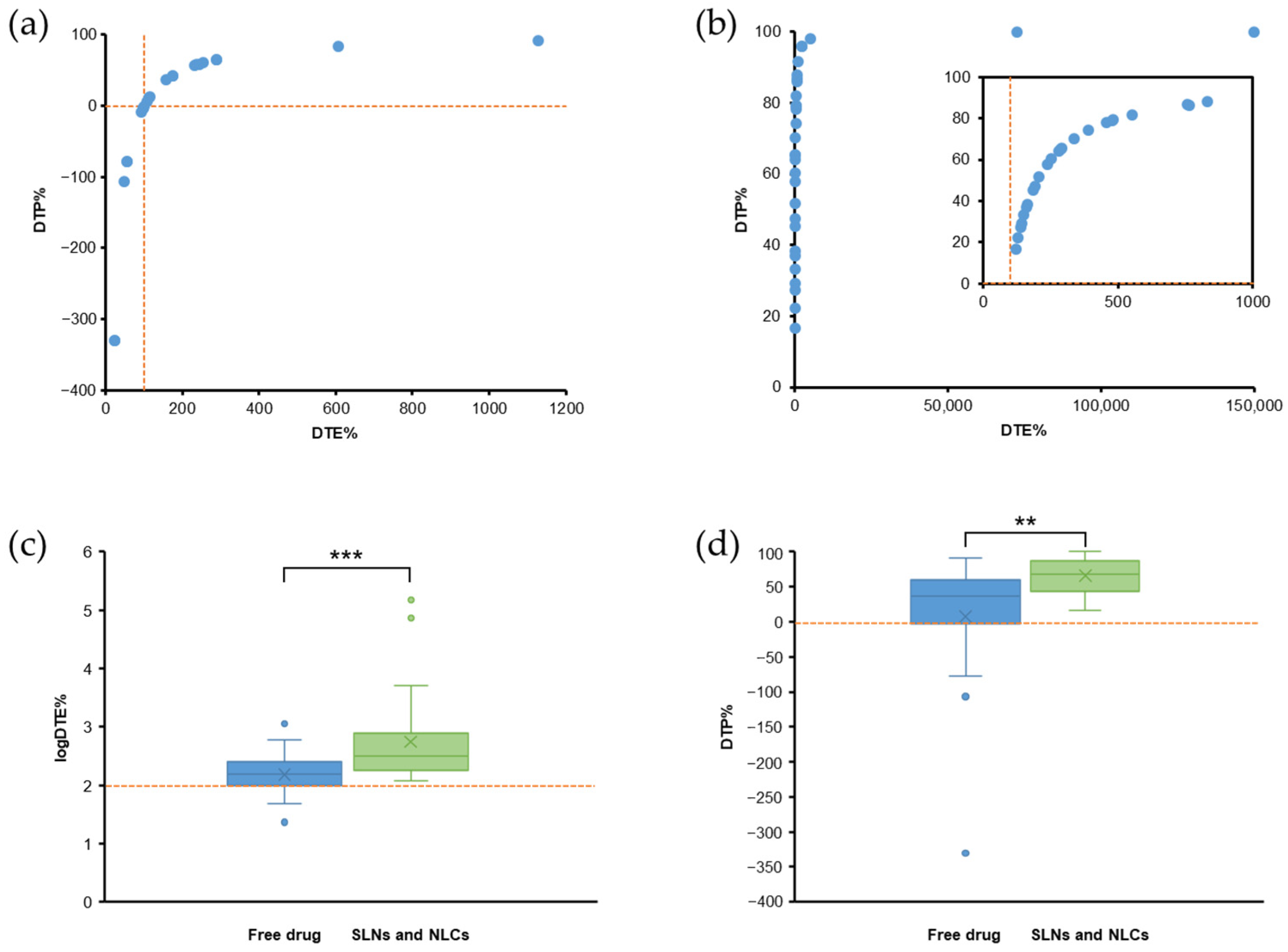
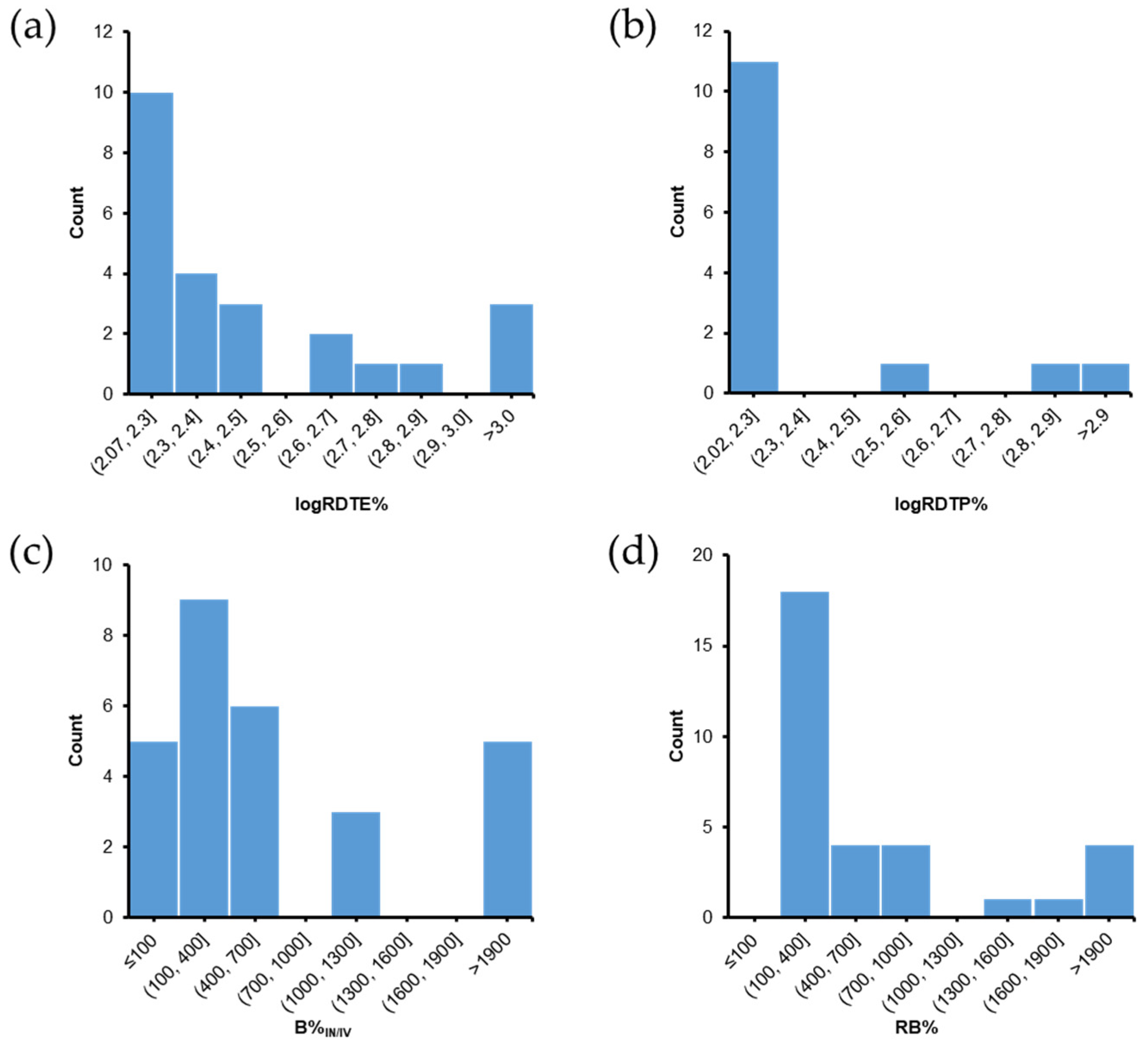
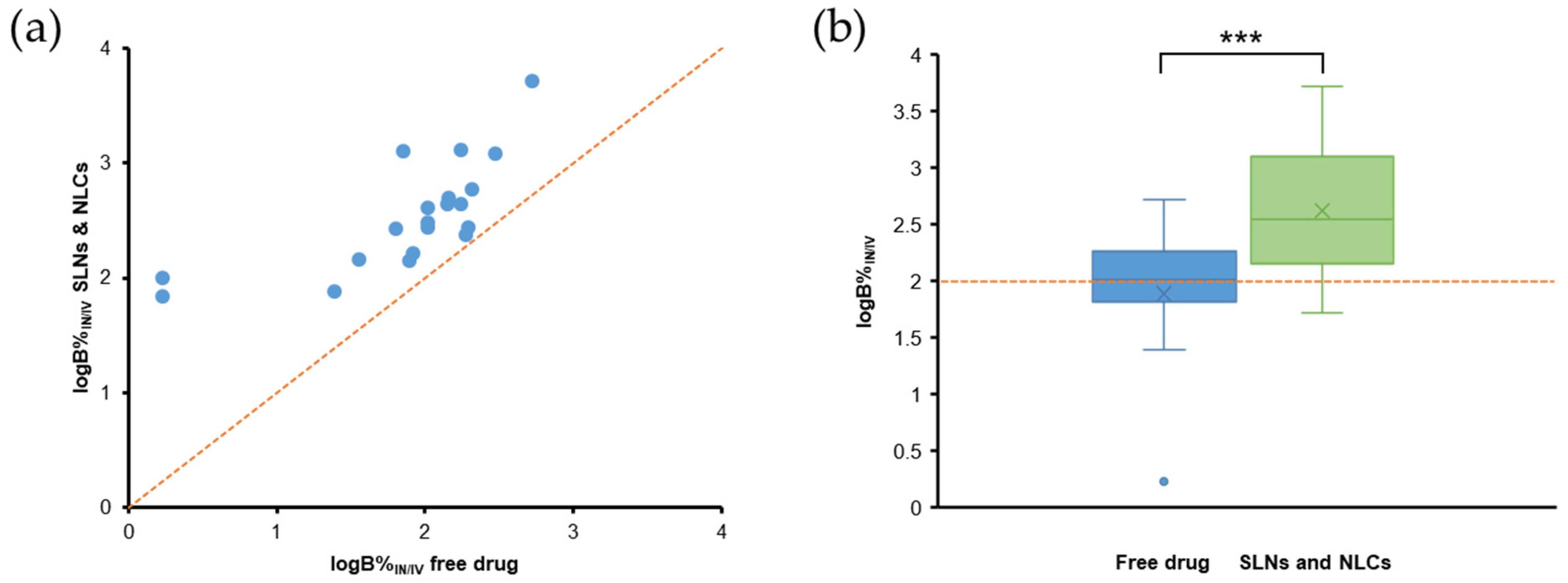
5.1. PK Studies with DTE% and DTP% Values
5.2. PK and Biodistribution Studies without DTE% and DTP% Values
5.2.1. Comparisons Using Brain Bioavailability
5.2.2. Comparison Using Brain: Blood Concentration Ratios
5.2.3. Drug Accumulation in the Brain
5.3. PD Studies
5.4. Toxicity Studies
6. Evaluation of PK Parameters for Nose-to-Brain Delivery
7. Effects of Gelling Systems and Surface Modifications of SLNs and NLCs
7.1. Effects of Gelling Systems
7.2. Effects of Surface Modification of SLNs and NLCs
8. Challenges, Considerations, and Future Developments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, S.-S.; Oh, K.T.; Choi, H.-G.; Lim, S.-J. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Bhattmisra, S.K.; Zeeshan, F.; Shahzad, N.; Mujtaba, M.A.; Srikanth Meka, V.; Radhakrishnan, A.; Kesharwani, P.; Baboota, S.; Ali, J. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J. Drug Deliv. Sci. Technol. 2018, 43, 295–310. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood—Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatrics 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169-409X(96)00423-1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Brookes, A.; Ji, L.; Bradshaw, T.D.; Stocks, M.; Gray, D.; Butler, J.; Gershkovich, P. Is oral lipid-based delivery for drug targeting to the brain feasible? Eur. J. Pharm. Biopharm. 2022, 172, 112–122. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13, 1079. [Google Scholar] [CrossRef]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, L.; Xie, H.; Wang, Y.; Gao, S.; Zhang, L.; Bo, F.; Yang, S.; Feng, A. Primary Studies on Construction and Evaluation of Ion-Sensitive in situ Gel Loaded with Paeonol-Solid Lipid Nanoparticles for Intranasal Drug Delivery. Int. J. Nanomed. 2020, 15, 3137–3160. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, D.A.; Ibrahim, A.B.; Amin, M.M.; Khowessah, O.M.; Elzanfaly, E.S. Intranasal haloperidol-loaded miniemulsions for brain targeting: Evaluation of locomotor suppression and in-vivo biodistribution. Eur. J. Pharm. Sci. 2016, 92, 244–254. [Google Scholar] [CrossRef] [PubMed]
- El-Zaafarany, G.M.; Soliman, M.E.; Mansour, S.; Awad, G.A.S. Identifying lipidic emulsomes for improved oxcarbazepine brain targeting: In vitro and rat in vivo studies. Int. J. Pharm. 2016, 503, 127–140. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Trapani, A.; De Giglio, E.; Cometa, S.; Bonifacio, M.A.; Dazzi, L.; Di Gioia, S.; Hossain, M.N.; Pellitteri, R.; Antimisiaris, S.G.; Conese, M. Dopamine-loaded lipid based nanocarriers for intranasal administration of the neurotransmitter: A comparative study. Eur. J. Pharm. Biopharm. 2021, 167, 189–200. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Smart Drug Delivery Systems in the Treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- de Barros, C.T.; Rios, A.C.; Alves, T.F.R.; Batain, F.; Crescencio, K.M.M.; Lopes, L.J.; Zielińska, A.; Severino, P.; Mazzola, P.; Souto, E.B.; et al. Cachexia: Pathophysiology and Ghrelin Liposomes for Nose-to-Brain Delivery. Int. J. Mol. Sci. 2020, 21, 5974. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, F.; Chen, C.-C.V.; Mo, K.-C.; Hung, Y.; Guo, Z.-X.; Lin, C.-H.; Lin, M.-H.; Lin, Y.-H.; Chang, C.; et al. Manganese-enhanced MRI of rat brain based on slow cerebral delivery of manganese(II) with silica-encapsulated MnxFe1–xO nanoparticles. NMR Biomed. 2013, 26, 1176–1185. [Google Scholar] [CrossRef]
- Ray, S.; Cheng, C.A.; Chen, W.; Li, Z.; Zink, J.I.; Lin, Y.Y. Magnetic Heating Stimulated Cargo Release with Dose Control using Multifunctional MR and Thermosensitive Liposome. Nanotheranostics 2019, 3, 166–178. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Tagalpallewar, A.A.; Kokare, C.R. Agranulocytosis-Protective Olanzapine-Loaded Nanostructured Lipid Carriers Engineered for CNS Delivery: Optimization and Hematological Toxicity Studies. AAPS PharmSciTech 2019, 20, 22. [Google Scholar] [CrossRef]
- Uppuluri, C.T.; Ravi, P.R.; Dalvi, A.V. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int. J. Pharm. 2021, 606, 120881. [Google Scholar] [CrossRef]
- Singh, S.K.; Dadhania, P.; Vuddanda, P.R.; Jain, A.; Velaga, S.; Singh, S. Intranasal delivery of asenapine loaded nanostructured lipid carriers: Formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016, 6, 2032–2045. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Cunha, S.; Almeida, H.; Amaral, M.H.; Lobo, S.J.M.; Silva, A.C. Intranasal Lipid Nanoparticles for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2017, 23, 6553–6562. [Google Scholar] [CrossRef]
- Ahmad, J.; Rizwanullah, M.; Amin, S.; Warsi, H.M.; Ahmad, Z.M.; Barkat, A.M. Nanostructured Lipid Carriers (NLCs): Nose-to-Brain Delivery and Theranostic Application. Curr. Drug Metab. 2020, 21, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Nair, S.C.; Vinayan, K.P.; Mangalathillam, S. Nose to Brain Delivery of Phenytoin Sodium Loaded Nano Lipid Carriers: Formulation, Drug Release, Permeation and In Vivo Pharmacokinetic Studies. Pharmaceutics 2021, 13, 1640. [Google Scholar] [CrossRef]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov. Today 2018, 23, 1079–1088. [Google Scholar] [CrossRef]
- Singh, A.P.; Saraf, S.K.; Saraf, S.A. SLN approach for nose-to-brain delivery of alprazolam. Drug Deliv. Transl. Res. 2012, 2, 498–507. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.L.J.; Billa, N. Improved Bioavailability of Poorly Soluble Drugs through Gastrointestinal Muco-Adhesion of Lipid Nanoparticles. Pharmaceutics 2021, 13, 1817. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Mohamed, S.A.; AbuelEzz, N.Z. The Tiny Big World of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: An Updated Review. J. Microencapsul. 2021, 39, 72–94. [Google Scholar] [CrossRef]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [CrossRef]
- Qushawy, M.; Prabahar, K.; Abd-Alhaseeb, M.; Swidan, S.; Nasr, A. Preparation and Evaluation of Carbamazepine Solid Lipid Nanoparticle for Alleviating Seizure Activity in Pentylenetetrazole-Kindled Mice. Molecules 2019, 24, 3971. [Google Scholar] [CrossRef]
- Joshi, M.D.; Müller, R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009, 71, 161–172. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Nanostructured lipid carriers containing ondansetron hydrochloride by cold high-pressure homogenization method: Preparation, characterization, and pharmacokinetic evaluation. J. Drug Deliv. Sci. Technol. 2019, 53, 101185. [Google Scholar] [CrossRef]
- Nasiri, F.; Faghfouri, L.; Hamidi, M. Preparation, optimization, and in-vitro characterization of α-tocopherol-loaded solid lipid nanoparticles (SLNs). Drug Dev. Ind. Pharm. 2020, 46, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Preparation of Ondansetron Hydrochloride-Loaded Nanostructured Lipid Carriers Using Solvent Injection Method for Enhancement of Pharmacokinetic Properties. Pharm. Res. 2019, 36, 138. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Data on optimization and drug release kinetics of nanostructured lipid carriers containing ondansetron hydrochloride prepared by cold high-pressure homogenization method. Data Brief 2019, 26, 104475. [Google Scholar] [CrossRef]
- Agbo, C.P.; Ugwuanyi, T.C.; Ugwuoke, W.I.; McConville, C.; Attama, A.A.; Ofokansi, K.C. Intranasal artesunate-loaded nanostructured lipid carriers: A convenient alternative to parenteral formulations for the treatment of severe and cerebral malaria. J. Control. Release 2021, 334, 224–236. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; El Sisi, A.M. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics 2021, 13, 581. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Posadas, Y.; Miranda, J.; Uc, Y.P.; Ortega-Olvera, M.J.; Hernández, S. Strategies that Target Tight Junctions for Enhanced Drug Delivery. Curr. Pharm. Des. 2016, 22, 5313–5346. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-based in situ gels for brain targeting via the nasal route: Statistical optimization, in vitro, and in vivo evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y. Formulation and Evaluation of Neuroactive Drug Loaded Chitosan Nanoparticle for Nose to Brain Delivery: In-vitro Characterization and In-vivo Behavior Study. Curr. Drug Deliv. 2019, 16, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Gorki, V.; Singh, G.; Kaur, R.; Katare, O.P.; Nirmalan, N.; Singh, B. Intranasal delivery of polymer-anchored lipid nanoconstructs of artemether-lumefantrine in Plasmodium berghei ANKA murine model. J. Drug Deliv. Sci. Technol. 2021, 61, 102114. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil-Gadhe, A.; Palla, P. Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: In-vivo pharmacokinetic and toxicity study. Biomed. Pharmacother. 2017, 94, 150–164. [Google Scholar] [CrossRef]
- Patel, S.; Chavhan, S.; Soni, H.; Babbar, A.K.; Mathur, R.; Mishra, A.K.; Sawant, K. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J. Drug Target. 2011, 19, 468–474. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: Formulation, optimization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2017, 11, 1815–1825. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Patel, H.P.; Gandhi, P.A.; Chaudhari, P.S.; Desai, B.V.; Desai, D.T.; Dedhiya, P.P.; Maulvi, F.A.; Vyas, B.A. Clozapine loaded nanostructured lipid carriers engineered for brain targeting via nose-to-brain delivery: Optimization and in vivo pharmacokinetic studies. J. Drug Deliv. Sci. Technol. 2021, 64, 102533. [Google Scholar] [CrossRef]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef]
- Chandra Bhatt, P.; Srivastava, P.; Pandey, P.; Khan, W.; Panda, B.P. Nose to brain delivery of astaxanthin-loaded solid lipid nanoparticles: Fabrication, radio labeling, optimization and biological studies. RSC Adv. 2016, 6, 10001–10010. [Google Scholar] [CrossRef]
- Kumar, M.; Kakkar, V.; Mishra, A.K.; Chuttani, K.; Kaur, I.P. Intranasal delivery of streptomycin sulfate (STRS) loaded solid lipid nanoparticles to brain and blood. Int. J. Pharm. 2014, 461, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 167, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.; Choudhury, H.; Kokare, C. Neutropenia and leukopenia protective intranasal olanzapine-loaded lipid-based nanocarriers engineered for brain delivery. Appl. Nanosci. 2019, 9, 151–168. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Yasir, M.; Chauhan, I.; Zafar, A.; Verma, M.; Noorulla, K.M.; Tura, A.J.; Alruwaili, N.K.; Haji, M.J.; Puri, D.; Gobena, W.G.; et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: Formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J. Drug Deliv. Sci. Technol. 2021, 61, 102164. [Google Scholar] [CrossRef]
- Sarma, A.; Das, M.K.; Chakraborty, T.; Das, S. Nanostructured lipid carriers (NLCs)-based intranasal Drug Delivery System of Tenofovir disoproxil fumerate (TDF) for brain targeting. Res. J. Pharm. Technol. 2020, 13, 5411–5424. [Google Scholar]
- Deshkar, S.S.; Jadhav, M.S.; Shirolkar, S.V. Development of Carbamazepine Nanostructured Lipid Carrier Loaded Thermosensitive Gel for Intranasal Delivery. Adv. Pharm. Bull. 2021, 11, 150–162. [Google Scholar] [CrossRef]
- Eskandari, S.; Varshosaz, J.; Minaiyan, M.; Tabbakhian, M. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: In vivo pharmacodynamic studies using rat electroshock model. Int. J. Nanomed. 2011, 6, 363–371. [Google Scholar] [CrossRef][Green Version]
- Alam, T.; Pandit, J.; Vohora, D.; Aqil, M.; Ali, A.; Sultana, Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: In vitro characterization and in vivo efficacy in epilepsy. Expert Opin. Drug Deliv. 2015, 12, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhandari, S.; Deshmukh, R.; Yadav, A.K.; Mishra, N. Development and characterization of embelin-loaded nanolipid carriers for brain targeting. Artif. Cells Nanomed. Biotechnol. 2017, 45, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N. Superparamagnetic Iron Oxide–Loaded Lipid Nanocarriers Incorporated in Thermosensitive In Situ Gel for Magnetic Brain Targeting of Clonazepam. J. Pharm. Sci. 2018, 107, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Taymouri, S.; Minaiyan, M.; Ebrahimi, F.; Tavakoli, N. In-vitro and in-vivo evaluation of chitosan-based thermosensitive gel containing lorazepam NLCs for the treatment of status epilepticus. IET Nanobiotechnol. 2020, 14, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Bariya, A.; Allam, A.; Othman, S.; Butani, S.B. In situ nanostructured hydrogel of resveratrol for brain targeting: In vitro-in vivo characterization. Drug Deliv. Transl. Res. 2018, 8, 1460–1470. [Google Scholar] [CrossRef]
- Rajput, A.P.; Butani, S.B. Resveratrol anchored nanostructured lipid carrier loaded in situ gel via nasal route: Formulation, optimization and in vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 51, 214–223. [Google Scholar] [CrossRef]
- Butani, S. Fabrication of an ion-sensitive in situ gel loaded with nanostructured lipid carrier for nose to brain delivery of donepezil. Asian J. Pharm. 2018, 12, 4. [Google Scholar]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to Brain Delivery of Rivastigmine by In Situ Gelling Cationic Nanostructured Lipid Carriers: Enhanced Brain Distribution and Pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef]
- Anand, A.; Arya, M.; Kaithwas, G.; Singh, G.; Saraf, S.A. Sucrose stearate as a biosurfactant for development of rivastigmine containing nanostructured lipid carriers and assessment of its activity against dementia in C. elegans model. J. Drug Deliv. Sci. Technol. 2019, 49, 219–226. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, T.; Jain, A.; Kaur, H.; Katare, O.P.; Singh, B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids Surf. B Biointerfaces 2021, 205, 111838. [Google Scholar] [CrossRef]
- Hangargekar, S.R.; Mohanty, P.K.; Rai, J.P. Preclinical Screening of Antidepressant Activity of Formulated Sertraline Hydrochloride-Loaded Solid Lipid Nanoparticles in Rats. J. Pharm. Res. Int. 2021, 33, 134–138. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: Pharmacodynamic studies and estimation in blood and brain. J. Psychiatr. Res. 2012, 46, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Silva, S.; Gouveia, F.; Bicker, J.; Falcão, A.; Fortuna, A. QbD-driven development of intranasal lipid nanoparticles for depression treatment. Eur. J. Pharm. Biopharm. 2020, 153, 106–120. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal Administration of TAT-Conjugated Lipid Nanocarriers Loading GDNF for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Herrán, E.; Ruiz-Ortega, J.A.; Miguelez, C.; Igartua, M.; Lafuente, J.V.; Pedraz, J.L.; Ugedo, L.; Hernández, R.M. Intranasal Administration of Chitosan-Coated Nanostructured Lipid Carriers Loaded with GDNF Improves Behavioral and Histological Recovery in a Partial Lesion Model of Parkinson’s Disease. J. Biomed. Nanotechnol. 2016, 12, 2220–2280. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Rajput, P.V.; Belgamwar, V.S.; Tekade, A.R.; Surana, S.J. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: Application of factorial design approach. Drug Deliv. 2013, 20, 47–56. [Google Scholar] [CrossRef]
- Gautam, D.; Singh, S.; Maurya, P.; Singh, M.; Kushwaha, S.; Saraf, A.S. Appraisal of Nano-lipidic Astaxanthin Cum Thermoreversible Gel and its Efficacy in Haloperidol Induced Parkinsonism. Curr. Drug Deliv. 2021, 18, 1515–1527. [Google Scholar] [CrossRef]
- Mishra, N.; Sharma, S.; Deshmukh, R.; Kumar, A.; Sharma, R. Development and Characterization of Nasal Delivery of Selegiline Hydrochloride Loaded Nanolipid Carriers for the Management of Parkinson’s Disease. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 46–56. [Google Scholar] [CrossRef]
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; El-Massik, M.A.E.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Kokare, C.R. Nanostructured lipid carriers engineered for intranasal delivery of teriflunomide in multiple sclerosis: Optimization and in vivo studies. Drug Dev. Ind. Pharm. 2019, 45, 839–851. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv. 2013, 20, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Sara, U.V.S.; Som, I. Development of a new HPLC method for in vitro and in vivo studies of haloperidol in solid lipid nanoparticles. Braz. J. Pharm. Sci. 2017, 53, e16047. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S. Solid lipid nanoparticles for nose to brain delivery of haloperidol: In vitro drug release and pharmacokinetics evaluation. Acta Pharm. Sin. B 2014, 4, 454–463. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- de Sousa Marcial, S.P.; Carneiro, G.; Leite, E.A. Lipid-based nanoparticles as drug delivery system for paclitaxel in breast cancer treatment. J. Nanoparticle Res. 2017, 19, 340. [Google Scholar] [CrossRef]
- Jain, K.; Sood, S.; Gowthamarajan, K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015, 22, 940–954. [Google Scholar] [CrossRef]
- Yasir, M.; Chauhan, I.; Haji, M.J.; Tura, A.J.; Saxena, P.K. Formulation and evaluation of glyceryl behenate based solid lipid nanoparticles for the delivery of donepezil to brain through nasal route. Res. J. Pharm. Technol. 2018, 11, 2836–2844. [Google Scholar] [CrossRef]
- Yasir, M.; Sara, U.V.S.; Chauhan, I.; Gaur, P.K.; Singh, A.P.; Puri, D.A. Solid lipid nanoparticles for nose to brain delivery of donepezil: Formulation, optimization by Box–Behnken design, in vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1838–1851. [Google Scholar] [CrossRef]
- Silva, S.; Bicker, J.; Fonseca, C.; Ferreira, N.R.; Vitorino, C.; Alves, G.; Falcão, A.; Fortuna, A. Encapsulated Escitalopram and Paroxetine Intranasal Co-Administration: In Vitro/In Vivo Evaluation. Front. Pharmacol. 2021, 12, 751321. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hady, M.; Sayed, O.M.; Akl, M.A. Brain uptake and accumulation of new levofloxacin-doxycycline combination through the use of solid lipid nanoparticles: Formulation; Optimization and in-vivo evaluation. Colloids Surf. B Biointerfaces 2020, 193, 111076. [Google Scholar] [CrossRef] [PubMed]
- Devkar, T.B.; Tekade, A.R.; Khandelwal, K.R. Surface engineered nanostructured lipid carriers for efficient nose to brain delivery of ondansetron HCl using Delonix regia gum as a natural mucoadhesive polymer. Colloids Surf. B Biointerfaces 2014, 122, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Abd-algaleel, S.A.; Metwally, A.A.; Abdel-Bar, H.M.; Kassem, D.H.; Hathout, R.M. Synchronizing In Silico, In Vitro, and In Vivo Studies for the Successful Nose to Brain Delivery of an Anticancer Molecule. Mol. Pharm. 2021, 18, 3763–3776. [Google Scholar] [CrossRef]
- Masjedi, M.; Azadi, A.; Heidari, R.; Mohammadi-Samani, S. Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: Preparation, optimization, characterization and pharmacokinetic evaluation. J. Pharm. Pharmacol. 2020, 72, 1341–1351. [Google Scholar] [CrossRef]
- Khan, A.; Imam, S.S.; Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Brain Targeting of Temozolomide via the Intranasal Route Using Lipid-Based Nanoparticles: Brain Pharmacokinetic and Scintigraphic Analyses. Mol. Pharm. 2016, 13, 3773–3782. [Google Scholar] [CrossRef]
- Praveen, S.; Gowda, D.; Siddaramaiah, H.; Hemalatha, S. Ziprasidone hydrochloride loaded nanostructured lipid carriers (NLCS) for intranasal delivery: Optimization and in vivo studies. Int. J. Appl. Pharm. 2020, 12, 31–41. [Google Scholar]
- Noorulla, K.M.; Yasir, M.; Muzaffar, F.; Roshan, S.; Ghoneim, M.M.; Almurshedi, A.S.; Tura, A.J.; Alshehri, S.; Gebissa, T.; Mekit, S.; et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In-Vivo preclinical evaluation. J. Drug Deliv. Sci. Technol. 2021, 67, 102939. [Google Scholar] [CrossRef]
- Tripathi, D.; Sonar, P.K.; Parashar, P.; Chaudhary, S.K.; Upadhyay, S.; Saraf, S.K. Augmented Brain Delivery of Cinnarizine Through Nanostructured Lipid Carriers Loaded in situ Gel: In vitro and Pharmacokinetic Evaluation. BioNanoScience 2021, 11, 159–171. [Google Scholar] [CrossRef]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Fahmy, U.A.; Ahmed, O.A.A.; Badr-Eldin, S.M.; Aldawsari, H.M.; Okbazghi, S.Z.; Awan, Z.A.; Bakhrebah, M.A.; Alomary, M.N.; Abdulaal, W.H.; Medina, C.; et al. Optimized Nanostructured Lipid Carriers Integrated into In Situ Nasal Gel for Enhancing Brain Delivery of Flibanserin. Int. J. Nanomed. 2020, 15, 5253–5264. [Google Scholar] [CrossRef] [PubMed]
- Jazuli, I.; Annu; Nabi, B.; Moolakkadath, T.; Alam, T.; Baboota, S.; Ali, J. Optimization of Nanostructured Lipid Carriers of Lurasidone Hydrochloride Using Box-Behnken Design for Brain Targeting: In Vitro and In Vivo Studies. J. Pharm. Sci. 2019, 108, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Palagati, S.; Sv, S.; Kesavan, B.R. Application of computational tools for the designing of Oleuropein loaded nanostructured lipid carrier for brain targeting through nasal route. DARU J. Pharm. Sci. 2019, 27, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, X.; Du, J.; Liu, M.; Feng, J.; Hu, K. Improved brain delivery of pueraria flavones via intranasal administration of borneol-modified solid lipid nanoparticles. Nanomedicine 2019, 14, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Sivadasu, P.; Gowda, D.V.; Subramani, N.K.; Malgur, B.; Vishweshwaraiah, S.S.; Hatna, S. Direct brain targeted nanostructured lipid carriers for sustained release of schizophrenic drug: Formulation, characterization and pharmacokinetic studies. Brain 2020, 9, 12. [Google Scholar] [CrossRef]
- Singh, A.; Ubrane, R.; Prasad, P.; Ramteke, S. Preparation and Characterization of Rizatriptan Benzoate Loaded Solid Lipid Nanoparticles for Brain Targeting. Mater. Today Proc. 2015, 2, 4521–4543. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Improved brain pharmacokinetics following intranasal administration of N,N,N-trimethyl chitosan tailored mucoadhesive NLCs. Mater. Technol. 2020, 35, 249–266. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability. BioMed Res. Int. 2017, 2017, 5984014. [Google Scholar] [CrossRef]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Karri, V.V.S.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Drechsler, M.; Mariani, P.; Contado, C.; Ruokolainen, J.; Ratano, P.; Campolongo, P.; Trezza, V.; Nastruzzi, C.; et al. Cannabinoid antagonist in nanostructured lipid carriers (NLCs): Design, characterization and in vivo study. Mater. Sci. Eng. C 2015, 48, 328–336. [Google Scholar] [CrossRef]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Fan, J.; Fu, B.M.; Calderan, L.; Mannucci, S.; Boschi, F.; Nastruzzi, C. Nanoformulations for dimethyl fumarate: Physicochemical characterization and in vitro/in vivo behavior. Eur. J. Pharm. Biopharm. 2017, 115, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Imran, M.; Kesharwani, P.; Khanna, K.; Karwasra, R.; Sharma, N.; Rawat, S.; Sharma, D.; Ahmad, F.J.; Jain, G.K.; et al. Intranasal delivery of Naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int. J. Pharm. 2021, 599, 120428. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-C.; Zhang, W.-J.; Zhu, J.-X.; Zhu, N.; Zhang, H.-M.; Wang, X.; Zhang, J.; Wang, Q.-Q. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. Int. J. Clin. Exp. Med. 2015, 8, 17590–17600. [Google Scholar] [PubMed]
- de Oliveira Junior, E.R.; Truzzi, E.; Ferraro, L.; Fogagnolo, M.; Pavan, B.; Beggiato, S.; Rustichelli, C.; Maretti, E.; Lima, E.M.; Leo, E.; et al. Nasal administration of nanoencapsulated geraniol/ursodeoxycholic acid conjugate: Towards a new approach for the management of Parkinson’s disease. J. Control. Release 2020, 321, 540–552. [Google Scholar] [CrossRef]
- Khanna, K.; Sharma, N.; Rawat, S.; Khan, N.; Karwasra, R.; Hasan, N.; Kumar, A.; Jain, G.K.; Nishad, D.K.; Khanna, S.; et al. Intranasal solid lipid nanoparticles for management of pain: A full factorial design approach, characterization & Gamma Scintigraphy. Chem. Phys. Lipids 2021, 236, 105060. [Google Scholar] [CrossRef]
- Joshi, A.S.; Patel, H.S.; Belgamwar, V.S.; Agrawal, A.; Tekade, A.R. Solid lipid nanoparticles of ondansetron HCl for intranasal delivery: Development, optimization and evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 2163–2175. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef]
- Mostafa, D.A.E.; Khalifa, M.K.; Gad, S.S. Zolmitriptan Brain targeting via intranasal route using solid lipid nanoparticles for migraine therapy: Formulation, Characterization, in-vitro and In-vivo Assessment. Int. J. Appl. Pharm. 2020, 10, 86–93. [Google Scholar] [CrossRef]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef]
- Du, W.; Li, H.; Tian, B.; Sai, S.; Gao, Y.; Lan, T.; Meng, Y.; Ding, C. Development of nose-to-brain delivery of ketoconazole by nanostructured lipid carriers against cryptococcal meningoencephalitis in mice. Colloids Surf. B Biointerfaces 2019, 183, 110446. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Carducci, F.; Servadio, M.; Melancia, F.; Ratano, P.; Campolongo, P.; Trezza, V.; Cortesi, R.; et al. Lipid nanoparticles for administration of poorly water soluble neuroactive drugs. Biomed. Microdevices 2017, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Soane, R.J.; Frier, M.; Perkins, A.C.; Jones, N.S.; Davis, S.S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Schipper, N.G.M.; Verhoef, J.C.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; De Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-β-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef]
- Cassano, R.; Trapani, A.; Di Gioia, M.L.; Mandracchia, D.; Pellitteri, R.; Tripodo, G.; Trombino, S.; Di Gioia, S.; Conese, M. Synthesis and characterization of novel chitosan-dopamine or chitosan-tyrosine conjugates for potential nose-to-brain delivery. Int. J. Pharm. 2020, 589, 119829. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef]
- Trapani, A.; Cometa, S.; De Giglio, E.; Corbo, F.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Hossain, M.N.; Di Gioia, S.; Trapani, G.; et al. Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 147. [Google Scholar] [CrossRef]
- Sosnik, A.; das Neves, J.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Vieira, A.C.C.; Chaves, L.L.; Pinheiro, S.; Pinto, S.; Pinheiro, M.; Lima, S.C.; Ferreira, D.; Sarmento, B.; Reis, S. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm. 2018, 536, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Ismail, R.; Csoka, I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: Status quo and outlook. Drug Discov. Today 2020, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-R.; Maeng, H.-J.; Chae, J.-B.; Chong, S.; Kim, D.-D.; Shim, C.-K.; Chung, S.-J. Lack of a Primary Physicochemical Determinant in the Direct Transport of Drugs to the Brain after Nasal Administration in Rats: Potential Involvement of Transporters in the Pathway. Drug Metab. Pharmacokinet. 2010, 25, 430–441. [Google Scholar] [CrossRef]

| Drug | Formulation | Animal | DTE% | DTP% | logRDTE% | logRDTP% | B%IN/IV | RB% | Other Outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Almotriptan | SLNs (Precirol, PVA), gel of 18% Plx 407 + 0.75% Na-CMC | Rats | 335.7 | 70.21 | 2.12 | 2.06 | 237.0 | 125.9 | Fast onset (Tmax,brain = 10 min); safety (biomarkers’ evaluation and histopathological examination) | [99] |
| Agomelatine | SLNs (Gelucire 43/01, PVA, sodium deoxycholate) | Rats | 190.0 | 47.37 | ND | ND | 83.3 | ND | Higher systemic bioavailability (2.76-fold vs. oral susp.) | [67] |
| SLNs in gel of 16% Plx 407 + 0.4% HPMC | Rats | 141.4 | 29.29 | ND | ND | 52.6 | ND | Higher systemic bioavailability (2.35-fold vs. oral susp.); prolonged half-life (plasma, 378.92 min) | [60] | |
| Artemether | NLCs (Trimyristin, medium chain triglyceride, Plx 188) | Rats | 278.2 | 64.02 | 2.07 | 2.05 | 444.8 | 254.5 | Higher brain:blood conc. ratio (vs. IN and IV drug sol.); safety (histopathological examination) | [108] |
| Asenapine | NLCs (GMS, oleic acid, Tween 80) | Rats | 207.2 | 51.73 | 2.07 | 2.08 | 276.7 | 267.8 | Higher Cmax,brain (1.4- and 1.8-fold vs. IN and IV drug sol., respectively) | [28] |
| Asenapine | NLCs (GMS, oleic acid, Tween 80), glycol CS coating | Rats | 288.3 | 65.31 | 2.22 | 2.18 | 407.9 | 394.8 | Higher Cmax,brain (1.8- and 2.3-fold vs. IN and IV drug sol., respectively); safety (histopathological examination and embryo fetal toxicity study) | [29] |
| Donepezil | SLNs (Glyceryl behenate, Tween 80, Plx 188) | Rats | 288.8 | 65.37 | 2.26 | 2.26 | 163.6 | 197.6 | Higher Cmax,brain (4.1-fold vs. IN drug sol. and 5.4-fold vs. IV drug sol.) | [109] |
| Donepezil | SLNs (GMS, Tween 80, Plx 188) | Rabbits, rats | 553.9 | 81.94 | 2.36 | 2.14 | 300.9 | 290.9 | Higher Cmax,brain (5.5-fold vs. IN drug sol. and 7.6-fold vs. IV drug sol.); higher drug in brain vs. IN drug sol. (gamma scintigraphy) | [110] |
| Duloxetine | NLCs (GMS, capryol PGMC, Plx 188, sodium taurocholate) | Rabbits, rats | 758.1 | 86.81 | 2.42 | 2.12 | 5219 | 984.9 | Higher drug conc. in brain (3.8- and 2.9-fold vs. IN drug sol.) | [73,92,102] |
| Efavirenz | NLCs (Precirol ATO 5, Captex P500, MYS-25) | Rats | 1205 (487.4) | 91.7 (88.2) | 3.40 | ND | 1272.5 | 1782.5 | Higher Cmax,brain (2.5-fold vs. IN drug susp.); higher AUC0-inf,brain (3.73-fold vs. IV NLCs); safety (biomarkers evaluation and histopathological examination) | [65] |
| Escitalopram + paroxetine | NLCs (Precirol ATO5, Lauroglycol 90, Tween 80, borneol), gel of 18% Plx 407, 0.2% Carbopol 974P | Mice | 25.4; 388 | −293.7; 74.23 | ND; 2.22 | ND; 2.12 | ND; 272.5 | ND; 138.3 | Higher Cmax,brain (4.8-fold vs. IN free drug gel and 5.9-fold vs. IV drug sol.) for paroxetine | [111] |
| Haloperidol | SLNs (GMS, Tween 80) | Rats | 2362.4 | 95.77 | 2.32 | 2.02 | 500.9 | 349.7 | Higher Cmax,brain (3.7-fold vs. IN drug sol. and 4.3-fold vs. IV drug sol.) | [103,104] |
| Levofloxacin + doxycycline | SLNs (Compritol 888 ATO, stearic acid, span 60, Plx 407), gel of 1% HPMC | Rats | 149.8; 161.9 | 33.28; 38.26 (40.24) | 2.19; 2.21 | ND | 76.4; ND | 311.0; ND | Lower Cmax,brain and AUC0–360min,brain of both drugs (vs. IV drug sol.) | [112] |
| Nicergoline | NLCs (Precirol ATO5, sesame oil, Tween 80) | Rats | 237.3 (187.3) | 57.87 (56.64) | 2.41 | ND | 266.3 | 417.6 | Higher AUC0–8h,brain (1.44-fold vs. IV NLCs) | [58] |
| Ondansetron | NLCs (GMS, Capryol 90, soya lecithin, Plx 188), coating with Delonix regia gum | Rats | 5062 (506) | 98.02 (97.14) | ND | ND | 3432 | ND | Higher Cmax,brain (4.1-fold vs. IV drug sol.); safety (histopathological examination) | [113] |
| Phenytoin | NLCs (Cholesterol, oleic acid, Plx 188) | Rats | 149,952 | 99.93 | 5.18 | ND | 4873 | 3025 | Safety (histopathological examination) | [36] |
| Rats | 72,615 | 99.86 | 4.87 | ND | 3629 | 2252 | ||||
| Piribedil | SLNs (Palmitic acid, polyvinyl alcohol) | Rats | 119.9 | 16.59 | 2.34 | ND | 112.3 | 312.6 | Reduced Cmax,plasma vs. IN drug susp. | [27] |
| SLNs in gel of 1.5% methyl cellulose | 137.5 | 27.29 | 2.40 | ND | 145.3 | 404.5 | ||||
| Risperidone | SLNs (Compritol 888 ATO, Plx 407) | Mice | 830.9 | 87.97 | ND | ND | 2278.6 | ND | Higher brain:blood ratio at 1 h (10-fold vs. IV drug sol. and 5-fold vs. IV SLNs); brain targeting confirmed by gamma scintigraphy images | [66] |
| Risperidone | NLCs (Stearic acid, oleic acid, Tween 80), CS coating | Rats | 252.7 | 60.4 | 2.37 | 2.98 | 440.3 | 308.9 | Higher drug permeation ex vivo (2.32-fold vs. drug susp.) | [63] |
| Ropinirole | Anionic NLCs (Compritol 888 ATO, Labrafac, PC, Plx 188, Tween 80, SDS), gel of 15% Plx 407, 12% Plx 188, 1% HPMC | Rats | 158.5 | 36.9 | 2.83 | ND | 69.8 | 4087.9 | Higher Cmax,brain (48- and 81.8-fold) and half-life (5- and 8.8-fold) for cationic and anionic NLC gels vs. IN drug sol., respectively; safety (histopathological examination) | [61] |
| Cationic NLCs (+stearic acid), gel of 15% Plx 407, 12% Plx 188, 1% HPMC | Rats | 128.6 | 22.3 | 2.74 | ND | 99.4 | 5820.3 | |||
| Sesamol | SLNs (GMS, Tween 80) | Rats | 764 | 86.1 | ND | ND | 590.4 | ND | Shorter Tmax,brain (10 min vs. 30 min for IV drug sol.); higher Cmax,brain (13.2-fold vs. IV drug sol.) | [114] |
| Sumatriptan | NLCs (Stearic acid, cholesterol, triolein, Brij 35, Brij 72) | Rats | 2416 (258.0) | 95.86 (61.23) | 2.60 | 2.06 | 1295 | 744.6 | Higher Cmax,brain (5.6-, 7.3-, and 9.4-fold vs. IN drug sol., IV drug sol., and IV NLCs, respectively); higher AUC0–4h,brain (7.70-fold vs. IV NLCs) | [115] |
| Tarenflurbil | SLNs (GMS, stearic acid, soya lecithin, Tween 20) | Rats | 183.2 | 45.4 | 2.20 | 2.55 | 142.3 | 182.1 | Higher Cmax,brain (1.5-, 1.7-, and 4.1-fold vs. IV drug sol., IN drug sol., and oral drug susp., respectively); higher drug level in brain (multiple-dose, ~2-fold vs. IV drug sol. and oral drug susp.) | [68] |
| Temozolomide | NLCs (Gelucire 44/14, α tocopherol, Tween 80) | Rats | 457.8 | 78.16 | 2.61 | 2.82 | 588.1 | 282.7 | Gamma scintigraphy images confirmed the brain accumulation of NLCs | [116] |
| Tenofovir | NLCs (Compretol 888 ATO, oleic acid, Tween 80, Plx 188) | Rats | 481.9 | 79.25 | 2.23 | 2.08 | 1204 | 402.7 | Higher Cmax,brain (3.2, 5.8- and 6.5-fold vs. IV NLCs, IV drug sol., and IN drug sol., respectively); higher AUC0-inf,brain (3.6-fold vs. IV NLCs); higher brain accumulation (confocal microscopic image, vs. IV NLCs); safety (histopathological examination) | [78] |
| Ziprasidone | NLCs (Gelucire 43/01, Capmul MCM, Labrasol, Transcutol P) | Rats | 476.8 | 79.0 (89.85) | ND | ND | ND | ND | Higher brain:blood conc. ratios and faster onset (10 min) vs. IV drug sol. | [117] |
| Drug | Formulation | Animal | Outcomes | Ref. |
|---|---|---|---|---|
| Almotriptan | NLCs (Compritol, Labrafil, Tween 80, Lauroglycol), CS coating | Rabbits | Higher Cmax,brain (7.6-fold) and AUC0–8h,brain (8.1-fold) vs. IN drug sol.; fast onset (Tmax,brain = 10 min); higher brain:blood conc. ratios (vs. IN drug sol.); safety (histopathological examination) | [70] |
| Alprazolam | SLNs (GMS, Tween 80, Plx 188) | Rabbits, rats | Higher AUC0–8h,brain (1.33-fold vs. IV SLNs and 1.99-fold vs. IN drug sol.); reduced drug accumulation in liver, spleen, intestine, and kidney (vs. IV SLNs) | [38] |
| Astaxanthin | NLCs (GMS, soybean oil, Plx 188), gel of 20% Plx 407 + 0.5% CS | Rats | Higher Cmax,brain (9.5-fold) and AUC0–24,brain (7.8-fold) vs. IN free drug gel | [97] |
| Buspirone | SLNs (Compritol 888 ATO, Tween 80, Plx 188) | Rats | Higher Cmax,brain (1.7-fold vs. IV drug sol. and 2.3-fold vs. IV SLNs); higher AUC0–24h,brain (2.2-fold vs. IN drug sol.) | [77] |
| Buspirone | NLCs (GMS, oleic acid, Tween 80), CS coating | Rats | Higher Cmax,brain (1.5-fold vs. IV drug sol. and 2.6-fold vs. IV NLCs); higher AUC0–12h,brain (2.2-fold vs. IV drug sol. and 3.1-fold vs. IV NLCs) | [118] |
| Cinnarizine | NLCs (Cetyl palmitate, oleic acid, 3% Plx 188 + soya lecithin), gel of 19% Plx 407 + 0.5% Plx 188 + 0.1% CS | Rats | Higher Cmax,brain (2.07-fold) and AUC0–4h,brain (2.23-fold) vs. IN drug sol. | [119] |
| Clozapine | NLCs (Precirol ATO 5, oleic acid, Tween 80) | Mice | Higher Cmax,brain (11.8-fold) and AUC0–12h,brain (6.15-fold) vs. oral clozapine tablet; safety (histopathological examination) | [69] |
| Curcumin | NLCs (Precirol ATO 5, Capmul MCM, Tween 80, soya lecithin) | Rats | Higher Cmax,brain (1.6-fold) and AUC0–48h,brain (2.2-fold) vs. IN drug susp.; safety (histopathological examination) | [120] |
| Donepezil | NLCs (Glyceryl distearate, Capmul MCM, Acrysol K150, Plx 188, Tween 80), gel of gellan gum | Rats | Higher AUC0–8h,brain (126-fold vs. oral tablet) | [87] |
| Flibanserin | NLCs (Compritol 888 ATO, sweet almond oil, PC, Gelucire 44/14), gel of 0.6% gellan gum | Rats | Higher Cmax,brain (3.5-fold) and AUC0-inf,brain (6.3-fold) vs. IN flibanserin gel; safety (histopathological examination) | [121] |
| Lurasidone | NLCs (Gelot 64, Capryol 90, Tween 80, Transcutol P) | Rats | Higher Cmax,brain (1.9- and 7.9-fold) and AUC0–24h,brain (2.96- and 9.3-fold) vs. IN drug sol. (IN) and oral drug susp., respectively. | [122] |
| Olanzapine | NLCs (Compritol 888 ATO, Labrafil M 1944 CS, Tween 80), gel of 17% Plx 407 + 0.3% HPMC | Mice | Higher Cmax,brain (3.98-fold) and AUC0–6h,brain (3.81-fold) vs. IV drug sol.; safety (hematological study and histopathological examination) | [26] |
| Rats | Higher AUC0-inf,brain vs. IN microemulsion gel and IV NLCs; safety (hematological study and histopathological examination) | [75] | ||
| Oleuropein | NLCs (Tefose, Capmul, Plx 188, Tween 80, soy lecithin) | Rats | Higher AUC0–6h,brain (2.23-fold vs. IV NLCs); safety (histopathological examination) | [123] |
| Pueraria flavones | SLNs (Borneol-stearic acid, lipoid E80, Plx 188) | Rats | Higher AUC0–8h,brain (8.31-fold) and Cmax,brain (8.29-fold) (IN borneol-stearic acid SLNs vs. IN SLNs) | [124] |
| Quetiapine | NLCs (Gelucire 44/14, oleic acid, Tween 80, Transcutol P) | Rats | Higher Cmax,brain (4.15-fold) and AUC0–6h,brain (3.57-fold) vs. IV NLCs; safety (histopathological examination) | [125] |
| Resveratrol | NLCs (Cetyl palmitate, Capmul MCM, Acrysol, Tween 80, Plx 188), gel of gellan and xanthan gum | Rats | Higher Cmax,brain (2.6-fold) and AUC0–8h,brain (1.4-fold) vs. oral drug susp.; safety (histopathological examination) | [85,86] |
| Rizatriptan | SLNs (Glycerol monostearate, lecithin, Plx 407) | Rats | Higher Cmax,cerebrospinal fluid (1.3- and 5.5-fold) and AUC0-inf,cerebrospinal fluid (1.7- and 3.0-fold) vs. IV drug sol. and oral tablet, respectively | [126] |
| Ropinirole | NLCs (Tristearin, flaxseed oil, TPGS, Lipoid S100), TMC coating | Mice | Higher Cmax,brain (1.7-fold vs. IV NLCs and 17.4-fold vs. IN drug sol.); higher AUC0–12h,brain (1.5-fold vs. IV NLCs and 13.7-fold vs. IN drug sol.); safety (histopathological examination) | [127] |
| Streptomycin | SLNs (Compritol 888 ATO, Tween 80, soy lecithin) | Rabbits; mice | Higher brain conc. (4.57-fold at 0.5 h and 6.0-fold at 24 h) and AUC0-inf,brain (3.6-fold) vs. drug sol. in mice; higher brain conc. in rabbits (gamma scintigraphy) | [72] |
| Teriflunomide | NLCs (Glyceryl dibehenate, glyceryl mono-linoleate, Gelucire 44/14), gel of 0.2% carbopol 974P + 0.2% gellan gum | Mice | Higher AUC0–8,brain (1.34-fold vs. IV NLCs); higher brain:blood conc. ratios (2–3- and 8–10-fold vs. IN and IV NLCs, respectively); safety (histopathological examination and biochemical markers) | [74] |
| Drug | Formulation | Animal | Outcomes | Ref. |
|---|---|---|---|---|
| Efavirenz | SLNs (Tripalmitin, Plx 188) | Rats | Higher brain:blood conc. ratio at 24 h (150-fold vs. oral tablet) | [128] |
| Pioglitazone | NLCs (Tripalmitin, Capmul MCM, stearyl amine, Tween 80, Plx 188) | Rats | Higher brain:blood conc. ratio (1.9- and 10.7-fold vs. IN and IV drug sol., respectively); safety (histopathological examination); | [129] |
| Rimonabant | NLCs (Tristearin, Miglyol 812N, Plx 188) | Rats | Higher brain:blood conc. ratio (vs. IN drug sol.) | [130] |
| Valproic acid | NLCs (Cetyl palmitate, soy lecithin, octyldodecanol, Plx 188) | Rats | Higher brain:plasma conc. ratio at 60 min (5.09-fold vs. IP NLCs) | [80] |
| Drug | Formulation | Animal | Outcomes | Ref. |
|---|---|---|---|---|
| Artemether + lumefantrine | NLCs (Gelucire 50/13, Lipoid S75, oleic acid, Capmul MCM, Tween 80), TMC coating | Mice | Higher drug conc. in mice brain (vs. IN and oral drug susp.) | [64] |
| Astaxanthin | SLNs (Stearic acid, Plx 188, lecithin) | Rats | Higher drug conc. in the brain (~2-fold vs. IV SLNs) | [71] |
| Dimethyl fumarate | SLNs (Tristearin, Tween 80, Plx 188) | Mice | Similar brain accumulation to SLNs (IP) at a 10-fold lower dose | [131] |
| Embelin | NLCs (Cetyl palmitate, octyldodecanol, Plx 188) | Rats | Higher drug conc. in the brain (vs. IN drug sol. and IV marketed formulation) | [82] |
| Ferulic acid | SLNs (Compritol, Plx 188), CS coating | Rats | Higher drug conc. in brain (6.91-fold for IN CS-SLNs and 5.42-fold for IN SLNs vs. IN drug susp.); safety (histopathological examination) | [90] |
| Lamotrigine | NLCs (GMS, oleic acid, Tween 80, Plx 188) | Rats | Higher drug conc. in the brain (1.4- and 5.1-fold vs. IN and oral drug sol.) | [81] |
| Naloxone | SLNs (GMS, Plx 407, Tween 80) | Rabbits, rats | Better brain deposition via gamma scintigraphy and biodistribution studies (vs. IN drug sol.); safety (weight variation, histopathological examination) | [132] |
| Paeonol | SLNs (GMS, soybean lecithin, Plx 407, Tween 80), gel of 0.4% deacetylated gellan gum + 0.3% HPMC | Rats | Higher brain accumulation (vs. IV SLNs) | [12] |
| Quetiapine | SLNs (GMS, span-80, butanol), gel of 21% Plx 407 + 5.6% Plx 188 | Rats | Drug conc. in the brain: similar to those for IV drug sol. and higher than those for oral drug sol.; better effect in improving hippocampal morphology change | [133] |
| Rivastigmine | NLCs (GMS, Capmul MCM C8, Lecithin and Tween 80), gel of 15% Plx 407 + 0.8% gellan gum | Rats, mice | Higher drug concentration in the brain at 1 h (4.6-, 8.6-, and 1.6-fold vs. IN drug sol., IV drug sol., and IV NLCs, respectively); safety (hematology and histopathological examination) | [88] |
| Geraniol-ursodeoxycholic acid | SLNs (Compritol ATO 888, Span 85, Tween 80, taurocholate sodium salt) | Rats | Detection of drug in the cerebrospinal fluid until 3 h; safety (histopathological examination) | [134] |
| hIGF-1 | NLCs (Precirol ATO5, Miglyol, Tween 80, Plx 188), CS coating | Mice | Brain accumulation (via fluorescence imaging); safety (histopathological examination) | [76] |
| Nalbuphine | SLNs (Phosphatidylcholine, Tween 80, Plx 407) | Rats | Detection of drug in brain from 10 min to 8 h | [135] |
| Ondansetron | SLNs (GMS, Plx 188, lecithin) | Rabbits | Rapid drug localization in brain (1 h, gamma scintigraphy); safety (histopathological examination) | [136] |
| Rosmarinic acid | SLNs (GMS, Tween 80, hydrogenated soya phosphatidyl choline) | Rats | Drug amount in brain (5.69 µg) | [137] |
| Zolmitriptan | SLNs (Steari acid, cholesterol, lecithin), gel of 3% HPMC | Rats | Accumulation of SLNs in brain until 24 h | [138] |
| Drug | Formulation | Animal Model | Outcomes | Ref. |
|---|---|---|---|---|
| Artemether + lumefantrine | NLCs (Gelucire 50/13, Lipoid S75, oleic acid, Capmul MCM, Tween 80), TMC coating | Plasmodium berghei ANKA-injected mice | Higher parasite suppression (95% vs. 82.5% (IN NLCs), 79.1% (IN drug susp.), and 46.3% (oral drug susp.)) | [64] * |
| Artesunate | NLCs (Compritol HD5 ATO, Phospholipon 90H, Miglyol 812 N, Transcutol HP, Tween 80, Plx 188) | Plasmodium berghei ANKA-injected mice | Similar activity (54.70% vs. 58.80%) and reduction in parasitaemia (33.28% vs. 42.18%) vs. IM NLCs | [57] |
| Asenapine | NLCs (GMS, oleic acid, Tween 80) | Rats with L-dopa and carbidopa-induced catalepsy | Better therapeutic and safety profiles (vs. IN drug sol.) | [28] * |
| Astaxanthin | NLCs (GMS, soybean oil, Plx 188), gel of 20% Plx 407 + 0.5% CS | Rats with haloperidol-induced catalepsy | Improved behaviors in rotarod test and akinesia measurement (vs. IN free drug gel) | [97] * |
| Cannabidiol | NLCs (Stearic acid, oleic acid, Span 20, cetylpyridinium chloride), gel of 17% Plx 407 + 3% Plx 188 | Mice with paclitaxel-induced neuropathic pain | Increased antinociceptive effects (vs. IN and oral drug sol.) | [139] |
| Carbamazepine | NLCs (Precirol, Capmul MCM, Tween 80, Span 20), gel of 20% Plx 407, 5% Plx 188, 0.2% CS | Rats with MES | Higher protection efficacy against seizure (vs. IN plain drug gel) | [79] |
| Cinnarizine | NLCs (Cetyl palmitate, oleic acid, 3% Plx 188 + soya lecithin), gel of 19% Plx 407 + 0.5% Plx 188 + 0.1% CS | Formalin-induced acute nociception rats | Higher antinociceptive activity in neurogenic pain and inflammatory pain (vs. IN drug sol.) | [119] * |
| Clonazepam | SLNs (GMS, stearic acid, compritol, oleic acid, glycerol oleate), gel of 15% Plx 407 + 0.75% sodium alginate | Mice with pentylenetetrazole-induced epilepsy | Prolonged onset times for convulsion (7.5- and 1.5-fold) and death (14- and 5-fold) for IN SPION-NLC gel and IN NLC gel vs. control | [83] |
| Donepezil | NLCs (Glyceryl distearate, Capmul MCM, Acrysol K150, Plx 188, Tween 80), gel of gellan gum | Rats with scopolamine-induced amnesia | Improved cognitive function (vs. oral tablet) | [87] * |
| Duloxetine | NLCs (GMS, capryol PGMC, Plx 188, sodium taurocholate) | Rats, locomotor activity and forced swimming tests | Improved locomotor activity, increased swimming and climbing time, reduced immobility period vs. drug sol. (IN and IV); | [73,92,102] * |
| Embelin | NLCs (Cetyl palmitate, octyldodecanol, Plx 188) | Rats with pentylenetetrazole-induced epilepsy | Reduced malondialdehyde and nitrite and increased glutathione (vs. IN drug sol. and IV marketed formulation) | [82] * |
| Ferulic acid | SLNs (Compritol, Plx 188), CS coating | Rats with streptozocin-induced Alzheimer’s disease | Improved cognitive ability and biochemical parameters (IN CS-SLNs > IN SLNs > IN, oral drug susp., oral SLNs) | [90] * |
| Fluoxetine | NLCs (Precirol ATO5, Lauroglycol 90, Tween 80) | Mice, marble-burying and forced swimming tests | Reduced depressive and anxiety-like behaviors (better than oral drug solution) | [93] |
| GDNF | NLCs (Precirol ATO5, Miglyol, Tween 80, Plx 188), CS coating | 6-hydroxydopamine partially lesioned rats | Increased behavioral improvement, neuroprotective, and neuro-restorative effects (vs. oral drug sol.) | [95] |
| GDNF | NLCs (Precirol ATO5, Mygliol, Tween 80, Plx 188), coating with transactivator of transcription (TAT) peptide-CS conjugate | Mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease | Better motor recovery and immunohistochemistry data (vs. IN GDNF sol.) | [94] |
| Ketoconazole | NLCs (Compritol 888 ATO, Miglyol 812 N, Solutol HS15, Tween 80) | Mice infected with fungal cells | Reduced fungal burden in brain (vs. IN drug sol.) | [140] |
| Lamotrigine | NLCs (GMS, oleic acid, Tween 80, Plx 188) | Rats with MES | Improved behavioral abnormalities, decreased malondialdehyde, and increased glutathione (vs. IN and oral drug sol.) | [81] * |
| Lorazepam | SLNs (GMS, oleic acid, Plx 407), gel of CS and β-glycerol phosphate | Rats with pentylenetetrazole-induced epilepsy | Reduced occurrence seizures (vs. IN NLC dispersion and IP drug sol.) | [84] |
| Nalbuphine | SLNs (Phosphatidylcholine, Tween 80, Plx 407) | Thermal allodynia induced rats | Better analgesic effect and early onset of action (vs. IM drug sol.) | [135] * |
| Resveratrol | NLCs (Cetyl palmitate, Capmul MCM, Acrysol, Tween 80, Plx 188), gel of gellan and xanthan gum | Rats scopolamine-induced amnesia | Improved memory function (vs. oral drug sol.) | [85,86] * |
| Risperidone | NLCs (Stearic acid, oleic acid, Tween 80), CS coating | Rats with haloperidol-induced catalepsy | Greater bio-efficacy (vs. IN and IV drug susp.) | [63] * |
| Rivastigmine | NLCs (Compritol 888 ATO, triacetin, sucrose acetate, Plx) | Rats with scopolamine-induced amnesia | Improve escape latency and transfer latency (vs. IN drug sol.) | [89] |
| Rivastigmine | NLCs (GMS, Capmul MCM C8, Lecithin and Tween 80), gel of 15% Plx 407 + 0.8% gellan gum | Mice with scopolamine-induced amnesia | Faster regain of memory loss (vs. IN and IV drug sol.) | [88] * |
| Ropinirole | SLNs (Dynasan 114, stearylamine, Plx 188, soy lecithin) | Mice with chlorpromazine-induced Parkinsonism-like signs | Better anti-tremor activity with a 3.3-fold lower dose (vs. oral tablet); safety (histopathological examination) | [96] |
| Rosmarinic acid | SLNs (GMS, Tween 80, hydrogenated soya phosphatidyl choline) | Rats with 3-nitropropionic acid-induced neurotoxicity | Increased protection against striatal oxidative stress (vs. IV SLNs) | [137] * |
| Selegiline | NLCs (Stearylamine, olive oil, Tween 80, Plx 188) | Rats with rotenone-induced Parkinson’s disease | Better restored behavior (vs. IN drug sol.); reduced malondialdehyde and nitrite and increased glutathione (vs. IN drug sol.) | [98] |
| Sertraline | SLNs (GMS, Plx 188, Tween 80) | Rats, tail suspension test and forced swimming test | Similar reduction in immobility duration (vs. IN free drug) but at a 2.5-fold higher dose. | [91] |
| Teriflunomide | NLCs (Compritol 888 ATO, maisine 35–1, Gelucire 44/14); gel of 17% Plx 407 + 0.3% HPMC | Rats with cuprizone-induced demyelination | Rapid remyelination and improved behaviors (vs. oral NLCs); safety (microscopic examination, hepatic biomarkers) | [100] |
| URB597 | SLNs (Tristearin, Plx 188) | Rats, social behavioural study | Similar behavioral effects (vs. IP drug sol.) | [141] |
| Valproic acid | NLCs (Cetyl palmitate, soy lecithin, octyldodecanol, Plx 188) | Rats with MES | Similar protective effects (vs. IP drug sol.) with a 37.5-fold lower dose | [80] * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. https://doi.org/10.3390/pharmaceutics14030572
Nguyen T-T-L, Maeng H-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics. 2022; 14(3):572. https://doi.org/10.3390/pharmaceutics14030572
Chicago/Turabian StyleNguyen, Thi-Thao-Linh, and Han-Joo Maeng. 2022. "Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery" Pharmaceutics 14, no. 3: 572. https://doi.org/10.3390/pharmaceutics14030572
APA StyleNguyen, T.-T.-L., & Maeng, H.-J. (2022). Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics, 14(3), 572. https://doi.org/10.3390/pharmaceutics14030572







