Sustained Release of Co-Amorphous Matrine-Type Alkaloids and Resveratrol with Anti-COVID-19 Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Co-Amorphous Systems
2.3. Solid-State Characterization
2.3.1. PXRD
2.3.2. mDSC
2.3.3. FTIR
2.4. NMR
2.5. Dissolution Experiments
2.5.1. Equilibrium Solubility Determination
2.5.2. In Vitro Release Experiment
2.6. Scanning Electron Microscopy
2.7. Physical Stability
3. Results and Discussion
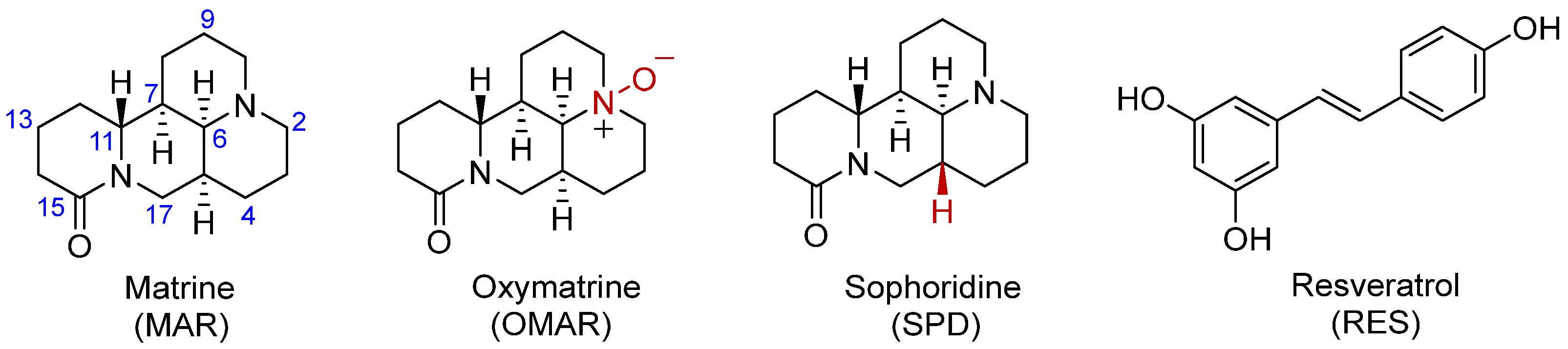
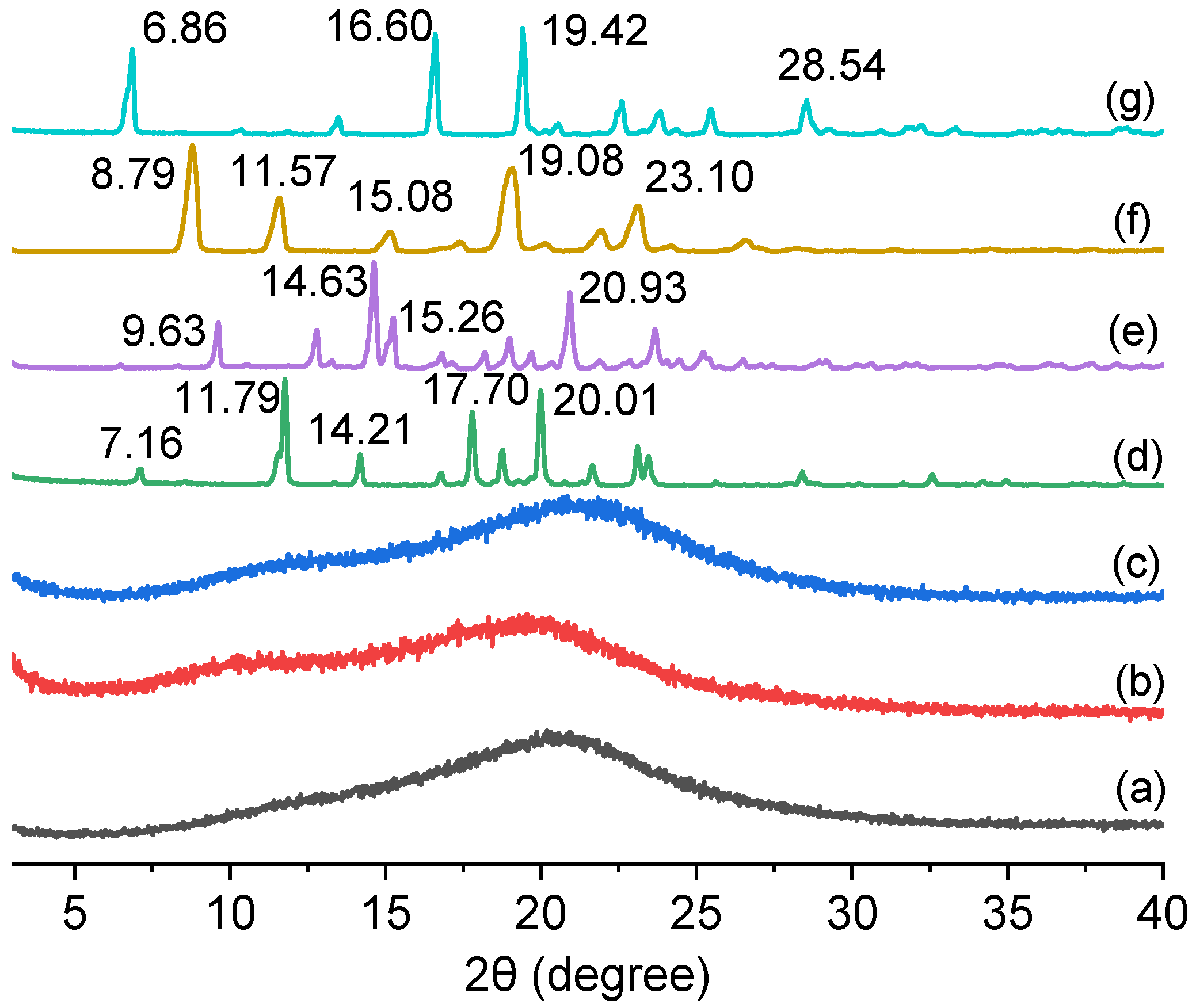
3.1. The Design and Preparation of Co-Amorphous Drug Systems of Matrine, Oxymatrine, and Sophoridine with Resveratrol and Their PXRD Characterization
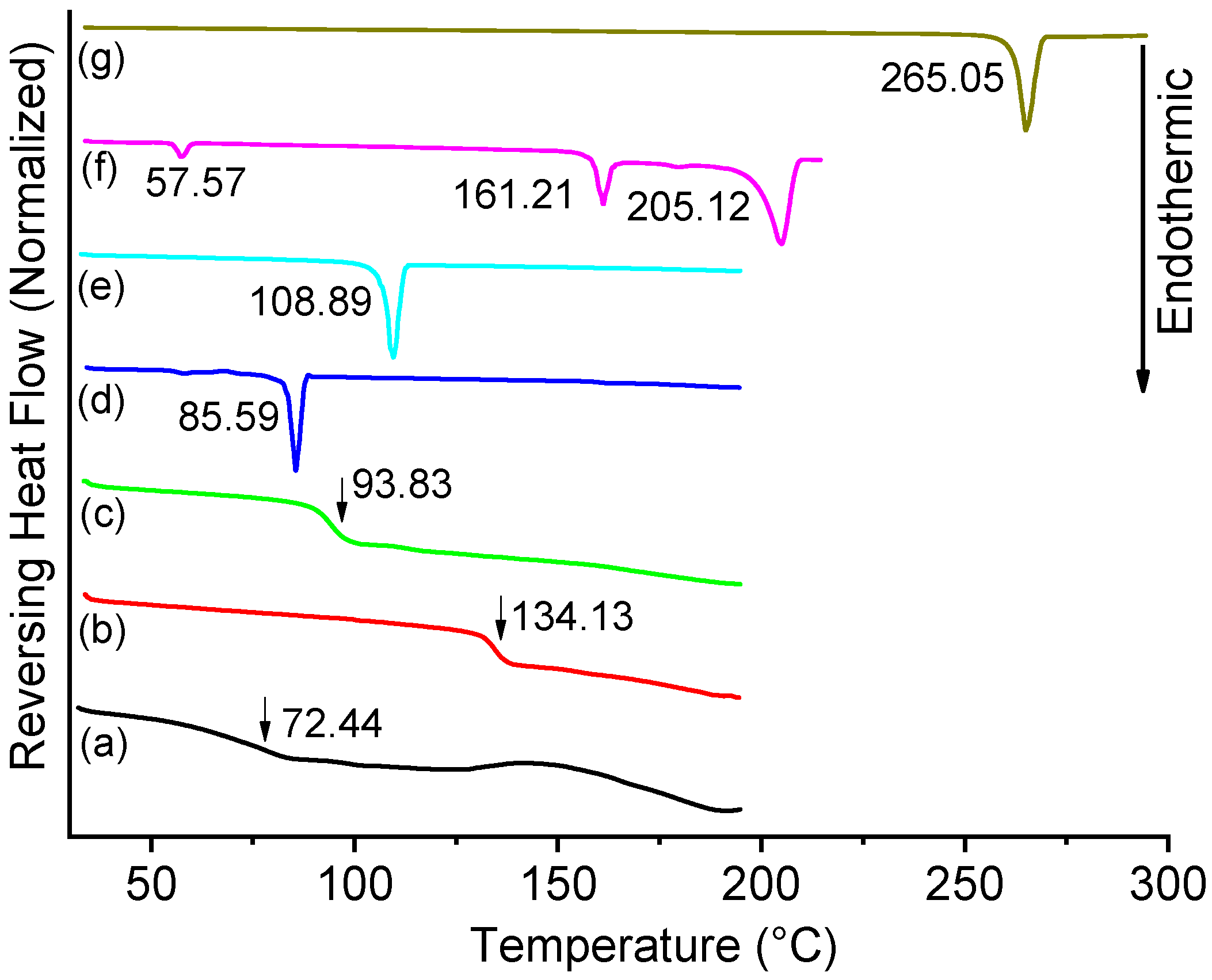
3.2. mDSC Study of the Amorphous MAR-RES, OMRA-RES, and SPD-RES Samples
3.3. FTIR
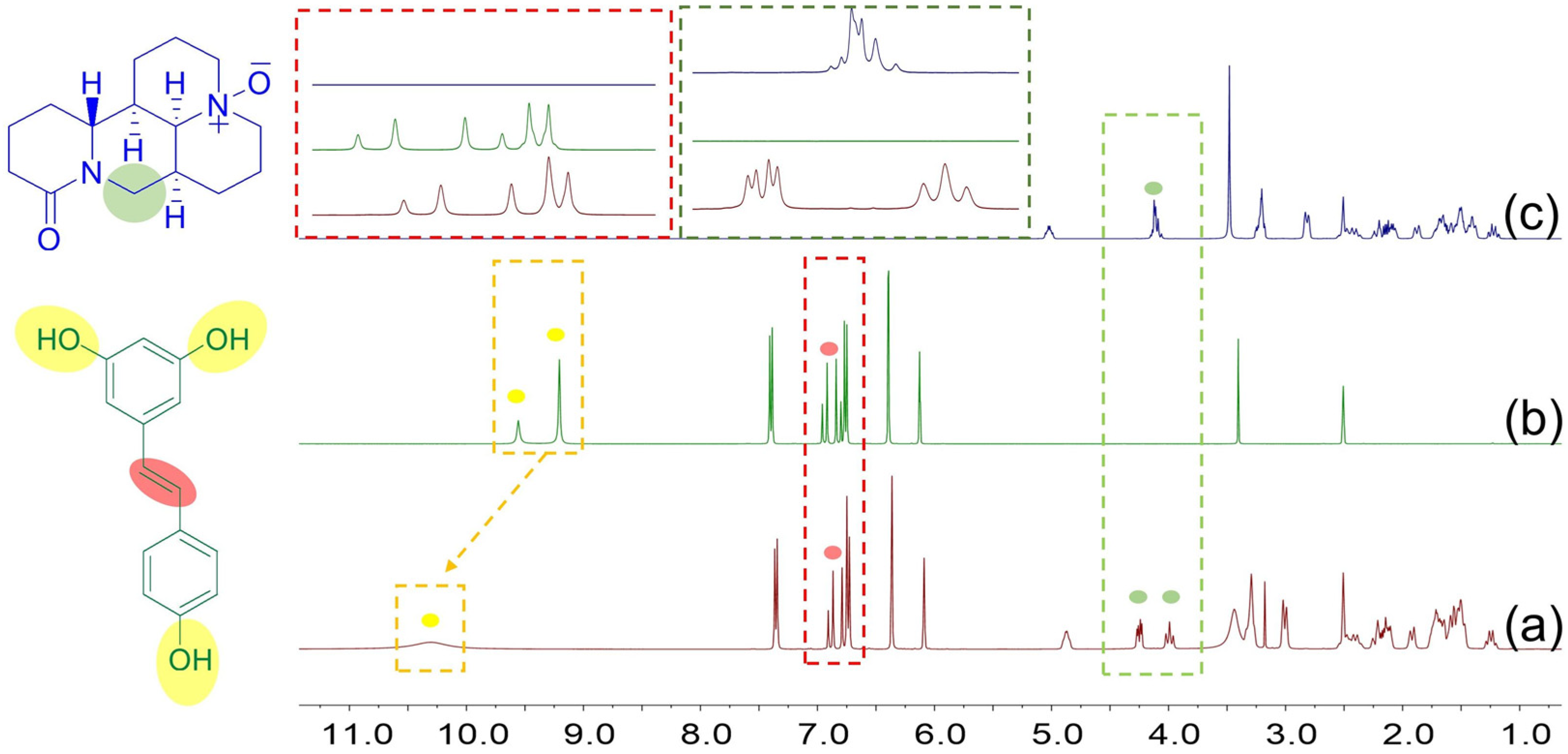
3.4. The Molecular Interactions of Resveratrol and Oxymatrine in DMSO-d6
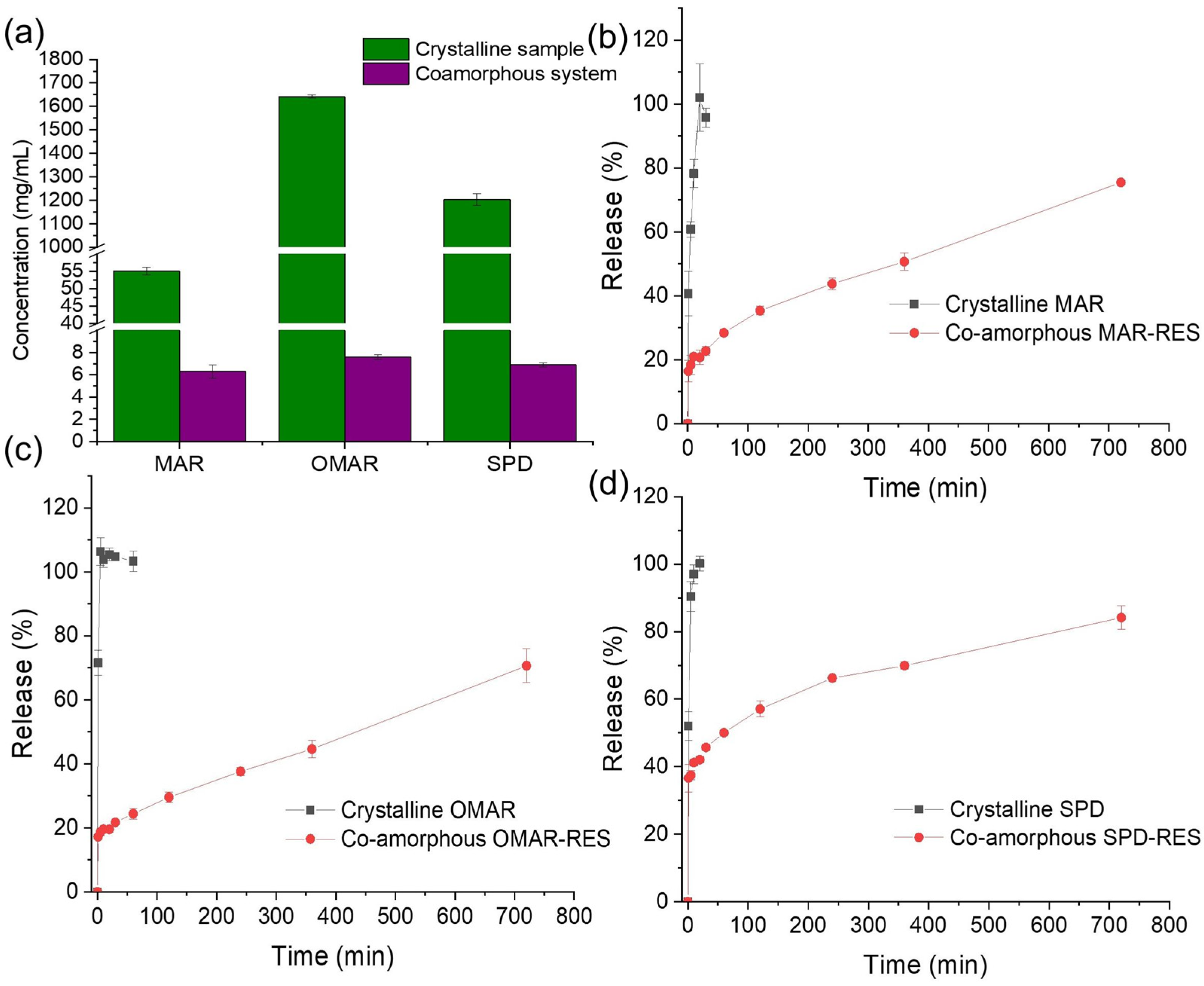
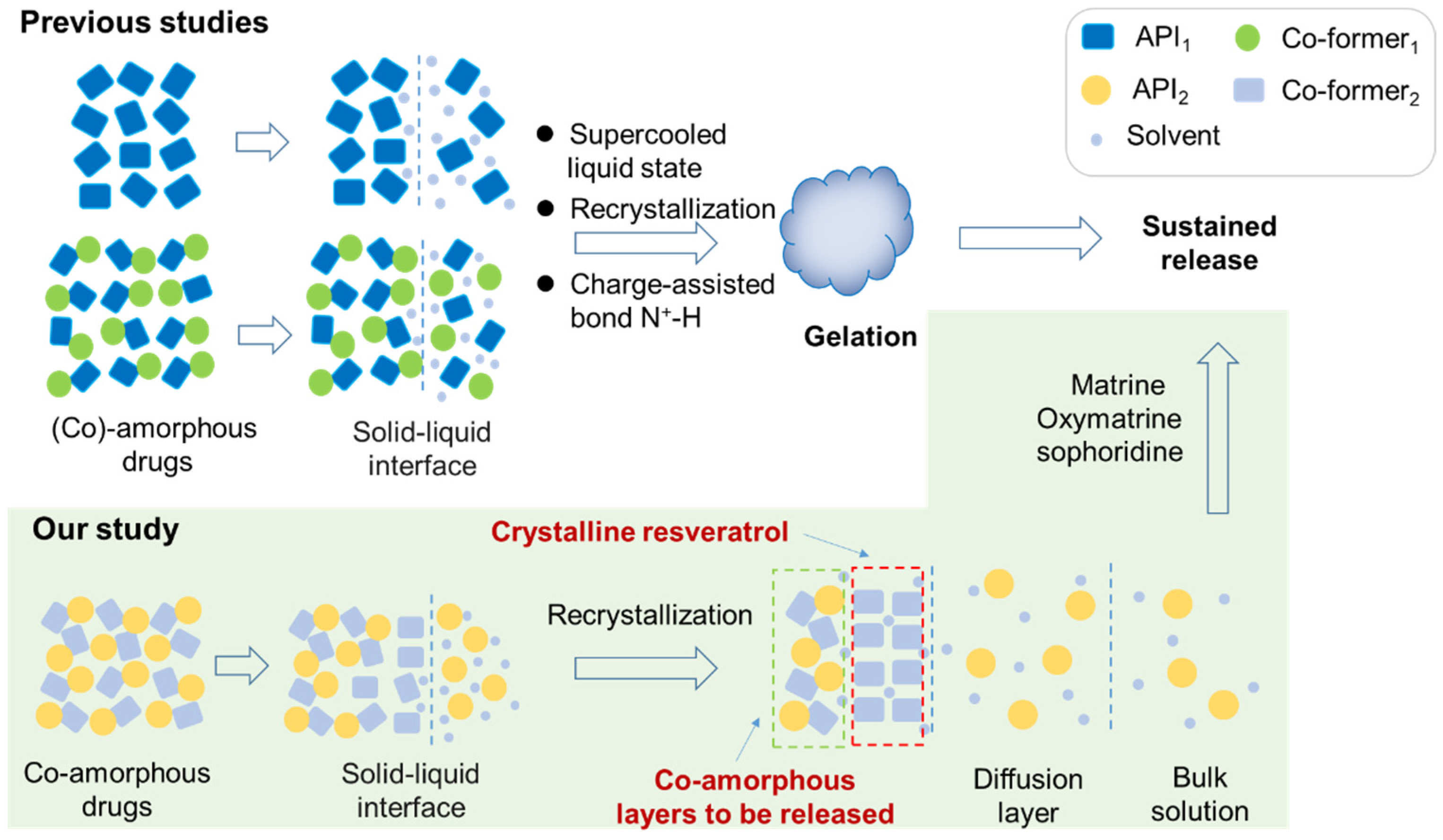
3.5. Dissolution Experiments
3.6. Physical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19). Dashboard. Available online: https://covid19.who.int/ (accessed on 10 December 2021).
- COVID-19 Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 10 December 2021).
- Riva, L.; Yuan, S.F.; Yin, X.; Martin-Sancho, L.; Matsunaga, N.; Pache, L.; Burgstaller-Muehlbacher, S.; De Jesus, P.D.; Teriete, P.; Hull, M.V.; et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020, 586, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.D.; Jeon, S.; Kim, S.; Lee, S.Y. Drugs repurposed for COVID-19 by virtual screening of 6218 drugs and cell-based assay. Proc. Natl. Acad. Sci. USA 2021, 118, e2024302118. [Google Scholar] [CrossRef] [PubMed]
- Christy, M.P.; Uekusa, Y.; Gerwick, L.; Gerwick, W.H. Natural products with potential to treat RNA virus pathogens including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.F.; Shao, S.; Feng, Y.; Ye, F.; Sun, X.; Wang, Q.L.; Yu, F.; Wang, Q.S.; Huang, B.Y.; Niu, P.H.; et al. Mechanism of microbial metabolite leupeptin in the treatment of COVID-19 by traditional chinese medicine herbs. mBio 2021, 12, e0222021. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-K. Plant solutions for the COVID-19 pandemic and beyond: Historical reflections and future perspectives. Mol. Plant. 2020, 13, 803–807. [Google Scholar] [CrossRef]
- Li, X.; Tang, Z.W.; Wen, L.; Jiang, C.; Feng, Q.S. Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J. Ethnopharmacol. 2021, 269, 113682. [Google Scholar] [CrossRef]
- Yang, M.W.; Chen, F.; Zhu, D.J.; Li, J.Z.; Zhu, J.L.; Zeng, W.; Qu, S.L.; Zhang, Y. Clinical efficacy of Matrine and Sodium Chloride Injection in treatment of 40 cases of COVID-19 (in Chinese). Zhongguo Zhong Yao Za Zhi 2020, 45, 2221–2231. [Google Scholar] [CrossRef]
- Zuniga, M.; Gomes, C.; Carsons, S.E.; Bender, M.T.; Cotzia, P.; Miao, Q.R.; Lee, D.C.; Rodriguez, A. Autoimmunity to annexin A2 predicts mortality among hospitalised COVID-19 patients. Eur. Resp. J. 2021, 58, 2100918. [Google Scholar] [CrossRef]
- Patil, P.; Shetty, P.; Kuriakose, N.; Gollapalli, P.; Shetty, S.; Bhandary, R.; Vishwanatha, J.K.; Ghate, S.D. Molecular insights on the possible role of Annexin A2 in COVID-19 pathogenesis and post-infection complications. Int. J. Mol. Sci. 2021, 22, 1028. [Google Scholar] [CrossRef]
- Wang, D.Y.; Cao, Y.; Zheng, L.Y.; Lv, D.Y.; Chen, L.D.; Xing, X.R.; Zhu, Z.Y.; Li, X.Y.; Chai, Y.F. Identification of Annexin A2 as a target protein for plant alkaloid matrine. Chem. Commun. 2017, 53, 5020–5023. [Google Scholar] [CrossRef]
- Ye, X.W.; Deng, Y.L.; Zhang, X.; Liu, M.M.; Liu, Y.; Xie, Y.T.; Wan, Q.; Huang, M.; Zhang, T.; Xi, J.H.; et al. Study on the mechanism of treating COVID-19 with Shenqi Wan based on network pharmacology. Drug Dev. Ind. Pharm. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Z.; Li, Y.B.; Yang, Y.; Li, M.; Du, Y.; Zhang, Y.Y.; Wang, J.; Shi, Y.J. Study on mechanism of matrine in treatment of COVID-19 combined with liver injury by network pharmacology and molecular docking technology. Drug Deliv. 2021, 28, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.P.; Xu, Y.; Han, D.; Feng, F.C.; Wang, Z.C.; Gu, C.; Zhou, X.M.; Wu, Q. Potential mechanism underlying the effect of matrine on COVID-19 patients revealed through network pharmacological approaches and molecular docking analysis. Arch. Physiol. Biochem. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Liu, J.; Ouyang, L. Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: Bioactivities, structure-activity relationships and preliminary molecular mechanisms. Eur. J. Med. Chem. 2020, 188, 111972. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Deng, X.X.; Gao, Q.X.; Wu, X.L.; Han, L.; Gao, X.L.; Zhao, S.P.; Chen, W.B.; Zhou, R.R.; Li, Z.Y.; et al. Sophora alopecuroides L.: An ethnopharmacological, phytochemical, and pharmacological review. J. Ethnopharmacol. 2020, 248, 112172. [Google Scholar] [CrossRef]
- Dai, J.P.; Wang, Q.W.; Su, Y.; Gu, L.M.; Deng, H.X.; Chen, X.X.; Li, W.Z.; Li, K.S. Oxymatrine inhibits influenza a virus replication and inflammation via TLR4, p38 MAPK and NF-kappa B pathways. Int. J. Mol. Sci. 2018, 19, 965. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Ding, G.T.; Zhang, H.Y.; Wang, H.P.; Jin, Z.J.; Yang, G.X.; Han, Y.H.; Zhang, X.; Li, G.Y.; Li, W.H. Antiviral activity of sophoridine against enterovirus 71 in vitro. J. Ethnopharmacol. 2019, 236, 124–128. [Google Scholar] [CrossRef]
- McCreary, M.R.; Schnell, P.M.; Rhoda, D.A. Randomized double-blind placebo-controlled proof-of-concept trial of Resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Res. Sq. 2021. Preprint. [Google Scholar] [CrossRef]
- Liao, M.T.; Wu, C.C.; Wu, S.F.V.; Lee, M.C.; Hu, W.C.; Tsai, K.W.; Yang, C.H.; Lu, C.L.; Chiu, S.K.; Lu, K.C. Resveratrol as an adjunctive therapy for excessive oxidative stress in aging COVID-19 patients. Antioxidants 2021, 10, 1440. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, S.; Yin, T.J.; Kulkarni, K.H.; Teng, Y.; You, M.; Hu, M. Biopharmaceutical and pharmacokinetic characterization of matrine as determined by a sensitive and robust UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2010, 51, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Dong, L.N.; Peng, X.J.; Li, Y.; Shi, J.; Zhou, F.Y.; Liu, Z.Q. Pharmacokinetic characterization of oxymatrine and matrine in rats after oral administration of radix Sophorae tonkinensis extract and oxymatrine by sensitive and robust UPLC-MS/MS method. J. Pharm. Biomed. Anal. 2013, 83, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Jiang, Q.; Zhang, M.Y.; Chen, S.Y.; Lou, J.S.; Chen, Y.J.; Wang, F.; Wang, R.Y. Establishment of quantitative methodology for sophoridine analysis and determination of its pharmacokinetics and bioavailability in rat. Drug Dev. Ind. Pharm. 2021, 47, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Moinuddin, S.M.; Cai, T. Advances in coamorphous drug delivery systems. Acta Pharm. Sin. B 2019, 9, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Wei, Y.F.; Lu, Y.; Wang, R.Z.; Zhang, J.J.; Gao, Y.; Qian, S. Co-amorphous systems for the delivery of poorly water-soluble drugs: Recent advances and an update. Expert Opin. Drug Deliv. 2020, 17, 1411–1435. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Indomethacin co-amorphous drug-drug systems with improved solubility, supersaturation, dissolution rate and physical stability. Int. J. Pharm. 2021, 600, 120448. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Wu, T.; Feng, Y.; Jiang, C.P.; Du, H.Z.; Lu, S. Apigenin-oxymatrine binary co-amorphous mixture: Enhanced solubility, bioavailability, and anti-inflammatory effect. Food Chem. 2021, 373, 131485. [Google Scholar] [CrossRef]
- Heng, W.L.; Wei, Y.F.; Xue, Y.F.; Cheng, H.; Zhang, L.H.; Zhang, J.J.; Gao, Y.; Qian, S. Gel formation induced slow dissolution of amorphous Indomethacin. Pharm. Res. 2019, 36, 159. [Google Scholar] [CrossRef]
- Qian, S.; Wang, S.S.; Li, Z.; Wang, X.J.; Ma, D.; Liang, S.J.; Gao, Y.; Zhang, J.J.; Wei, Y.F. Charge-assisted bond N(+)H mediates the gelation of amorphous lurasidone hydrochloride during dissolution. Int. J. Pharm. 2017, 518, 335–341. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.X.; Zhang, H.L.; Duan, Y.W.; Huang, Y. Co-amorphous systems of sinomenine with nonsteroidal anti-inflammatory drugs: A strategy for solubility improvement, sustained release, and drug combination therapy against rheumatoid arthritis. Int. J. Pharm. 2021, 606, 120894. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.X.; Zhang, H.L.; Duan, Y.W.; Huang, Y. Sinomenine-phenolic acid coamorphous drug systems: Solubilization, sustained release, and improved physical stability. Int. J. Pharm. 2021, 598, 120389. [Google Scholar] [CrossRef]
- Bui, R.; Labat, M.; Aubert, J.E. Comparison of the saturated salt solution and the dynamic vapor sorption techniques based on the measured sorption isotherm of barley straw. Constr. Build. Mater. 2017, 141, 140–151. [Google Scholar] [CrossRef]
- Lobmann, K.; Laitinen, R.; Strachan, C.; Rades, T.; Grohganz, H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs—Part 2: Molecular interactions. Eur. J. Pharm. Biopharm. 2013, 85, 882–888. [Google Scholar] [CrossRef]
- Wu, W.Q.; Grohganz, H.; Rades, T.; Lobmann, K. Comparison of co-former performance in co-amorphous formulations: Single amino acids, amino acid physical mixtures, amino acid salts and dipeptides as co-formers. Eur. J. Pharm. Sci. 2021, 156. [Google Scholar] [CrossRef] [PubMed]
- Fael, H.; Demirel, A.L. Tannic acid as a co-former in co-amorphous systems: Enhancing their physical stability, solubility and dissolution behavior. Int. J. Pharm. 2020, 581, 119284. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.H.; Li, H.; Zhu, B.; Hong, M.H.; Ren, G.B. Cocrystals of Oxymatrine: Reducing Hygroscopicity and Affecting the Dissolution Rate. Cryst. Growth Des. 2021, 21, 3874–3888. [Google Scholar] [CrossRef]
- Yue, P.F.; Yuan, H.L.; Li, X.Y.; Yang, M.; Zhu, W.F. Process optimization, characterization and evaluation in vivo of oxymatrine-phospholipid complex. Int. J. Pharm. 2010, 387, 139–146. [Google Scholar] [CrossRef]
- Ruan, J.H.; Liu, J.; Zhu, D.; Gong, T.; Yang, F.M.; Hao, X.J.; Zhang, Z.R. Preparation and evaluation of self-nanoemulsified drug delivery systems (SNEDDSs) of matrine based on drug-phospholipid complex technique. Int. J. Pharm. 2010, 386, 282–290. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Guo, Y.J.; Xu, R.J.; Fang, X.P.; Xiao, X.C.; Jiang, C.P.; Lu, S. Coamorphous system of Florfenicol-Oxymatrine for improving the solubility and dissolution rate of Florfenicol: Preparation, characterization and molecular dynamics simulation. J. Pharm. Sci. 2021, 110, 2544–2554. [Google Scholar] [CrossRef]
- Lei, W.; Xing-De, W.; Juan, H.; Gen-Tao, L.; Li-Yan, P.; Yan, L.; Liu-Dong, S.; Qin-Shi, Z. A new Quinolizidine alkaloid from Sophora flavescens. Chem. Nat. Compd. 2014, 50, 876–879. [Google Scholar] [CrossRef]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from the roots of Sophora flavescens. Arch. Pharm. Res. 2005, 28, 190–194. [Google Scholar] [CrossRef]
- Pineda-Sanabria, S.E.; Robertson, I.M.; Sykes, B.D. Structure of trans-resveratrol in complex with the cardiac regulatory protein troponin C. Biochemistry 2011, 50, 1309–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M.A.; Ali, A.A.; Maghrabi, I.A. Clozapine-carboxylic acid plasticized co-amorphous dispersions: Preparation, characterization and solution stability evaluation. Acta Pharm. 2015, 65, 133–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Chen, X.; Li, D.; Zhang, H.; Duan, Y.; Huang, Y. Sustained Release of Co-Amorphous Matrine-Type Alkaloids and Resveratrol with Anti-COVID-19 Potential. Pharmaceutics 2022, 14, 603. https://doi.org/10.3390/pharmaceutics14030603
Hu D, Chen X, Li D, Zhang H, Duan Y, Huang Y. Sustained Release of Co-Amorphous Matrine-Type Alkaloids and Resveratrol with Anti-COVID-19 Potential. Pharmaceutics. 2022; 14(3):603. https://doi.org/10.3390/pharmaceutics14030603
Chicago/Turabian StyleHu, Dandan, Xin Chen, Duanxiu Li, Hailu Zhang, Yanwen Duan, and Yong Huang. 2022. "Sustained Release of Co-Amorphous Matrine-Type Alkaloids and Resveratrol with Anti-COVID-19 Potential" Pharmaceutics 14, no. 3: 603. https://doi.org/10.3390/pharmaceutics14030603





