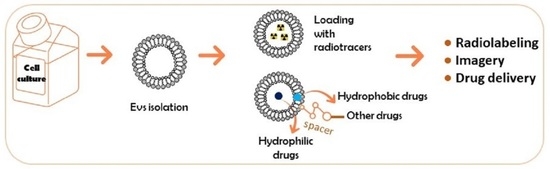
Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery
Abstract
:1. Introduction
2. Chemical Modification for In Vivo Tracking Extracellular Vesicles
2.1. Covalent-Binding Method
2.2. Bifunctional Chelators for Membrane Radiolabeling
3. Chemical Modifications on Extracellular Vesicle-Mediated Delivery Cargo
3.1. Covalent Binding Approach
3.2. Non-Covalent Binding
3.3. Hydrophobic Insertion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA-PEG | aminoethylanisamide-PEG |
| AAV | adeno-associated viruses |
| Abs | antibodies |
| Ac4ManNAz | N-acetoxy-N-acetyl-4-chlorobenzenesulfonamide |
| ADIBO | aza-dibenzyl cyclooctyne-fluorescent dyes |
| ASGPR | asialoglycoprotein |
| BAP-TM | biotin acceptor peptide transmembrane |
| BFC | bifunctional chelators |
| BBB | blood-brain barrier |
| CT | computed tomography |
| CuAAC | copper-catalyzed azide-alkyne cycloaddition |
| D-SMCNC-Exo | drug-loaded SMCNC-Exo |
| DOTA | dodecane tetraacetic acid |
| DTPA-anhydride | diethylenetriaminepentaacetic dianhydride |
| DDS | drug delivery systems |
| ELVs | exosome-like vesicles |
| EVs | extracellular vesicles |
| Exo | exosomes |
| GFP | green fluorescent protein |
| HMPAO | hexamethylene-propylene amine oxime |
| hUCB-MNC SEVs | human umbilical cord blood mononuclear cell-derived SEVs |
| MF | magnetic field |
| MDE | milk-derived exosomes |
| MPS | mononuclear phagocytic system |
| MVs | microvesicles |
| MPS | mononuclear phagocytic system |
| MRI | magnetic resonance imaging |
| PET | positron emission tomography |
| SMCNC-Exo | superparamagnetic nanoparticle cluster |
| SPAAC | strain-promoted azide-alkyne click chemistry |
| SPECT | single-photon emission computed tomography |
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Sharma, P.K.; Malviya, R. Role of blood retinal barrier in drug absorption. Pharm. Anal. Acta 2018, 9, 5. [Google Scholar]
- Anselmo, A.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering Nanoparticles to Lungs while Avoiding Liver and Spleen through Adsorption on Red Blood Cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.; Liu, M.; Tan, T.; Yang, Q.; Wang, Y.; Men, L.; Zhao, L.; Zhang, H.; Wang, S.; Xie, T.; et al. Emerging Significance and Therapeutic Potential of Extracellular vesicles. Int. J. Biol. Sci. 2021, 17, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Orefice, N. Development of New Strategies Using Extracellular Vesicles Loaded with Exogenous Nucleic Acid. Pharmaceutics 2020, 12, 705. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Jansen, F.; Li, Q.; Pfeifer, A.; Werner, N. Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease. JACC Basic Transl. Sci. 2017, 2, 790–807. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Sork, H.; Corso, G.; Krjutskov, K.; Johansson, H.J.; Nordin, J.; Wiklander, O.P.B.; Lee, Y.X.F.; Westholm, J.O.; Lehtiö, J.; Wood, M.J.A.; et al. Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome. Sci. Rep. 2018, 8, 10813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Altinoglu, S.; Takeda, Y.S.; Xu, Q. Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLoS ONE 2015, 10, e0141860. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconjugate Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic Applications of Extracellular Vesicles: Clinical Promise and Open Questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef]
- Lee, T.S.; Kim, Y.; Zhang, W.; Song, I.H.; Tung, C.-H. Facile metabolic glycan labeling strategy for exosome tracking. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 1091–1100. [Google Scholar] [CrossRef]
- Dommerholt, J.; Rutjes, F.P.J.T.; Van Delft, F.L. Strain-Promoted 1,3-Dipolar Cycloaddition of Cycloalkynes and Organic Azides. Top. Curr. Chem. 2016, 374, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Sletten, E.M.; Bertozzi, C.R. From mechanism to mouse: A tale of two bioorthogonal reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.-S.; Jeong, J.M.; et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99mTc-HMPAO. Sci. Rep. 2015, 5, 15636. [Google Scholar] [CrossRef]
- Goins, B.; Klipper, R.; Rudolph, A.S.; O’Cliff, R.; Blumhardt, R.; Phillips, W.T. Biodistribution and imaging studies of technetium-99m-labeled liposomes in rats with focal infection. J. Nucl. Med. 1993, 34, 2160–2168. [Google Scholar] [PubMed]
- Varga, Z.; Gyurkó, I.; Pálóczi, K.; Buzás, E.I.; Horváth, I.; Hegedűs, N.; Máthé, D.; Szigeti, K. Radiolabeling of Extracellular Vesicles with 99mTc for Quantitative In Vivo Imaging Studies. Cancer Biother. Radiopharm. 2016, 31, 168–173. [Google Scholar] [CrossRef] [Green Version]
- González, M.I.; Martín-Duque, P.; Desco, M.; Salinas, B. Radioactive Labeling of Milk-Derived Exosomes with 99mTc and In Vivo Tracking by SPECT Imaging. Nanomaterials 2020, 10, 1062. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Cossío, U.; de Angulo, A.R.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscher, C.; Dennis, J.W.; Nabi, I.R. Glycosylation, galectins, and cellular signaling. Curr. Opin. Cell. Biol. 2011, 23, 383–392. [Google Scholar] [CrossRef]
- Stéen, E.J.L.; Edem, P.; Norregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef] [PubMed]
- Faruqu, F.N.; Wang, J.T.-W.; Xu, L.; McNickle, L.; Chong, E.M.-Y.; Walters, A.; Gurney, M.; Clayton, A.; Smyth, L.A.; Hider, R.; et al. Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice—A Novel and Universal Approach. Theranostics 2019, 9, 1666–1682. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Alves, V.; Rondão, T.; Sereno, J.; Neves, Â.; Lino, M.; Ribeiro, A.; Abrunhosa, A.J.; Ferreira, L.S. A Positron-emission tomography (PET)/magnetic resonance imaging (MRI) platform to track in vivo small extracellular vesicles. Nanoscale 2019, 11, 13243–13248. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Kim, Y.-H.; Chung, S.-J.; Lee, C.-H.; Rhee, S.; Pratx, G.; Chung, J.-K.; Youn, H. Identification of Lymphatic and Hematogenous Routes of Rapidly Labeled Radioactive and Fluorescent Exosomes through Highly Sensitive Multimodal Imaging. Int. J. Mol. Sci. 2020, 21, 7850. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Li, T.; Wen, X.; Wu, S.Y.; Xiong, C.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.; Sood, A.K.; et al. Copper-64 Labeled PEGylated Exosomes for In Vivo Positron Emission Tomography and Enhanced Tumor Retention. Bioconjugate Chem. 2019, 30, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Mäkilä, E.; Kaasalainen, M.H.; Liu, D.; Sarparanta, M.; Airaksinen, A.J.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Copper-free azide–alkyne cycloaddition of targeting peptides to porous silicon nanoparticles for intracellular drug uptake. Biomaterials 2013, 35, 1257–1266. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, L.; Zhu, C.; Zheng, Q.; Wang, G.; Tong, J.; Fang, Y.; Xia, Y.; Cheng, G.; He, X.; et al. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018, 78, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Brennan, J.L.; Hatzakis, N.; Tshikhudo, T.R.; Dirvianskyte, N.; Razumas, V.; Patkar, S.; Vind, J.; Svendsen, A.; Nolte, R.; Rowan, A.; et al. Bionanoconjugation via Click Chemistry: The Creation of Functional Hybrids of Lipases and Gold Nanoparticles. Bioconjugate Chem. 2006, 17, 1373–1375. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Patel, H.M. Tissue specific opsonins for phagocytic cells and their different affinity for cholesterol-rich liposomes. FEBS Lett. 1988, 233, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a ph-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Uemoto, S.; Tabata, Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017, 57, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanatani, I.; Ikai, T.; Okazaki, A.; Jo, J.-I.; Yamamoto, M.; Imamura, M.; Kanematsu, A.; Yamamoto, S.; Ito, N.; Ogawa, O.; et al. Efficient gene transfer by pullulan–spermine occurs through both clathrin- and raft/caveolae-dependent mechanisms. J. Control. Release 2006, 116, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV Vector as a Novel Gene Delivery System. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Maurya, S.; Bammidi, S.; Jayandharan, G.R. AAV6 Vexosomes Mediate Robust Suicide Gene Delivery in a Murine Model of Hepatocellular Carcinoma. Mol. Ther. Methods Clin. Dev. 2020, 17, 497–504. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gu, N.; Zhang, X.-E.; Wang, D.-B. Light-Inducible Exosome-Based Vehicle for Endogenous RNA Loading and Delivery to Leukemia Cells. Adv. Funct. Mater. 2019, 29, 1807189. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011, 8, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular Consequences of Copper Complexes Used To Catalyze Bioorthogonal Click Reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef]
- Li, S.; Cai, H.; He, J.; Chen, H.; Lam, S.; Cai, T.; Zhu, Z.; Bark, S.J.; Cai, C. Extent of the Oxidative Side Reactions to Peptides and Proteins During the CuAAC Reaction. Bioconjugate Chem. 2016, 27, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Riguera, R.; Fernandez-Megia, E. Reliable and Efficient Procedures for the Conjugation of Biomolecules through Huisgen Azide-Alkyne Cycloadditions. Angew. Chem. Int. Ed. 2011, 50, 8794–8804. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, N.; Yoshioka, Y.; Kikuchi, S.; Okamura, A.; Azuma, N.; Ochiya, T. Challenges for the Development of Extracellular Vesicle-Based Nucleic Acid Medicines. Cancers 2021, 13, 6137. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.; de Jong, O.G.; Schiffelers, R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021, 173, 252–278. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes, and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2021, 11, 2100639. [Google Scholar] [CrossRef]


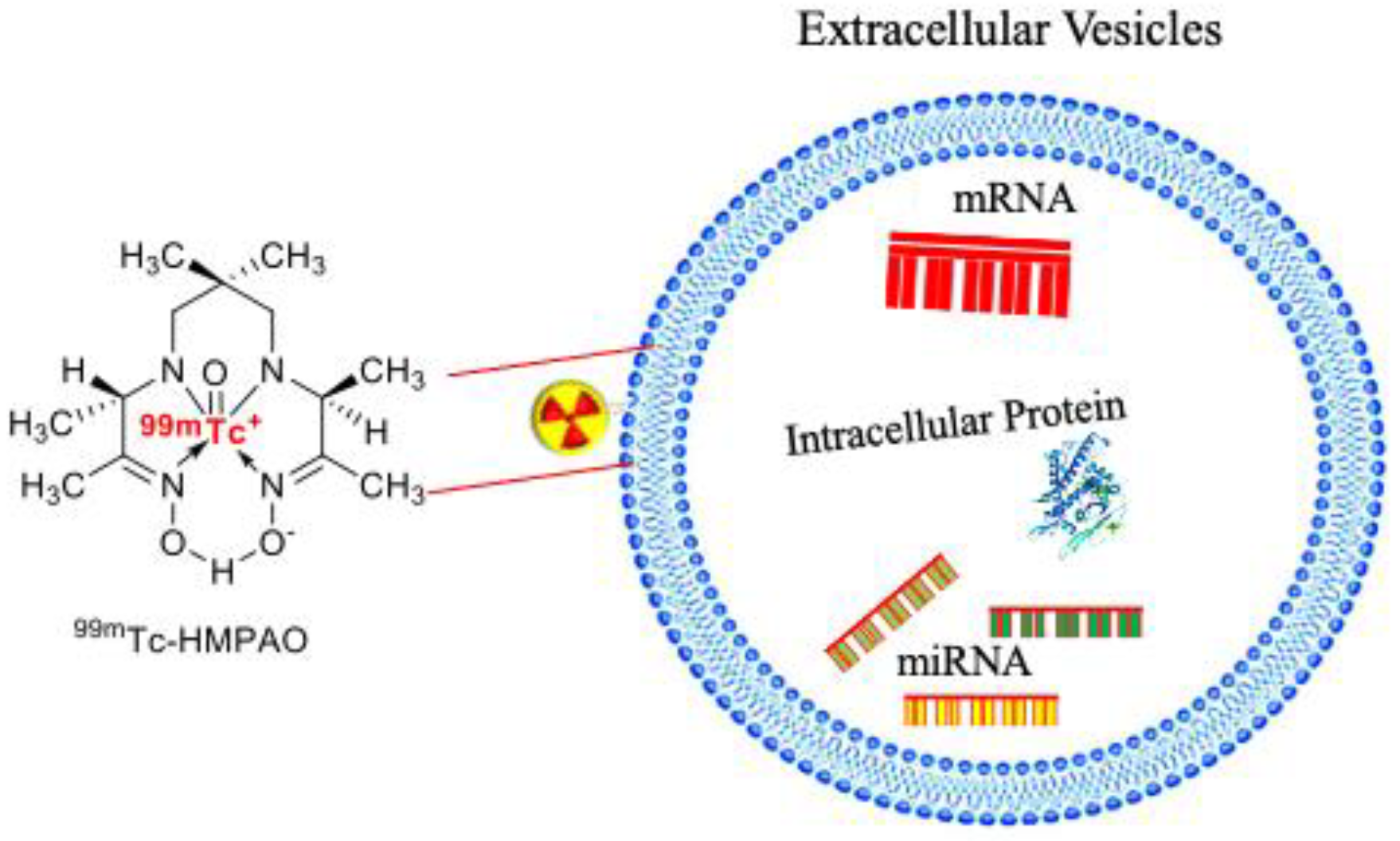

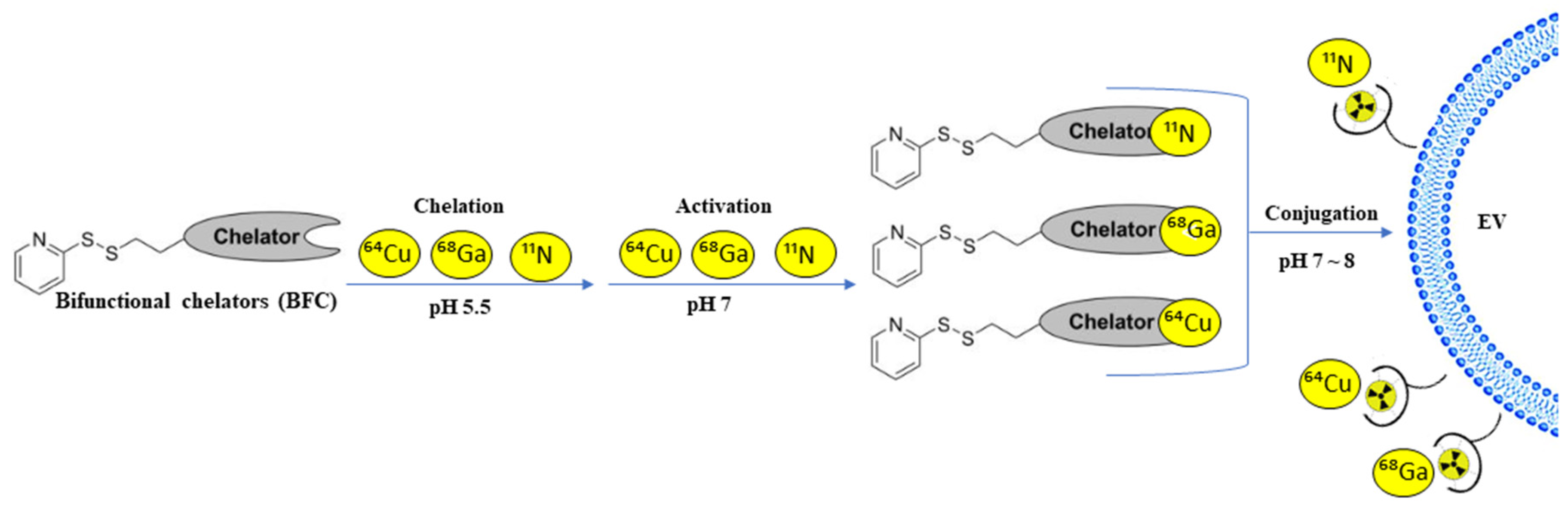
| Source of Exosomes | Purpose | Method | Results | References |
|---|---|---|---|---|
| Cy.5.5 exosomes derived from MCF7 and MDA-MB-231 cells | In vivo biodistribution of the azido-containing exosomes | Cooper-free click chemistry with a strain-promoted azide–alkyne click (SPAAC) | Better distribution of the labeled exosomes in tumor than blood and muscles; accumulation in the liver and intestines | [17] |
| Macrophage-derived exosome-mimetic nanovesicles (ENVs) | Biodistribution of ENVs in vivo | Incorporating 99mTc on the EVs membrane surface with click chemistry | 99mTc-HMPAO-ENVs accumulates in the liver, spleen, salivary gland | [20] |
| Erythrocyte-derived EVs | Erythrocyte-derived EVs’ biodistribution under SPECT/CT | Radiolabeling by 99mTc-tricarbonyl complexes with click chemistry | Accumulation of the 99mTc-Exos in the liver and spleen | [22] |
| Milk-derived exosomes (MDE) | A cheaper method with higher efficiency to study EVs biodistribution | Radiochemical labeling of MDE with reduced 99mTc (IV) injected intravenously, intraperitoneally, and intranasally | IV: reduced 99mTc-MDE accumulated in the liver and urinary bladder and distributed in aorta and lungs IP: reduced 99mTc-MDE distributed in the abdominal cavity, spleen, and thyroid IN: Biodistribution in the nasal cavity, trachea, and lung | [23] |
| Mouse liver proliferative cell-derived EVs | Impact of glycosylation modification on the biodistribution of EVs in mice | EVs were treated with neuraminidase and labeled with ¹²⁴I | Distribution primarily in liver and lung and slightly in the thyroid gland | [24] |
| Source of Exosomes | Purpose | Method | Results | References |
|---|---|---|---|---|
| Melanoma (B16F10)-derived exosomes (EXOB16) | A novel, reliable, and universal method for the radiolabeling of exosomes | 111Indium-chelated labeling of EV | Better radiolabeling efficiency and radiochemical stability Distribution in liver, spleen, and bladder | [27] |
| Human umbilical cord blood mononuclear cell-derived small EVs (hUCB-MNC SEVs) | Biodistribution of the new hUCB-MNC SEVs showed by PET/MRI | 2-step surface modification method of small EVs with 64Cu2+ | Biodistribution in liver > lungs > kidney > stomach > brain (striatum, prefrontal cortex, and the cerebellum) | [28] |
| 4T1 breast cancer-derived exosomes | Adequate imaging method for the in vivo tracking of EVs between PET, optical imaging, ex vivo radioactivity quantification | Exosomes were either radiolabeled with a BFC-64Cu or -68 Ga or fluorescently labeled | PET imaging and ex vivo radioactivity quantification could see the biodistribution of the BFC-4T1-EXOs with more detail than optical imaging | [29] |
| 4T1 breast cancer-derived exosomes | Impact of PEGylation of EVs on their pharmacokinetics | Radiolabeling of PEG conjugated Exosomes | The efficient PEGylation method provides an exciting improvement in the pharmacokinetics of EVs, even in the tumor | [30] |
| Source of Exosomes | Purpose | Method | Results | References |
|---|---|---|---|---|
| 4T1 breast cancer-derived exosomes | See whether the linkage of azide-fluor 545 on the surface of an EV would change its function | 4T1 EXOs were functionalized with a terminal alkyl group after click chemistry | No modification of the natural functions of the EV was impaired by being chemically modified | [14] |
| Dendritic cell-derived EVs | Improving the delivery of paclitaxel to target cancer cells | Conjugation of an aptamer on the surface of EVs using covalent binding | The surface modification showed a 6-fold and 3-fold treatment efficacy in vitro and in vivo | [33] |
| Human red blood cells (RBCs) as a source of EVs | Study of a permanent covalent bond between peptides or specific nanobodies and EVs’ surfaces | Simple enzymatic method on EVs targeting several cancer cells | Epidermal growth factor receptor (EGFR)-targeting peptide or anti-EGFR nanobody improved their accumulation in EGFR+ cancer cells | [36] |
| Source of Exosomes | Purpose | Method | Results | References |
|---|---|---|---|---|
| CD63-GFP-containing exosomes derived from HeLa cells and Chines Hamster Ovary (CHO)-K1 cells | A simple technique for enhancing exosomes cellular uptake and cytosolic release | Electrostatic interaction between a positively charged lipofectamine and the negatively charged surface membrane of an EV | LTX increased the cellular uptake of GFP-GALA-Exos 15-fold by HeLa cells and 175-fold by CHO-K1 cells | [37] |
| Mesenchymal stem cells (MSC)-derived exosomes | Reach injured liver sites | EVs surface modified with cationized pullulan | Excellent cellular uptake in HepG2 cells and good distribution in the liver Enhanced anti-inflammatory effect of +pull-MSC Exos | [38] |
| Vexosomes are formed by the natural association between adeno-associated viruses and exosomes | Influence of magnetic beads on the targeting of vexosomes | Vexosomes were bound to streptavidin-conjugated magnetic beads | After activation of the magnetic field, two times more vexosomes joined the magnetic region | [40] |
| Reticulocyte-derived exosomes (REXOs) | Study of a new targeted drug delivery system | Transferrin conjugated superparamagnetic nanoparticle cluster bound to the transferrin of REXOs loaded with doxorubicin via hydrophobic effects | The entire suppression of the tumor growth factor was possible only under MF | [42] |
| Source of Exosomes | Purpose | Method | Results | References |
|---|---|---|---|---|
| Primary bone marrow stemmed macrophage-derived exosomes | Targeting of paclitaxel delivery to pulmonary metastases for systemic administration | Incorporation of amino-ethylanisamide-PEG on the surface of EXOs allows the bond of the sigma receptors to lung cancer cells | Greater antineoplastic efficacy, high inhibition of tumor growth, and better survival time after systemic administration | [43] |
| Plasma-derived exosomes containing miRNA21 | Hydrophobic insertion of cholesterol to improve the therapeutic effects of exosome-based cancer therapy | Modification of loaded exosomes with the hydrophobic insertion of AS1411 aptamer interacting with proteins after a reversible light-inducible protein-protein interaction | Good internalization of the exosomes in leukemia cells and successful delivery of the miRNA21 loaded AS1411-Exos with significant induction of cellular apoptosis | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N’Diaye, E.-R.; Orefice, N.S.; Ghezzi, C.; Boumendjel, A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics 2022, 14, 653. https://doi.org/10.3390/pharmaceutics14030653
N’Diaye E-R, Orefice NS, Ghezzi C, Boumendjel A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics. 2022; 14(3):653. https://doi.org/10.3390/pharmaceutics14030653
Chicago/Turabian StyleN’Diaye, Elisa-Racky, Nicola Salvatore Orefice, Catherine Ghezzi, and Ahcène Boumendjel. 2022. "Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery" Pharmaceutics 14, no. 3: 653. https://doi.org/10.3390/pharmaceutics14030653
APA StyleN’Diaye, E.-R., Orefice, N. S., Ghezzi, C., & Boumendjel, A. (2022). Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics, 14(3), 653. https://doi.org/10.3390/pharmaceutics14030653