Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solubility of Formoterol Fumarate Dihydrate in Aqueous and Organic Media
2.2.2. Preparation of Polymeric Nanoparticles
2.2.3. Effect of Polymeric Nanoparticle Synthesis Parameters on Formoterol Drug Loading
2.2.4. Effect of Polymeric Nanoparticle Synthesis Parameters on Particle Size
2.2.5. Impact of Nanoparticle Synthesis Parameters on Zeta Potential
2.2.6. Impact of Nanoparticle Synthesis Parameters on In Vitro Drug Release
2.2.7. Characterization of Nanoparticle Surface Morphology
2.2.8. X-Ray Powder Diffraction (XRPD)
2.2.9. Thermal Analysis of Lyophilized Nanoparticles
2.2.10. Residual Water Content Analysis by Karl Fischer Titration
2.2.11. Statistical Analysis
3. Results
3.1. Solubility of Formoterol Fumarate Dihydrate in Aqueous and Organic Media
3.2. Effect of Polymeric Nanoparticle Synthesis Parameters on Formoterol Drug Loading
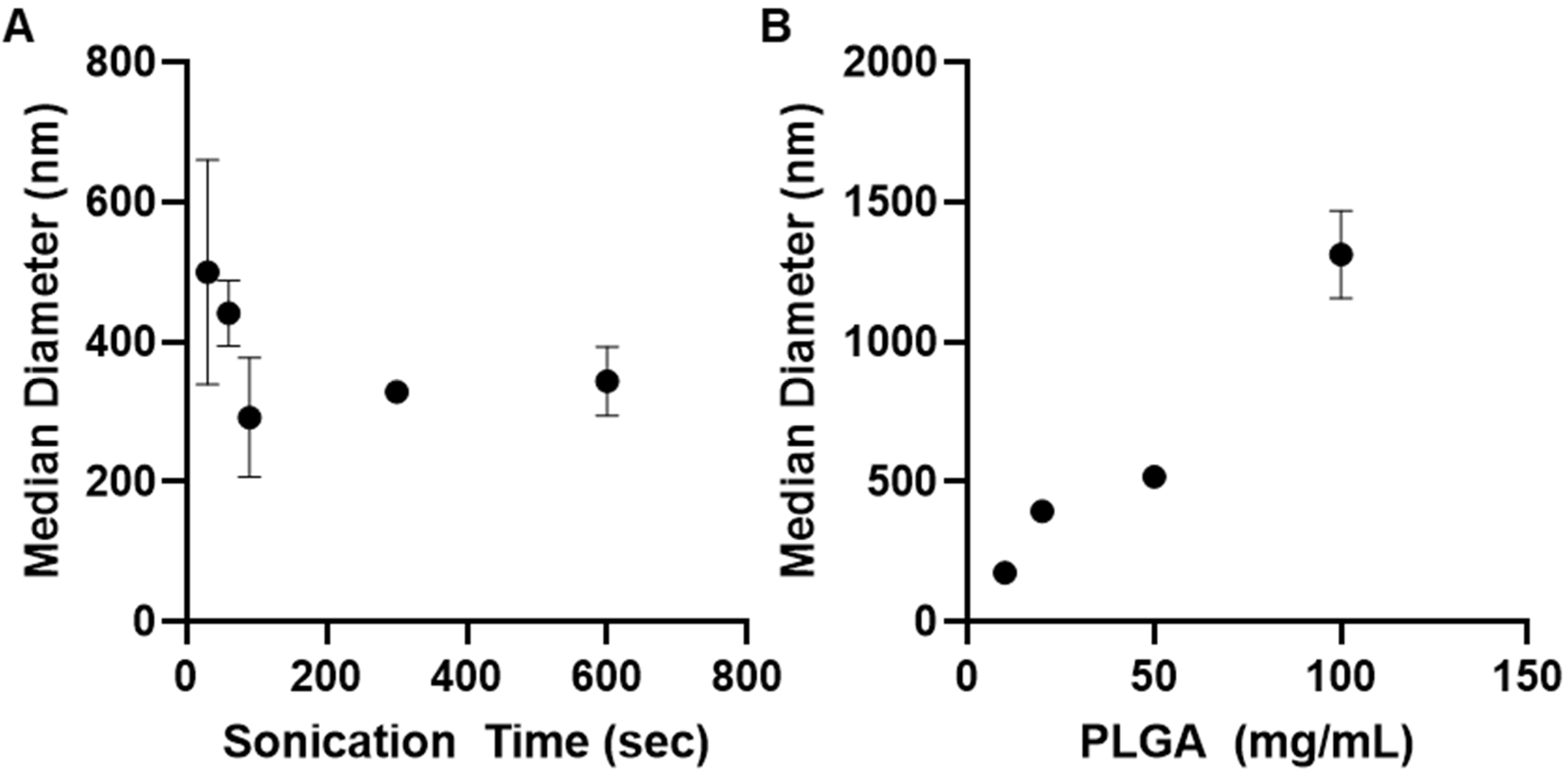
3.3. Effect of Polymeric Nanoparticle Synthesis Parameters on Particle Size
3.4. Impact of Nanoparticle Synthesis Parameters on Zeta Potential
3.5. Impact of Nanoparticle Synthesis Parameters on Drug Release
3.6. Characterization of Nanoparticle Surface Morphology
3.7. X-ray Powder Diffraction (XRPD)
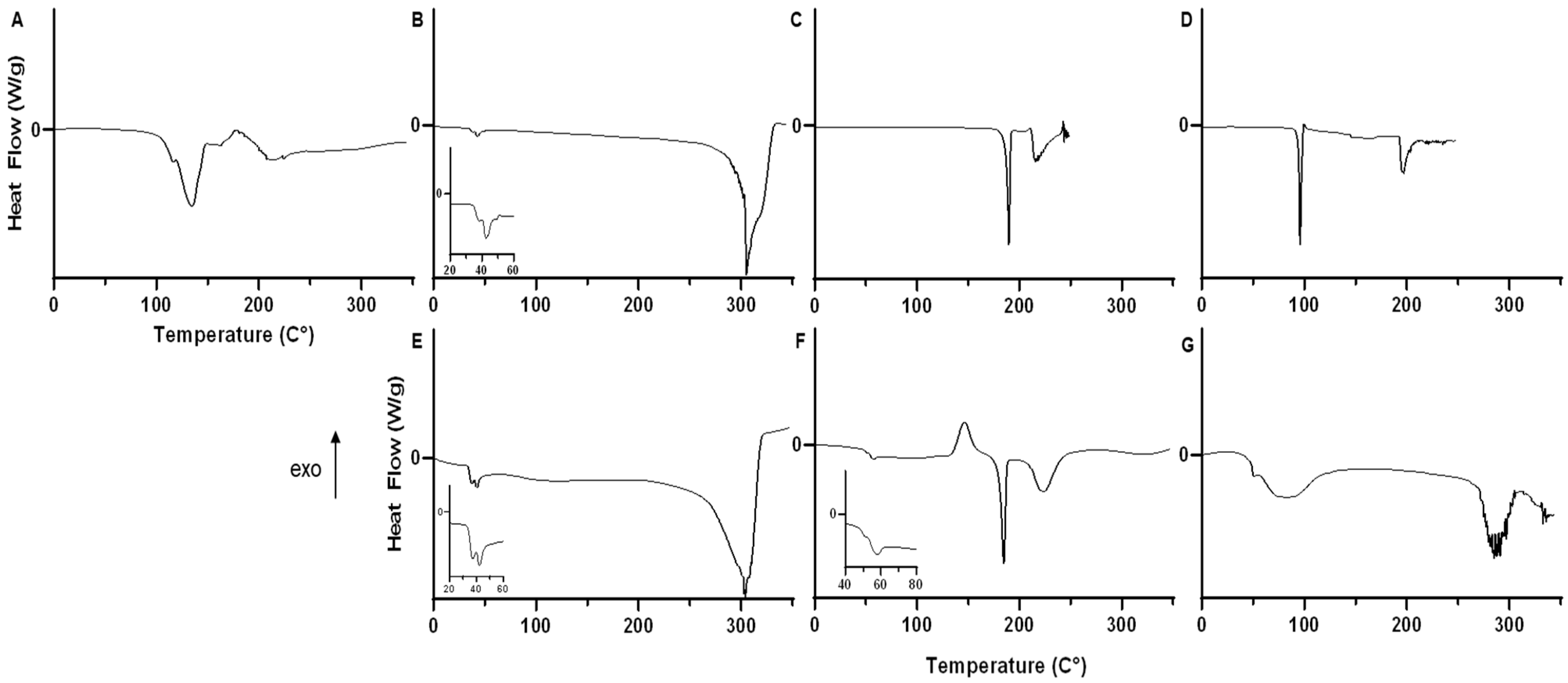
3.8. Thermal Analysis of Lyophilized Nanoparticles
3.9. Water Content Analysis by Karl Fischer Titration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, Y.S.; Park, C.W.; DeLuca, P.P.; Mansour, H.M. Sustained-Release Injectable Drug Delivery Systems. Pharm. Technol. Spec. Issue-Drug Deliv. 2010, 11, 6–13. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Muralidharan, P.; Mallory, E.; Malapit, M.; Hayes, D., Jr.; Mansour, H.M. Inhalable PEGylated Phospholipid Nanocarriers and PEGylated Therapeutics for Respiratory Delivery as Aerosolized Colloidal Dispersions and Dry Powder Inhalers. Pharmaceutics 2014, 6, 333–353. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.M.; Jaimes, E.A.; Heller, D.A. Nanomedicines for kidney diseases. Kidney Int. 2016, 90, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Han, S.J.; Williams, R.M.; D’Agati, V.; Jaimes, E.A.; Heller, D.A.; Lee, H.T. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020, 98, 76–87. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.H.; Chun, K.W.; Park, T.G. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir 2005, 21, 8852–8857. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Gauss, J.; Gessler, T.; Seeger, W.; Kissel, T.; Schmehl, T. Pulmonary targeting with biodegradable salbutamol-loaded nanoparticles. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jesinkey, S.R.; Funk, J.A.; Stallons, L.J.; Wills, L.P.; Megyesi, J.K.; Beeson, C.C.; Schnellmann, R.G. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2014, 25, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleveland, K.H.; Brosius, F.C.; Schnellmann, R.G. Regulation of mitochondrial dynamics and energetics in the diabetic renal proximal tubule by the β. Am. J. Physiol. Renal. Physiol. 2020, 319, F773–F779. [Google Scholar] [CrossRef] [PubMed]
- Scholpa, N.E.; Williams, H.C.; Wang, W.; Corum, D.; Narang, A.; Tomlinson, S.; Sullivan, P.; Rabchevsky, A.S.; Schnellmann, R. Pharmacological Stimulation of Mitochondrial Biogenesis Using the Food and Drug Administration-Approved β. J. Neurotrauma 2019, 36, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Scholpa, N.E.; Simmons, E.C.; Crossman, J.D.; Schnellmann, R.G. Time-to-treatment window and cross-sex potential of Beta 2-adrenergic receptor-induced mitochondrial biogenesis-mediated recovery after spinal cord injury. Toxicol. Appl. Pharmacol. 2021, 411, 115366. [Google Scholar] [CrossRef] [PubMed]
- Arif, E.; Solanki, A.K.; Srivastava, P.; Rahman, B.; Fitzgibbon, W.R.; Deng, P.; Budisavljevic, M.N.; Baicu, C.F.; Zile, M.R.; Megyesi, J.; et al. Mitochondrial biogenesis induced by the β2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 2019, 96, 656–673. [Google Scholar] [CrossRef]
- Vekaria, H.J.; Hubbard, W.B.; Scholpa, N.E.; Spry, M.L.; Gooch, J.L.; Prince, S.J.; Schnellmann, R.G.; Sullivan, P.G. Formoterol, a beta-2-adrenoreceptor agonist, induces mitochondrial biogenesis and promotes cognitive recovery after traumatic brain injury. Neurobiol. Dis. 2020, 140, 104866. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Cameron, R.B.; Gibbs, W.S.; Miller, S.R.; Dupre, T.V.; Megyesi, J.; Beeson, C.C.; Schnellmann, R.G. Proximal Tubule Beta-2 Adrenergic Receptor Mediates Formoterol-Induced Recovery of Mitochondrial and Renal Function After Ischemia-Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019, 369, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.A.; Leenen, F.H. Role of beta 1-receptors and vagal tone in cardiac inotropic and chronotropic responses to a beta 2-agonist in humans. Circulation 1989, 79, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodde, O.E. Beta 1- and beta 2-adrenoceptors in the human heart: Properties, function, and alterations in chronic heart failure. Pharmacol. Rev. 1991, 43, 203–242. [Google Scholar]
- Vyas, F.S.; Nelson, C.P.; Freeman, F.; Boocock, D.J.; Hargreaves, A.J.; Dickenson, J.M. β 2-adrenoceptor-induced modulation of transglutaminase 2 transamidase activity in cardiomyoblasts. Eur. J. Pharmacol. 2017, 813, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koziczak-Holbro, M.; Rigel, D.F.; Dumotier, B.; Sykes, D.A.; Tsao, J.; Nguyen, N.; Bösch, J.; Jourdain, M.; Flotte, L.; Adachi, Y. Pharmacological Characterization of a Novel 5-Hydroxybenzothiazolone-Derived β 2-Adrenoceptor Agonist with Functional Selectivity for Anabolic Effects on Skeletal Muscle Resulting in a Wider Cardiovascular Safety Window in Preclinical Studies. J. Pharmacol. Exp. Ther. 2019, 369, 188–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molenaar, P.; Chen, L.; Parsonage, W.A. Cardiac implications for the use of beta2-adrenoceptor agonists for the management of muscle wasting. Br. J. Pharmacol. 2006, 147, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Yang, C.; Wu, J.; Lu, H.; Zheng, X.; Zhang, Y.; Lv, Z.; Zheng, X.; Li, Z. Downregulation of β-Adrenoceptors in Isoproterenol-Induced Cardiac Remodeling through HuR. PLoS ONE 2016, 11, e0152005. [Google Scholar] [CrossRef]
- Dorn, G.W. Adrenergic pathways and left ventricular remodeling. J. Card. Fail. 2002, 8, S370–S373. [Google Scholar] [CrossRef]
- Brouri, F.; Findji, L.; Mediani, O.; Mougenot, N.; Hanoun, N.; le Naour, G.; Hamon, M.; Lechat, P. Toxic cardiac effects of catecholamines: Role of beta-adrenoceptor downregulation. Eur. J. Pharmacol. 2002, 456, 69–75. [Google Scholar] [CrossRef]
- He, J.; Chen, H.; Zhou, W.; Chen, M.; Yao, Y.; Zhang, Z.; Tan, N. Kidney targeted delivery of asiatic acid using a FITC labeled renal tubular-targeting peptide modified PLGA-PEG system. Int. J. Pharm. 2020, 584, 119455. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Chen, W.; Cao, W.; Zhang, C.; Wang, T.; Ding, M.; Zhao, S.; Wei, H.; Guo, H.; et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 2019, 219, 119368. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.V.; Keliher, E.J.; Core, A.B.; Brown, D.; Weissleder, R. Characterizing the interactions of organic nanoparticles with renal epithelial cells in vivo. ACS Nano 2015, 9, 3641–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallorz, E.L.; Blohm-Mangone, K.; Schnellmann, R.G.; Mansour, H.M. Formoterol PLGA-PEG Nanoparticles Induce Mitochondrial Biogenesis in Renal Proximal Tubules. AAPS J. 2021, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Aodah, A.; Pavlik, A.; Karlage, K.; Myrdal, P.B. Preformulation Studies on Piperlongumine. PLoS ONE 2016, 11, e0151707. [Google Scholar] [CrossRef]
- Akapo, S.O.; Asif, M. Validation of a RP-HPLC method for the assay of formoterol and its related substances in formoterol fumarate dihydrate drug substance. J. Pharm. Biomed. Anal. 2003, 33, 935–945. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, S.S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials 2006, 27, 4025–4033. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabsi, W.; Al-Obeidi, F.A.; Polt, R.; Mansour, H.M. Organic Solution Advanced Spray-Dried Microparticulate/Nanoparticulate Dry Powders of Lactomorphin for Respiratory Delivery: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. Pharmaceutics 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Tajber, L.; Corrigan, D.O.; Corrigan, O.I.; Healy, A.M. Spray drying of budesonide, formoterol fumarate and their composites—I. Physicochemical characterisation. Int. J. Pharm. 2009, 367, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jarring, K.; Larsson, T.; Stensland, B.; Ymén, I. Thermodynamic stability and crystal structures for polymorphs and solvates of formoterol fumarate. J. Pharm. Sci. 2006, 95, 1144–1161. [Google Scholar] [CrossRef]
- Sharafkhaneh, A.; Mattewal, A.S.; Abraham, V.M.; Dronavalli, G.; Hanania, N.A. Budesonide/formoterol combination in COPD: A US perspective. Int. J. Chron. Obstruct. Pulmon. Dis. 2010, 5, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, S.M.; Vidgren, M.T.; Herrala, J.; Turjanmaa, V.M.; Koskinen, M.O.; Nieminen, M.M. Possibilities of formoterol to enhance the peripheral lung deposition of the inhaled liposome corticosteroids. Respir. Med. 2002, 96, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyothi, N.V.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Ravi, S.; Peh, K.K.; Darwis, Y.; Murthy, B.K.; Singh, T.R.; Mallikarjun, C. Development and characterization of polymeric microspheres for controlled release protein loaded drug delivery system. Indian J. Pharm. Sci. 2008, 70, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Zhao, Y.; Hou, S.; Xu, F.; Zhao, R.; He, J.; Cai, Z.; Li, Y.; Chen, Q. Dual agents loaded PLGA nanoparticles: Systematic study of particle size and drug entrapment efficiency. Eur. J. Pharm. Biopharm. 2008, 69, 445–453. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Hildgen, P.; Banquy, X. Assessment of PEG on polymeric particles surface, a key step in drug carrier translation. J. Control. Release 2014, 185, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Müller, R.H. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm. Res. 2002, 19, 189–194. [Google Scholar] [CrossRef]
- Feng, S.; Huang, G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J. Control. Release 2001, 71, 53–69. [Google Scholar] [CrossRef]
- Vega, E.; Gamisans, F.; García, M.L.; Chauvet, A.; Lacoulonche, F.; Egea, M.A. PLGA nanospheres for the ocular delivery of flurbiprofen: Drug release and interactions. J. Pharm. Sci. 2008, 97, 5306–5317. [Google Scholar] [CrossRef]
- Ting, W.; Tong-Chun, B.; Wei, W.; Jian-Jun, Z.; Cheng-Wen, Z. Viscosity and activation parameters of viscous flow of sodium cholate aqueous solution. J. Mol. Liq. 2008, 142, 150–154. [Google Scholar]
- Mohsen-Nia, M.; Modarress, H. Viscometric study of aqueous poly(vinyl alcohol) (PVA) solutions as a binder in adhesive formulations. J. Adhes. Sci. Technol. 2012, 20, 1273–1280. [Google Scholar] [CrossRef]
- Santhanalakshmi, J.; Lakshmi, G.S.; Aswal, V.K.; Goyal, P.S. Small-angle neutron scattering study of sodium cholate and sodium deoxycholate interacting micelles in aqueous medium. Proc. Indian Acad. Sci. 2001, 113, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Maslova, V.A.; Kiselev, M.A. Structure of Sodium Cholate Micelles. Crystallogr. Rep. 2018, 63, 472–475. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Fonte, P.; Soares, S.; Sousa, F.; Costa, A.; Seabra, V.; Reis, S.; Sarmento, B. Stability study perspective of the effect of freeze-drying using cryoprotectants on the structure of insulin loaded into PLGA nanoparticles. Biomacromolecules 2014, 15, 3753–3765. [Google Scholar] [CrossRef]
- Holzer, M.; Vogel, V.; Mäntele, W.; Schwartz, D.; Haase, W.; Langer, K. Physico-chemical characterisation of PLGA nanoparticles after freeze-drying and storage. Eur. J. Pharm. Biopharm. 2009, 72, 428–437. [Google Scholar] [CrossRef]
- Kedward, C.J.; MacNaughtan, W.; Mitchell, J.R. Isothermal and non-isothermal crystallization in amorphous sucrose and lactose at low moisture contents. Carbohydr. Res. 2000, 329, 423–430. [Google Scholar] [CrossRef]
- van Eerdenbrugh, B.; Froyen, L.; Martens, J.A.; Blaton, N.; Augustijns, P.; Brewster, M.; van den Mooter, G. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze-dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int. J. Pharm. 2007, 338, 198–206. [Google Scholar] [CrossRef]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym. Int. 2004, 54, 36–46. [Google Scholar] [CrossRef]
- Kranz, H.; Ubrich, N.; Maincent, P.; Bodmeier, R. Physicomechanical properties of biodegradable poly(D,L-lactide) and poly(D,L-lactide-co-glycolide) films in the dry and wet states. J. Pharm. Sci. 2000, 89, 1558–1566. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Wang, B.; Tchessalov, S.; Cicerone, M.T.; Warne, N.W.; Pikal, M.J. Impact of sucrose level on storage stability of proteins in freeze-dried solids: II. Correlation of aggregation rate with protein structure and molecular mobility. J. Pharm. Sci. 2009, 98, 3145–3166. [Google Scholar] [CrossRef] [PubMed]


 mannitol,
mannitol,  trehalose, or
trehalose, or  sucrose as cryoprotectants. All data are presented as mean (n = 3) ± s.d.
sucrose as cryoprotectants. All data are presented as mean (n = 3) ± s.d.
 mannitol,
mannitol,  trehalose, or
trehalose, or  sucrose as cryoprotectants. All data are presented as mean (n = 3) ± s.d.
sucrose as cryoprotectants. All data are presented as mean (n = 3) ± s.d.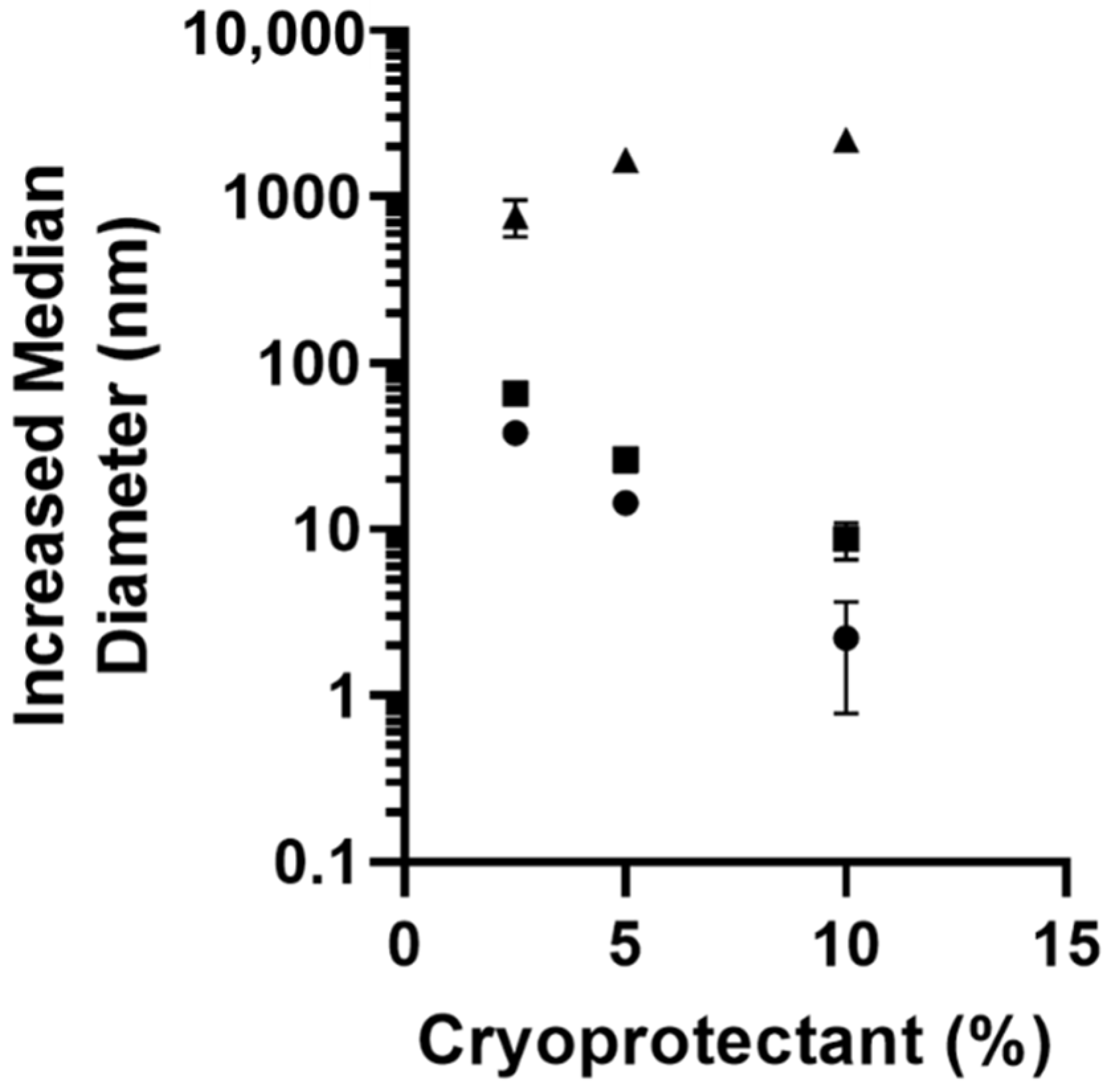
 PLGA-PEG-COOH,
PLGA-PEG-COOH,  PLGA-PEG,
PLGA-PEG,  PLGA-PEG-NH2. All data are presented as mean (n = 3) ± s.d.
PLGA-PEG-NH2. All data are presented as mean (n = 3) ± s.d.
 PLGA-PEG-COOH,
PLGA-PEG-COOH,  PLGA-PEG,
PLGA-PEG,  PLGA-PEG-NH2. All data are presented as mean (n = 3) ± s.d.
PLGA-PEG-NH2. All data are presented as mean (n = 3) ± s.d.
 oil-in-water single emulsion
oil-in-water single emulsion  water-in-oil-in-water double emulsion (B) nanoparticles prepared by double emulsion with
water-in-oil-in-water double emulsion (B) nanoparticles prepared by double emulsion with  1% PVA inner phase,
1% PVA inner phase,  12 mM sodium cholate inner phase,
12 mM sodium cholate inner phase,  10 mM sodium deoxycholate inner phase (C) nanoparticles prepared by double emulsion using
10 mM sodium deoxycholate inner phase (C) nanoparticles prepared by double emulsion using  10 mg/mL,
10 mg/mL,  20 mg/mL,
20 mg/mL,  50 mg/mL PLGA-PEG-NH2. Inserts of the first 10 h are included for (B,C). All data are presented as the mean of (n = 3) ± s.d.
50 mg/mL PLGA-PEG-NH2. Inserts of the first 10 h are included for (B,C). All data are presented as the mean of (n = 3) ± s.d.
 oil-in-water single emulsion
oil-in-water single emulsion  water-in-oil-in-water double emulsion (B) nanoparticles prepared by double emulsion with
water-in-oil-in-water double emulsion (B) nanoparticles prepared by double emulsion with  1% PVA inner phase,
1% PVA inner phase,  12 mM sodium cholate inner phase,
12 mM sodium cholate inner phase,  10 mM sodium deoxycholate inner phase (C) nanoparticles prepared by double emulsion using
10 mM sodium deoxycholate inner phase (C) nanoparticles prepared by double emulsion using  10 mg/mL,
10 mg/mL,  20 mg/mL,
20 mg/mL,  50 mg/mL PLGA-PEG-NH2. Inserts of the first 10 h are included for (B,C). All data are presented as the mean of (n = 3) ± s.d.
50 mg/mL PLGA-PEG-NH2. Inserts of the first 10 h are included for (B,C). All data are presented as the mean of (n = 3) ± s.d.



| Solvent | Formoterol Solubility (mg/mL) |
|---|---|
| DCM | 0.001 ± 0.0004 |
| Chloroform | 0.002 ± 0.001 |
| ACN | 0.005 ± 0.001 |
| Acetone | 0.051 ± 0.004 |
| Zero Order | First Order | Korsmeyer-Peppas | ||
|---|---|---|---|---|
| R2 | R2 | R2 | n | |
| o/w | 0.33 | 0.94 | 0.88 | 0.56 |
| w/o/w | 0.77 | 0.97 | 0.97 | 0.34 |
| PVA | 0.65 | 0.89 | 0.94 | 0.23 |
| NaCholate | 0.75 | 0.86 | 0.94 | 0.27 |
| NaDOCholate | 0.49 | 0.81 | 0.84 | 0.17 |
| 10 mg/mL | 0.75 | 0.86 | 0.94 | 0.27 |
| 20 mg/mL | 0.85 | 0.96 | 0.97 | 0.40 |
| 50 mg/mL | 0.92 | 0.96 | 0.98 | 0.42 |
| Tg (°C) | ∆Cp | Endotherm #1 | Endotherm #2 | Endotherm #3 | Endotherm #4 | Exotherm #1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | onset (°C) | mid (°C) | end (°C) | J/g °C | onset (°C) | peak (°C) | enthalpy (J/g) | onset (°C) | peak (°C) | enthalpy (J/g) | onset (°C) | peak (°C) | enthalpy (J/g) | onset (°C) | peak (°C) | enthalpy (J/g) | onset (°C) | peak (°C) | enthalpy (J/g) |
| Raw Formoterol Fumarate Dihydrate | 88.84 ± 1.87 | 104.62 ± 3.62 | 13.56 ± 1.04 | 116.28 ± 0.76 | 130.82 ± 2.73 | 136.7 ± 0.89 | |||||||||||||
| Raw PLGA-PEG-NH2 | 41.56 ± 1.34 | 42.43 ± 1.51 | 43.34 ± 1.93 | 0.69 ± 0.44 | 43.32 ± 2.16 | 47.97 ± 4.72 | 4.95 ± 3.34 | ||||||||||||
| Raw Sucrose | 187.01 ± 0.59 | 189.43 ± 0.23 | 129.47 ± 0.4 | 219.35 ± 5.56 | 220.72 ± 4.48 | 150 ± 50.01 | |||||||||||||
| Raw Trehalose | 94.46 ± 0.07 | 95.79 ± 0.05 | 94.54 ± 2.28 | 190.98 ± 1.75 | 193.3 ± 3.24 | 92.98 ± 14.66 | |||||||||||||
| Nanoparticle No Cryoprotectant | 35.3 ± 1.25 | 36.12 ± 0.8 | 36.48 ± 0.69 | 0.3 ± 0.2 | 42.16 ± 6.75 | 43.99 ± 6.73 | 1.98 ± 1.14 | 82.62 ± 2.34 | 112.95 ± 9.6 | 8.67 ± 3.24 | |||||||||
| Nanoparticle Sucrose Cryoprotectant | 50.09 ± 1.1 | 54.3 ± 0.21 | 54.82 ± 0.43 | 1.34 ± 0.38 | 53.45 ± 0.32 | 57.15 ± 0.33 | 4.13 ± 0.43 | 70.93 ± 2.3 | 96.02 ± 1.58 | 10.11 ± 0.56 | 180.03 ± 0.36 | 184.74 ± 0.03 | 86.59 ± 2.38 | 213.08 ± 5.68 | 222.78 ± 2.12 | 119.77 ± 1.16 | 136.56 ± 1.93 | 148.26 ± 3.06 | 79.36 ± 5.03 |
| Nanoparticle a Trehalose Cryoprotectant | 43.08 ± 3.39 | 44.68 ± 4.02 | 46.64 ± 2.94 | 1.21 ± 0.31 | 42.97 ± 1.61 | 50.17 ± 0.18 | 4.73 ± 3.73 | 58.99 ± 1.99 | 86.75 ± 3.45 | 16.89 ± 2.72 | |||||||||
| Nanoparticle | Water% (w/w) |
|---|---|
| PLGA-PEG (no cryoprotectant) | 1.38 ± 0.20 |
| PLGA-PEG-NH2 (no cryoprotectant) | 2.20 ± 0.61 |
| PLGA-PEG-NH2 (sucrose) | 0.78 ± 0.17 * |
| PLGA-PEG-NH2 (trehalose) | 0.80 ± 0.19 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallorz, E.L.; Encinas-Basurto, D.; Schnellmann, R.G.; Mansour, H.M. Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles. Pharmaceutics 2022, 14, 638. https://doi.org/10.3390/pharmaceutics14030638
Vallorz EL, Encinas-Basurto D, Schnellmann RG, Mansour HM. Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles. Pharmaceutics. 2022; 14(3):638. https://doi.org/10.3390/pharmaceutics14030638
Chicago/Turabian StyleVallorz, Ernest L., David Encinas-Basurto, Rick G. Schnellmann, and Heidi M. Mansour. 2022. "Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles" Pharmaceutics 14, no. 3: 638. https://doi.org/10.3390/pharmaceutics14030638






