Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Updated Review
Abstract
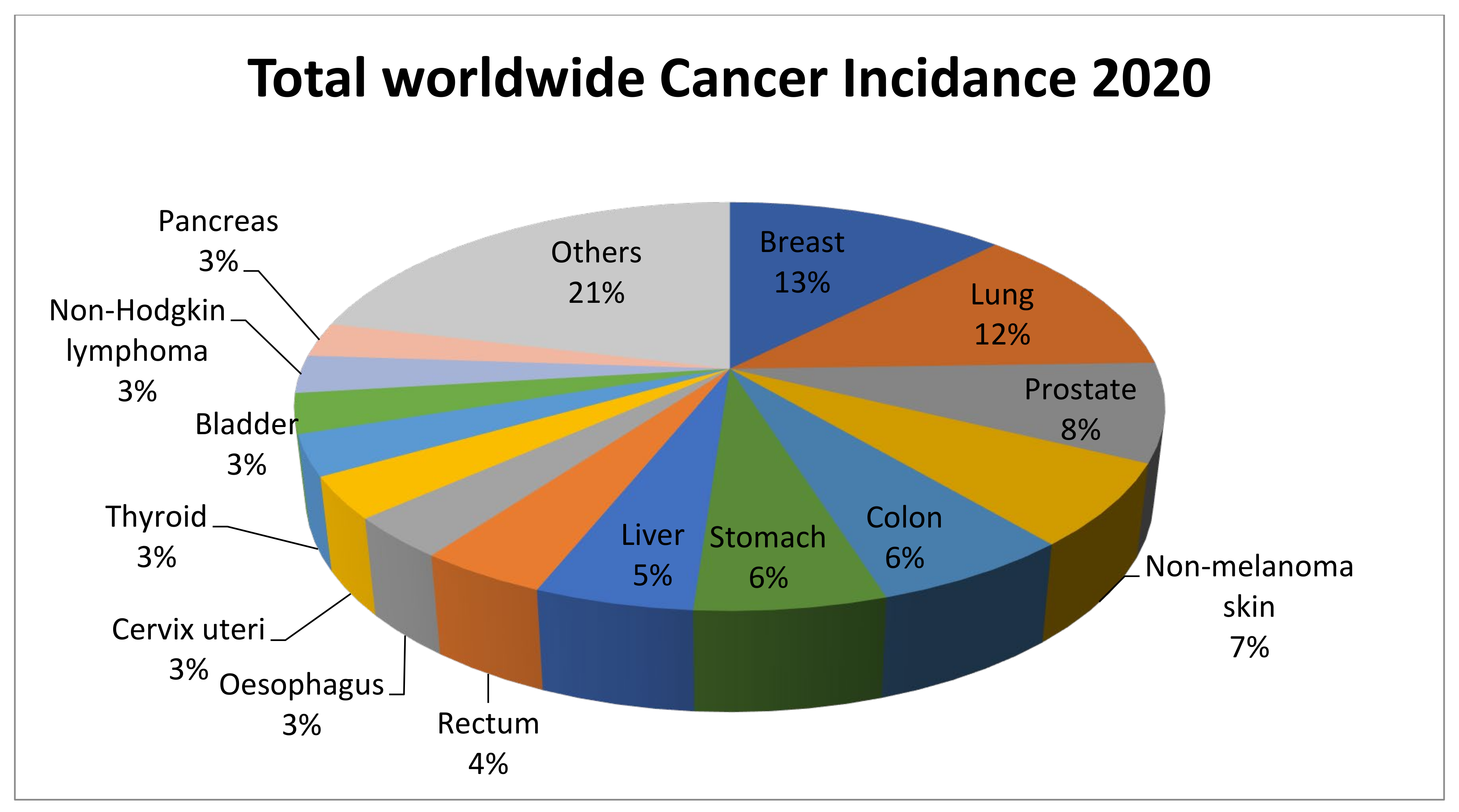
:1. Introduction
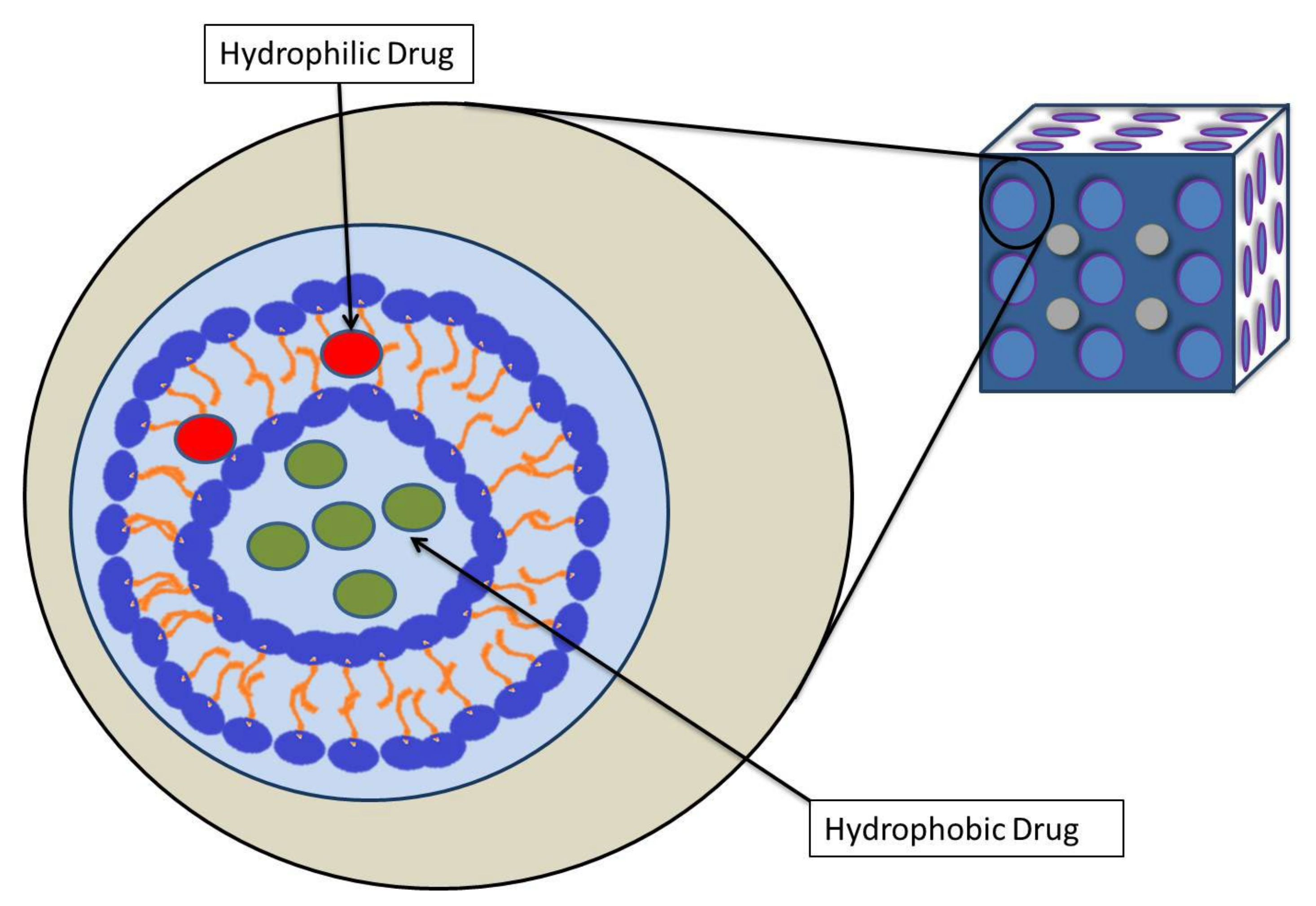
2. Cubosomes
3. Development of Cubosomes
3.1. Self-Assembly of Amphiphilic Liquids
3.2. Amphiphilic Lipids for Cubosomes
3.3. Stabilizers
3.4. Preparation of Cubosomes
3.5. Characterization of Cubosomes
4. Physiological Properites and Drug Delivery of Cubosome
5. Anticancer Activity of Cubosome
5.1. Cubosomes in Colorectal Cancer
5.2. Cubosomes in Liver Cancer
5.3. Cubosomes in Breast Cancer
5.4. Cubosomes in Lung Cancer
5.5. Cubosomes in Cervical Cancer
5.6. Cubosomes in Ovarian Cancer
5.7. Cubosomes in Skin Cancer
6. Cancer Theranostics and Cubosomes
7. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Compton, C. Cancer initiation, promotion, and progression and the acquisition of key behavioral traits. In Cancer: The Enemy from Within; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–48. [Google Scholar]
- Vokinger, K.N.; Hwang, T.J.; Grischott, T.; Reichert, S.; Tibau, A.; Rosemann, T.; Kesselheim, A.S. Prices and clinical benefit of cancer drugs in the USA and Europe: A cost–benefit analysis. Lancet Oncol. 2020, 21, 664–670. [Google Scholar] [CrossRef]
- Naoum, G.E.; Morkos, M.; Kim, B.; Arafat, W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol. Cancer 2018, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.-F.; Jiang, X.-N.; Gong, F.-L.; Guo, X.-L. New insights into molecular mechanisms of rosiglitazone in monotherapy or combination therapy against cancers. Chem. Biol. Interact. 2018, 296, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef]
- Skoetz, N.; Will, A.; Monsef, I.; Brillant, C.; Engert, A.; von Tresckow, B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst. Rev. 2017, 5, CD007941. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Krikorian, S.; Pories, S.; Tataronis, G.; Caughey, T.; Chervinsky, K.; Lotz, M.; Shen, A.H.; Weissmann, L. Adherence to oral chemotherapy: Challenges and opportunities. J. Oncol. Pharm. Pract. 2019, 25, 1590–1598. [Google Scholar] [CrossRef]
- Pisu, M.; Azuero, A.; Benz, R.; McNees, P.; Meneses, K. Out-of-pocket costs and burden among rural breast cancer survivors. Cancer Med. 2017, 6, 572–581. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar]
- Raniolo, S.; Vindigni, G.; Ottaviani, A.; Unida, V.; Iacovelli, F.; Manetto, A.; Figini, M.; Stella, L.; Desideri, A.; Biocca, S. Selective targeting and degradation of doxorubicin-loaded folate-functionalized DNA nanocages. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timucin, A.C.; Basaga, H.; Kutuk, O. Selective targeting of antiapoptotic BCL-2 proteins in cancer. Med. Res. Rev. 2019, 39, 146–175. [Google Scholar] [CrossRef] [PubMed]
- Manish, G.; Vimukta, S. Targeted drug delivery system: A review. Res. J. Chem. Sci. 2011, 1, 135–138. [Google Scholar]
- Khan, I.; Khan, M.; Umar, M.N.; Oh, D.-H. Nanobiotechnology and its applications in drug delivery system: A review. IET Nanobiotechnol. 2015, 9, 396–400. [Google Scholar] [CrossRef]
- Powers, K.W.; Palazuelos, M.; Moudgil, B.M.; Roberts, S.M. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 2007, 1, 42–51. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Ban, D.K.; Paul, S. Protein corona over silver nanoparticles triggers conformational change of proteins and drop in bactericidal potential of nanoparticles: Polyethylene glycol capping as preventive strategy. Colloids Surf. B Biointerfaces 2016, 146, 577–584. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.; Tomczak, P.; Ackland, S.J.A.o.o. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.-H.; Zeng, R.-F.; Fang, S.; Dai, Q.-S.; Li, H.-P.; Long, J.-T. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int. J. Pharm. 2014, 474, 112–122. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [Green Version]
- Sailor, M.J.; Park, J.H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Hosseini, S.F.; Jafari, S.M. Cubosomes and Hexosomes as Novel Nanocarriers for Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 1423–1437. [Google Scholar] [CrossRef]
- Kwon, T.K.; Kim, J.-C. Preparation and in vitro skin permeation of cubosomes containing hinokitiol. J. Dispers. Sci. Technol. 2010, 31, 1004–1009. [Google Scholar] [CrossRef]
- Lalu, L.; Tambe, V.; Pradhan, D.; Nayak, K.; Bagchi, S.; Maheshwari, R.; Kalia, K.; Tekade, R.K. Novel nanosystems for the treatment of ocular inflammation: Current paradigms and future research directions. J. Control. Release 2017, 268, 19–39. [Google Scholar]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A.J.I.J.o.P. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Bar, H.M.; Abd el Basset Sanad, R. Endocytic pathways of optimized resveratrol cubosomes capturing into human hepatoma cells. Biomed. Pharmacother. 2017, 93, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Patond, V.B.; Ghonge, A.B.; Narkhede, M.B. Cubosome-Review. Int. J. Trend Sci. Res. Dev. 2020, 4, 1116–1120. [Google Scholar]
- Rarokar, N.; Khedekar, P. Cubosomes: A vehicle for delivery of various therapeutic agents. MOJ Toxicol. 2018, 4, 19–21. [Google Scholar]
- Wakaskar, R.R. General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Papahadjopoulos-Sternberg, B.; Ollivon, M.; Bourgaux, C.J.L. Proteocubosomes: Nanoporous vehicles with tertiary organized fluid interfaces. Langmuir 2005, 21, 4138–4143. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. Lipid nanoparticles and their application in nanomedicine. Curr. Pharm. Biotechnol. 2016, 17, 662–672. [Google Scholar] [CrossRef]
- Demurtas, D.; Guichard, P.; Martiel, I.; Mezzenga, R.; Hébert, C.; Sagalowicz, L. Direct visualization of dispersed lipid bicontinuous cubic phases by cryo-electron tomography. Nat. Commun. 2015, 6, 8915. [Google Scholar] [CrossRef]
- Mathews, P.D.; Mertins, O.; Angelov, B.; Angelova, A. Cubosomal lipid nanoassemblies with pH-sensitive shells created by biopolymer complexes: A synchrotron SAXS study. J. Colloid Interface Sci. 2022, 607, 440–450. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, K.; Kaushik, N.; Kumar, A. Cubosomes: Versatile Nanosized Formulation for Efficient Delivery of Therapeutics. Curr. Drug Deliv. 2021, 19, 658–671. [Google Scholar] [CrossRef]
- Barriga, H.M.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Endo, M.; Sugiyama, H. Lipid-bilayer-assisted two-dimensional self-assembly of DNA origami nanostructures. Nat. Commun. 2015, 6, 8052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaballa, S.A.; El Garhy, O.H.; Abdelkader, H. Cubosomes: Composition, preparation, and drug delivery applications. J. Adv. Biomed. Pharm. Sci. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Abdelkader, H.; Alani, A.W.; Alany, R.G. Recent advances in non-ionic surfactant vesicles (niosomes): Self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug Deliv. 2014, 21, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Kaasgaard, T.; Drummond, C.J. Ordered 2-D and 3-D nanostructured amphiphile self-assembly materials stable in excess solvent. Phys. Chem. Chem. Phys. 2006, 8, 4957–4975. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.; Marčelja, S.; Horn, R.G. Physical principles of membrane organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.D.; Singh, G.; Singh, G.; Singhal, K.; Kant, S.; Bedi, N. Cubosomes as Potential Nanocarrier for Drug Delivery: A Comprehensive Review. J. Pharm. Res. Int. 2021, 33, 118–135. [Google Scholar] [CrossRef]
- Van Dalsen, L.; Weichert, D.; Caffrey, M. In meso crystallogenesis. Compatibility of the lipid cubic phase with the synthetic digitonin analogue, glyco-diosgenin. J. Appl. Crystallogr. 2020, 53, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Iskandar, W.F.N.W.; Salim, M.; Hashim, R.; Zahid, N.I. Stability of cubic phase and curvature tuning in the lyotropic system of branched chain galactose-based glycolipid by amphiphilic additives. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 623, 126697. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Huang, J.; Tian, C.; Xia, M.; Liu, L.; Li, Z.; Cao, J.; Gui, S.; Chu, X. A novel phytantriol-based lyotropic liquid crystalline gel for efficient ophthalmic delivery of pilocarpine nitrate. AAPS PharmSciTech 2019, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Barkate, A.R.; Gadekar, D.N. Cubosomes: The Novel Drug Delivery System. World J. Pharm. Res. 2020, 9, 1170–1185. [Google Scholar]
- Tajik Ahmadabad, B. Physicochemical Characterization of Novel Functionalized Lyotropic Liquid Crystalline Carriers for Therapeutic Nucleotide Delivery. Ph.D. Thesis, University of Melbourne, Parkville, Australia, 2017. [Google Scholar]
- Tan, A.; Hong, L.; Du, J.D.; Boyd, B.J. Self-assembled nanostructured lipid systems: Is there a link between structure and cytotoxicity? Adv. Sci. 2019, 6, 1801223. [Google Scholar] [CrossRef] [Green Version]
- Wörle, G.; Drechsler, M.; Koch, M.H.; Siekmann, B.; Westesen, K.; Bunjes, H. Influence of composition and preparation parameters on the properties of aqueous monoolein dispersions. Int. J. Pharm. 2007, 329, 150–157. [Google Scholar] [CrossRef]
- Van‘t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space–ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef]
- Wadsten-Hindrichsen, P.; Bender, J.; Unga, J.; Engström, S. Aqueous self-assembly of phytantriol in ternary systems: Effect of monoolein, distearoylphosphatidylglycerol and three water-miscible solvents. J. Colloid Interface Sci. 2007, 315, 701–713. [Google Scholar] [CrossRef]
- Cho, A.; La, Y.; Jeoung, S.; Moon, H.R.; Ryu, J.-H.; Shin, T.J.; Kim, K.T. Mix-and-match assembly of block copolymer blends in solution. Macromolecules 2017, 50, 3234–3243. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Nonionic amphiphilic linear dendritic block copolymers. solvent-induced self-Assembly and morphology tuning. Macromolecules 2019, 52, 5563–5573. [Google Scholar] [CrossRef]
- Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Thakkar, H.P. Cubosomes: Novel Nanocarriers for Drug Delivery. In Nanocarriers: Drug Delivery System; Springer: Berlin/Heidelberg, Germany, 2021; pp. 227–254. [Google Scholar]
- Bryant, S.J.; Bathke, E.K.; Edler, K.J. Bottom-up cubosome synthesis without organic solvents. J. Colloid Interface Sci. 2021, 601, 98–105. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Osmani, R.A.; Harkare, B.R.; Ghodake, P.P. Cubosomes: The inimitable nanoparticulate drug carriers. Sch. Acad. J. Pharm. 2013, 2, 481–486. [Google Scholar]
- Gorbenko, G.; Trusova, V. Protein aggregation in a membrane environment. Adv. Protein Chem. Struct. Biol. 2011, 84, 113–142. [Google Scholar]
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and evaluation of cubosomes/cubosomal gels for ocular delivery of beclomethasone dipropionate for management of uveitis. Pharm. Res. 2020, 37, 198. [Google Scholar] [CrossRef]
- Malheiros, B.; de Castro, R.D.; Lotierzo, M.C.; Casadei, B.R.; Mariani, P.; Barbosa, L.R. Influence of hexadecylphosphocholine (Miltefosine) in phytantriol-based cubosomes: A structural investigation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127720. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.H. Recent Progress in Polymer Cubosomes and Hexosomes. Macromol. Rapid Commun. 2021, 42, 2100194. [Google Scholar] [CrossRef]
- Helvig, S.; Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Recent advances in cryo-TEM imaging of soft lipid nanoparticles. Aims Biophys. 2015, 2, 116–130. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Müllertz, A. Development of Self-Emulsifying Drug Delivery Systems (SEDDS) for Oral Bioavailability Enhancement of Poorly Soluble Drugs. Drug Deliv. Strateg. Poorly Water-Soluble Drugs 2013, 7, 225–245. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, J.; Cortez-Jugo, C.; Han, Y.; Ma, Y.; Pan, S.; Hanssen, E.; Richardson, J.J.; Caruso, F. Ordered mesoporous metal–phenolic network particles. J. Am. Chem. Soc. 2019, 142, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, A.; Vento, F.; Pellegrino, A.L.; Ranc, V.; Piperno, A.; Mazzaglia, A.; Mineo, P. Polymer-based graphene derivatives and microwave-assisted silver nanoparticles decoration as a potential antibacterial agent. Nanomaterials 2020, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Khoo, S.-M.; Whittaker, D.V.; Davey, G.; Porter, C.J. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef]
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl monooleate/Poloxamer 407 cubic nanoparticles as oral drug delivery systems: I. In vitro evaluation and enhanced oral bioavailability of the poorly water-soluble drug simvastatin. AAPS PharmSciTech 2009, 10, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Mohsen, A.M.; Younis, M.M.; Salama, A.; Darwish, A.B. Cubosomes as a potential oral drug delivery system for enhancing the hepatoprotective effect of Coenzyme Q10. J. Pharm. Sci. 2021, 110, 2677–2686. [Google Scholar] [CrossRef]
- Chung, H.; Kim, J.-s.; Um, J.; Kwon, I.C.; Jeong, S. Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia 2002, 45, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef]
- Nasr, M.; Younes, H.; Abdel-Rashid, R.S. Formulation and evaluation of cubosomes containing colchicine for transdermal delivery. Drug Deliv. Transl. Res. 2020, 10, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H.; Younus, M.; Hook, S.M.; Boyd, B.J.; Rizwan, S.B. Cubosomes enhance drug permeability across the blood–brain barrier in zebrafish. Int. J. Pharm. 2021, 600, 120411. [Google Scholar] [CrossRef]
- Elsenosy, F.M.; Abdelbary, G.A.; Elshafeey, A.H.; Elsayed, I.; Fares, A.R. Brain Targeting of Duloxetine HCL via Intranasal Delivery of Loaded Cubosomal Gel: In vitro Characterization, ex vivo Permeation, and in vivo Biodistribution Studies. Int. J. Nanomed. 2020, 15, 9517. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid–PEG conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.W.; Acharya, D.P.; Kennedy, D.F.; Mulet, X.; Evans, R.A.; Pereira, S.M.; Wark, K.L.; Boyd, B.J.; Nguyen, T.-H.; Hinton, T.M. Metal-free and MRI visible theranostic lyotropic liquid crystal nitroxide-based nanoparticles. Biomaterials 2012, 33, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-H.; Crowston, J.G.; Huber, F.; Saubern, S.; McLean, K.M.; Hartley, P.G. The influence of dipalmitoyl phosphatidylserine on phase behaviour of and cellular response to lyotropic liquid crystalline dispersions. Biomaterials 2010, 31, 9473–9481. [Google Scholar] [CrossRef]
- Hinton, T.M.; Grusche, F.; Acharya, D.; Shukla, R.; Bansal, V.; Waddington, L.J.; Monaghan, P.; Muir, B.W. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol. Res. 2014, 3, 11–22. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Mano, M.; Lampis, S.; Angius, R.; Carnerup, A.M.; Schmidt, J.; Diaz, G.; Giacca, M.; Talmon, Y. Nanoparticles from lipid-based liquid crystals: Emulsifier influence on morphology and cytotoxicity. J. Phys. Chem. B 2010, 114, 3518–3525. [Google Scholar] [CrossRef]
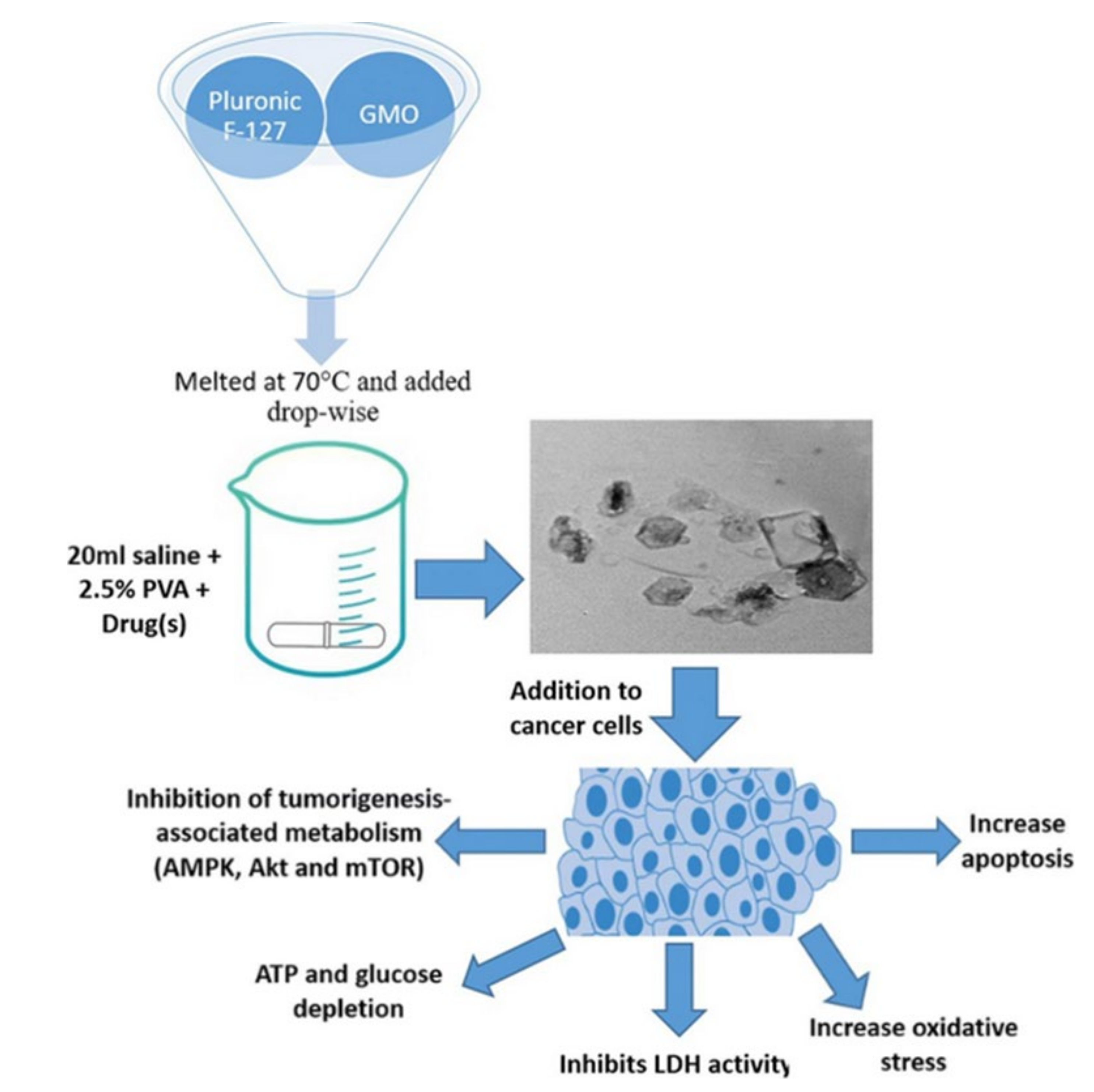
- Saber, M.M.; Al-Mahallawi, A.M.; Nassar, N.N.; Stork, B.; Shouman, S.A.J.B. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer 2018, 18, 822. [Google Scholar] [CrossRef] [Green Version]
- Magdy, M.; Almahallawi, A.; Nassar, N.; Shouman, S.J.C.T. Pluronic based cubosomes enhance metformin cytotoxicity in colon cancer cell lines. Clin. Ther. 2017, 39, e27. [Google Scholar] [CrossRef]
- Radbeh, Z.; Asefi, N.; Hamishehkar, H.; Roufegarinejad, L.; Pezeshki, A. Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (cornelian cherry) bioactive compounds. Biomed. Pharmacother. 2020, 125, 109906. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Z.-h.; Li, S.-l.; Sun, E.; Tan, X.-b.; Song, J.; Jia, X.-b. A nanostructured liquid crystalline formulation of 20 (S)-protopanaxadiol with improved oral absorption. Fitoterapia 2013, 84, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Han, S.; Yang, C.; Liu, L.; Zhao, S.; Wang, X.; Liu, B.; Pan, H.; Liu, Y. Glycyrrhetinic acid modified MOFs for the treatment of liver cancer. Nanotechnology 2020, 31, 325602. [Google Scholar] [CrossRef]
- Saber, S.; Nasr, M.; Saad, A.S.; Mourad, A.A.; Gobba, N.A.; Shata, A.; Hafez, A.-M.; Elsergany, R.N.; Elagamy, H.I.; El-Ahwany, E. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: A new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biome. Pharmacother. 2021, 142, 112029. [Google Scholar] [CrossRef]
- Luo, Q.; Lin, T.; Zhang, C.Y.; Zhu, T.; Wang, L.; Ji, Z.; Jia, B.; Ge, T.; Peng, D.; Chen, W. A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: Preparation, cytotoxicity and intracellular uptake. Int. J. Pharm. 2015, 493, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, P.; Giorgini, E.; Gambini, V.; Rossi, B.; Vaccari, L.; Vita, F.; Francescangeli, O.; Marchini, C.; Pisani, M. Lyotropic liquid-crystalline nanosystems as drug delivery agents for 5-fluorouracil: Structure and cytotoxicity. Langmuir 2017, 33, 12369–12378. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Sarieddine, R.; Alwattar, J.K.; Chouaib, R.; Gali-Muhtasib, H. Anticancer activity of thymoquinone cubic phase nanoparticles against human breast cancer: Formulation, cytotoxicity and subcellular localization. Int. J. Nanomed. 2020, 15, 9557. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Inhalable bedaquiline-loaded cubosomes for the treatment of non-small cell lung cancer (NSCLC). Int. J. Pharm. 2021, 607, 121046. [Google Scholar] [CrossRef]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Natesan, S.; Kandasamy, R. pH responsive delivery of lumefantrine with calcium phosphate nanoparticles loaded lipidic cubosomes for the site specific treatment of lung cancer. Chem. Phys. Lipids 2019, 224, 104763. [Google Scholar] [CrossRef]
- Cytryniak, A.; Nazaruk, E.; Bilewicz, R.; Górzyńska, E.; Żelechowska-Matysiak, K.; Walczak, R.; Mames, A.; Bilewicz, A.; Majkowska-Pilip, A.J.N. lipidic cubic-phase nanoparticles (cubosomes) loaded with doxorubicin and labeled with 177Lu as a potential tool for combined chemo and internal radiotherapy for cancers. Nanomaterials 2020, 10, 2272. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, U.A.; Fahmy, O.; Alhakamy, N.A. Optimized Icariin Cubosomes Exhibit Augmented Cytotoxicity against SKOV-3 Ovarian Cancer Cells. Pharmaceutics 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Luwor, R.B.; Ahmed, N.; Escalona, R.; Tan, F.H.; Fong, C.; Ratcliffe, J.; Scoble, J.A.; Drummond, C.J.; Tran, N. Paclitaxel-loaded self-assembled lipid nanoparticles as targeted drug delivery systems for the treatment of aggressive ovarian cancer. ACS Appl. Mater. Interfaces 2018, 10, 25174–25185. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and In Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. Formulation and evaluation of resveratrol loaded cubosomal nanoformulation for topical delivery. Curr. Drug Deliv. 2021, 18, 607–619. [Google Scholar] [CrossRef]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C.J.N. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Barani, M.; Bilal, M.; Rahdar, A.; Arshad, R.; Kumar, A.; Hamishekar, H.; Kyzas, G.Z. Nanodiagnosis and nanotreatment of colorectal cancer: An overview. J. Nanoparticle Res. 2021, 23, 18. [Google Scholar] [CrossRef]
- Moawad, M.M.S. Pharmacological study of the combinatorial cytotoxic effect of certain drugs in human colon cancer cell lines. Ph.D. Theses, Cairo University, Giza, Egypt, 2018. [Google Scholar]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Noorani, L.; Stenzel, M.; Liang, R.; Pourgholami, M.H.; Morris, D.L. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J. Nanobiotechnology 2015, 13, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; Wang, Q. Breast Cancer Risk Genes-Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [PubMed]
- Hussain, Z.; Khan, J.A.; Murtaza, S. Nanotechnology: An Emerging Therapeutic Option for Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 163–175. [Google Scholar] [CrossRef]
- Di Paolo, A.; Danesi, R.; Falcone, A.; Cionini, L.; Vannozzi, F.; Masi, G.; Allegrini, G.; Mini, E.; Bocci, G.; Conte, P. Relationship between 5-fluorouracil disposition, toxicity and dihydropyrimidine dehydrogenase activity in cancer patients. Ann. Oncol. 2001, 12, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Teymoordash, S.N.; Asemi, Z. Melatonin: A new inhibitor agent for cervical cancer treatment. J. Cell. Physiol. 2019, 234, 21670–21682. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: Combination therapy for effective cancer treatment. Int. J. Nanomedicine 2017, 12, 6487–6502. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. In Sunlight, Vitamin D and Skin Cancer; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–139. [Google Scholar]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M. Basics in nanoarchitectonics. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–21. [Google Scholar]
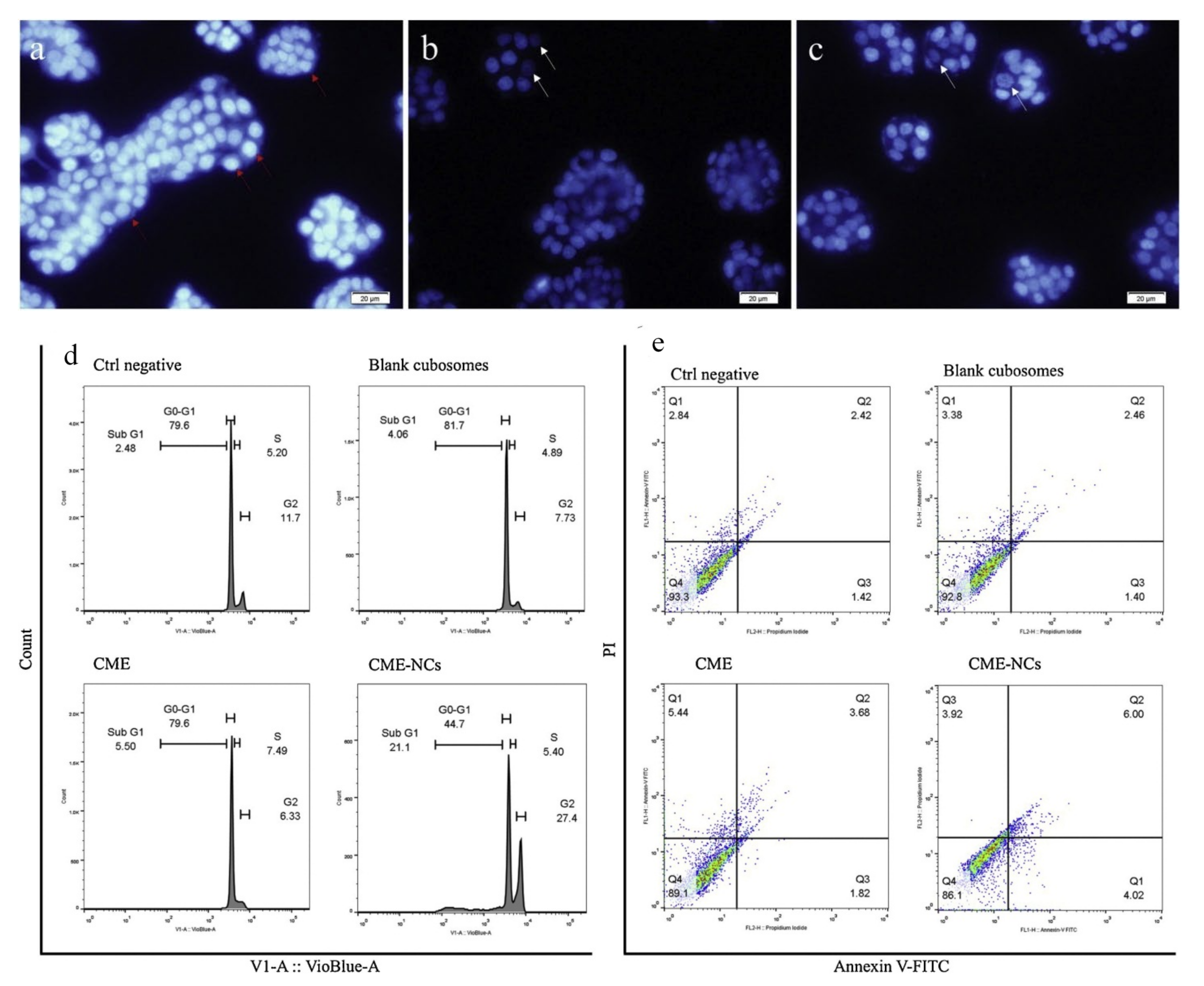
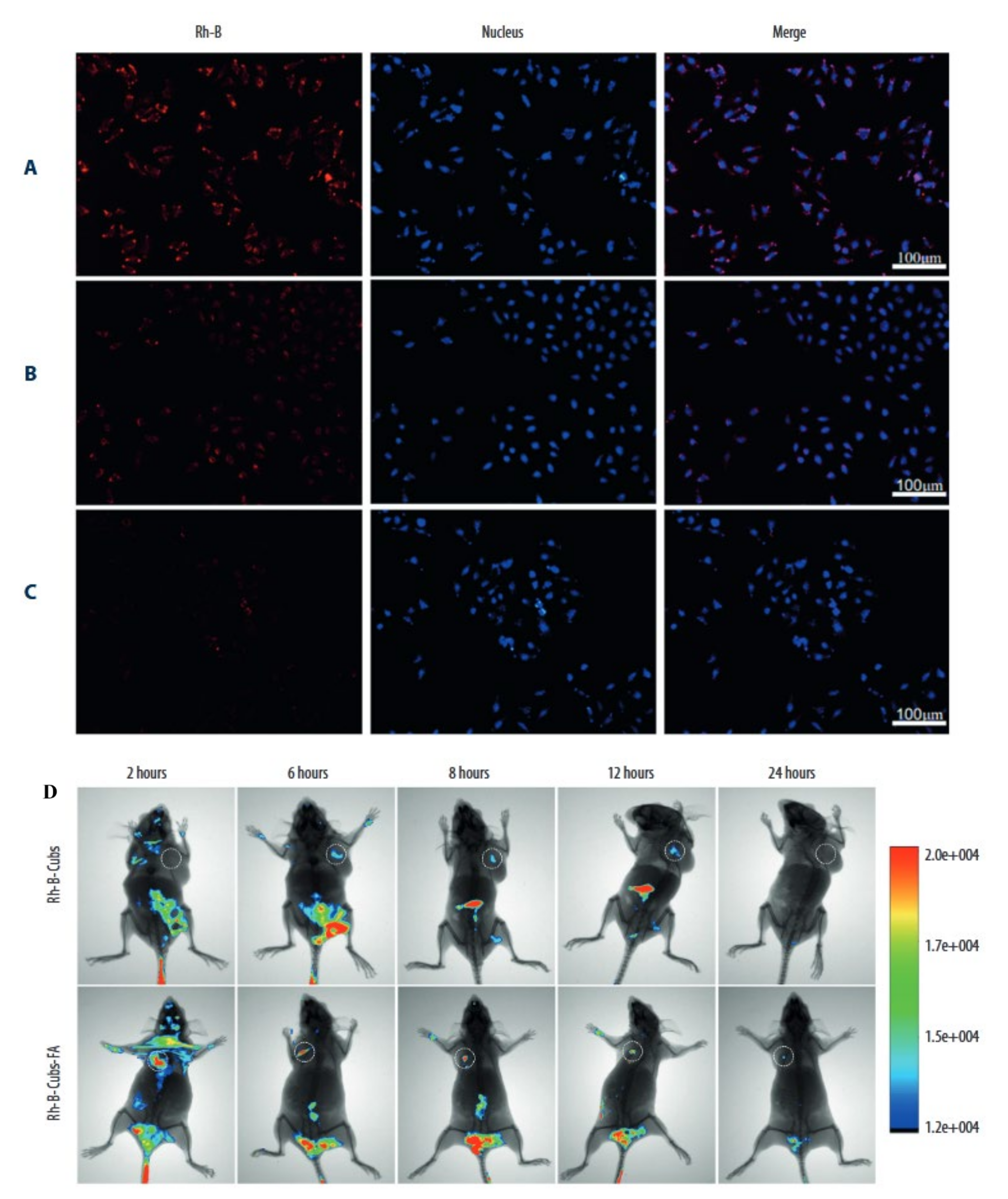
- Tian, Y.; Li, J.-C.; Zhu, J.-X.; Zhu, N.; Zhang, H.-M.; Liang, L.; Sun, L. Folic acid-targeted etoposide cubosomes for theranostic application of cancer cell imaging and therapy. Med. Sci. Monit. 2017, 23, 2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Park, S.H.; Kim, J.-C.J.B.; Engineering, B. In vitro Anti-cancer Efficacy and Cellular Interaction of Cubic Phases Containing Cinnamic Acid, Poly (ethyleneimine), and Doxorubicin. Biotechnol. Bioprocess Eng. 2020, 25, 235–245. [Google Scholar] [CrossRef]
- Godlewska, M.; Majkowska-Pilip, A.; Stachurska, A.; Biernat, J.F.; Gaweł, D.; Nazaruk, E. Voltammetric and biological studies of folate-targeted non-lamellar lipid mesophases. Electrochim. Acta 2019, 299, 1–11. [Google Scholar] [CrossRef]

| Sl No. | Cancer/ Cells Type | Chemicals/Drugs | Polymer Used | Stabilizer | Findings | Ref |
|---|---|---|---|---|---|---|
| 1 | Colorectal/HCT-116 | Cisplatin | GMO | Pluronic F127 | Cisplatin-loaded nano-cubosomes decreased the cell viability of HCT 116 and augmentation of their cytotoxicity in the presence of metformin. | [94] |
| 2 | Colorectal/HCT-116 and Caco-2 | Metformin | GMO | Pluronic F127 | The cubosomes formulation significantly lowered the IC50 concentration at which viable cells were destroyed compared to metformin alone. | [95] |
| 3 | Colorectal/HT-29 | Cornelian cherry | GMO | Poloxamer® 407 | After 24 and 48 hours of incubation, Cornus mas extract cubosome improved IC50 value 1.33 and 1.47 times higher than free Cornus mas extract. The cubosome formulation stopped G1 phase cell growth and produced apoptosis in the cancer cell line HT-29. | [96] |
| 4 | Colorectal/Caco-2 | 20(S)- protopanaxadiol | GMO | Poloxamer® 407 | The PPD-cubosome showed higher bioavailability, and better release was which is likely owing to greater absorption by the cubic nanoparticles. | [97] |
| 5 | Hepatic/HepG2 | 5-Fluorouracil | GMO | Poloxamer® 407 | 5-FU-loaded cubosomes performed well in vitro cell culture. The cubosomes formulation also boosted bio distribution concentration of 5-FU in the liver compared to the 5-FU solution alone in the rat. | [98] |
| 6 | Hepatic/rat model | Albendazole | GMO | Poloxamer® 407 | The cubosome formulation of the drug resulted in a two-fold increase in bioavailability and greater tumor regression in a rat model of cancer. | [99] |
| 7 | Hepatic/SMMC-7721 | Gambogenic acid | GMO | Poloxamer® 407 | The prepared spherical or ellipsoidal monocellular cubosomes showed remarkable cytotoxicity in the SMMC-7721 cells. | [100] |
| 8 | Hepatic/HepG2 | Resveratrol | GMO | Poloxamer® 407 | The cubosome formulation had higher cytotoxicity against hepatic HepG2 cells in vitro, and superior cell internalization of drugs was observed. | [35] |
| 9 | Breast/MDA-MB-231 | 5- Fluorouracil | Phytantriol | Pluronic F127 | In vitro cytotoxicity testing in the MDA-MB-231 cell line demonstrated that cubosomes containing 5-fluorouracil exhibit more cytotoxicity in the chosen cells than the medication alone. | [101] |
| 10 | Breast/MDA-MB-231/MCF-7 | Thymoquinone | GMO | Poloxamer® 407 | A dose and time-dependent increase in apoptotic cells was observed when treated with Thymoquinone-cubosome formulation against Thymoquinone alone. | [102] |
| 11 | Lung/A549 | Bedaquiline | GMO | Poloxamer 188 | The findings revealed that the cubosome formulation containing the medication exhibited considerable cytotoxicity in A549 cells, in addition to inducing apoptotic cell death, and had anti-invasive properties. | [103] |
| 12 | Lung/A549 | Lumefantrine | GMO | Poloxamer | In A549 cells, the cubosomes formulation demonstrated significantly greater anticancer and anti-angiogenesis action than the medication alone. | [104] |
| 13 | Cervical/Hela | Doxorubicin | GMO | Pluronic F127 | There was somewhat higher IC50 (15 MBq/mL) but statistically significant cytotoxicity at shorter time points, such as 24 h, with the cubosomes formulation. | [105] |
| 14 | Cervical/Hela | Paclitaxel | GMO | PF108-B | The biotinylated cubosome facilitated drug uptake at the cellular level. | [106] |
| 15 | Ovary/SKOV-3 and Caov 3 | Icariin | GMO | Poloxamer® 407 | The findings indicate that Icariin-cubosomes exhibit considerably increased cytotoxicity in both SKOV-3 and Caov 3 cells, but not in normal EA.hy926 endothelial cells. | [107] |
| 16 | Ovary/HEY | Paclitaxel | GMO | Pluronic F127 | The paclitaxel cubosomes demonstrated increased cytotoxicity in ovarian cells (HEY) and a 50% reduction in tumor burden in an animal xenograft model with more safety feature. | [108] |
| 17 | Skin/A431 cells | Paclitaxel | GMO | Pluronic F127 | Loaded paclitaxel accumulated preferentially at the tumor location. Additionally, when paclitaxel was loaded, the average tumor size was decreased to half of its original size when compared to the medication alone. | [109] |
| 18 | Skin/mice | Resveratrol | GMO | Pluronic F127 | The formulation improved skin permeability and deposition at the place of application in the mouse skin layer. | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almoshari, Y. Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Updated Review. Pharmaceutics 2022, 14, 600. https://doi.org/10.3390/pharmaceutics14030600
Almoshari Y. Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Updated Review. Pharmaceutics. 2022; 14(3):600. https://doi.org/10.3390/pharmaceutics14030600
Chicago/Turabian StyleAlmoshari, Yosif. 2022. "Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Updated Review" Pharmaceutics 14, no. 3: 600. https://doi.org/10.3390/pharmaceutics14030600
APA StyleAlmoshari, Y. (2022). Development, Therapeutic Evaluation and Theranostic Applications of Cubosomes on Cancers: An Updated Review. Pharmaceutics, 14(3), 600. https://doi.org/10.3390/pharmaceutics14030600






