Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model
Abstract
:1. Introduction
2. Materials and Methods
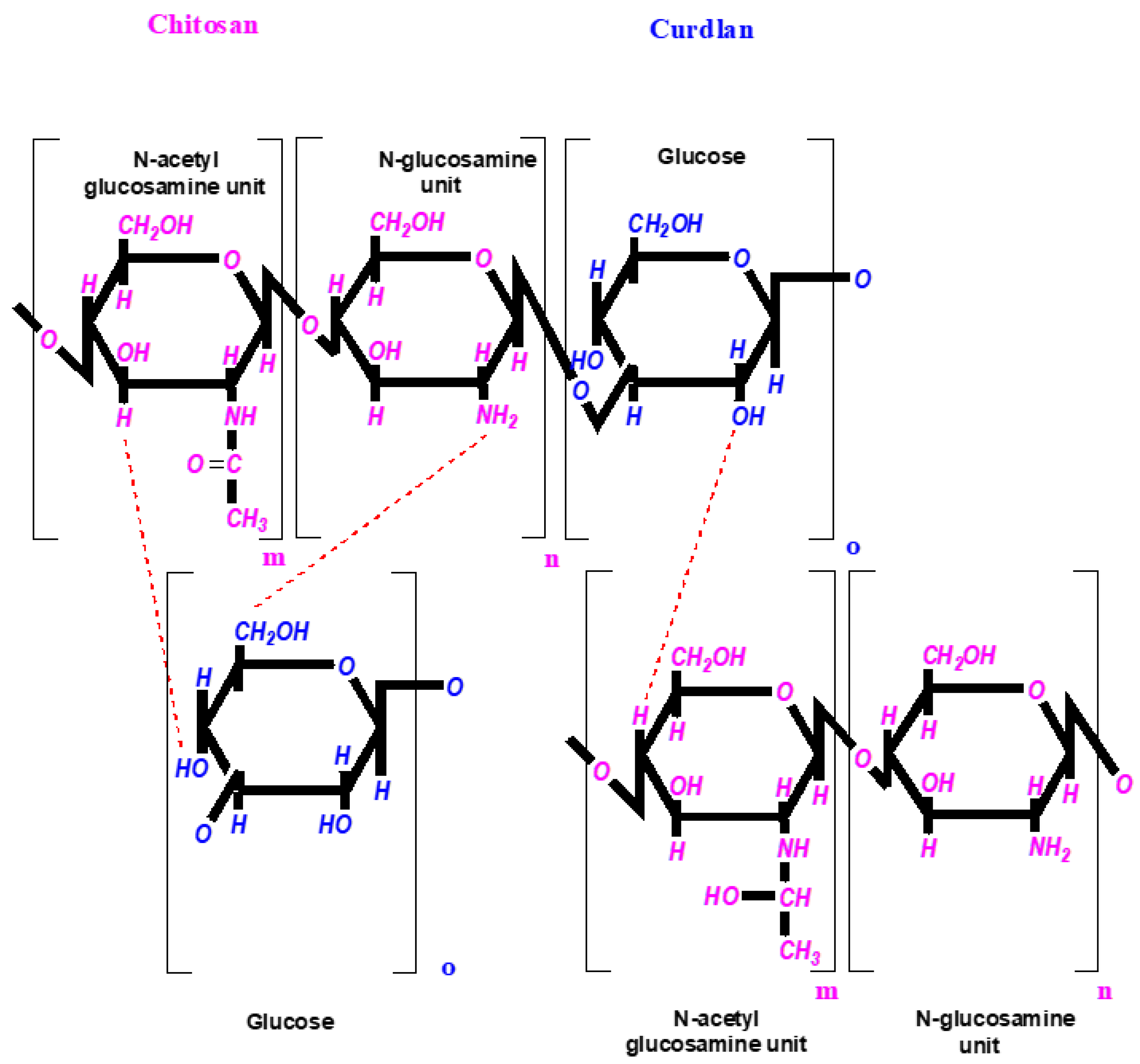
2.1. Preparation of Foam-like Curdlan–Chitosan (CUR/CS) and Curdlan–Chitosan-Ag NPs (CUR/CS/Ag) Biomaterials
2.2. Characterization
2.3. Water Absorbance
2.4. In Vivo Assay
3. Results
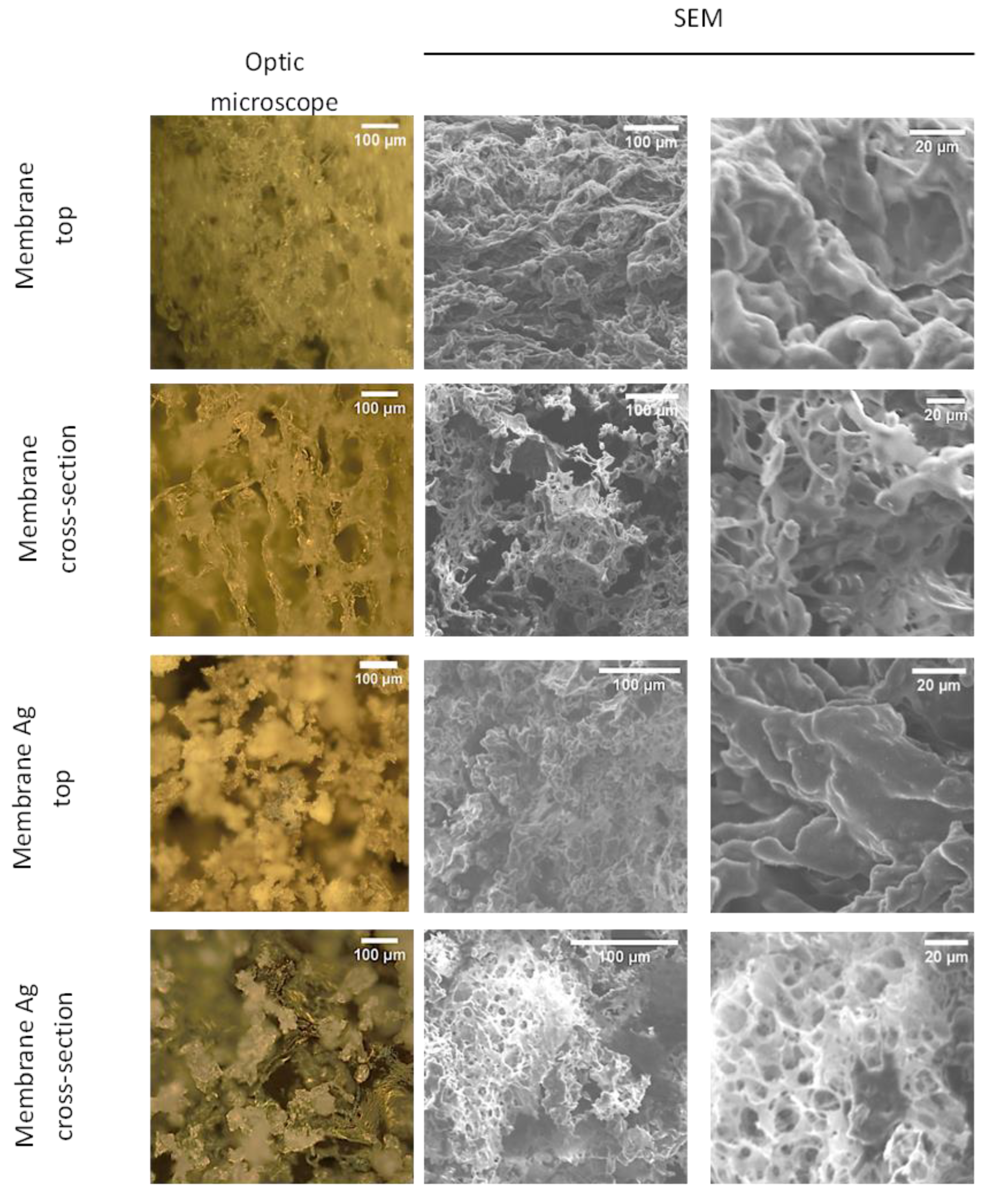
3.1. Fabrication of CUR/CS and CUR/CS/Ag Foams and Their Structural Analysis
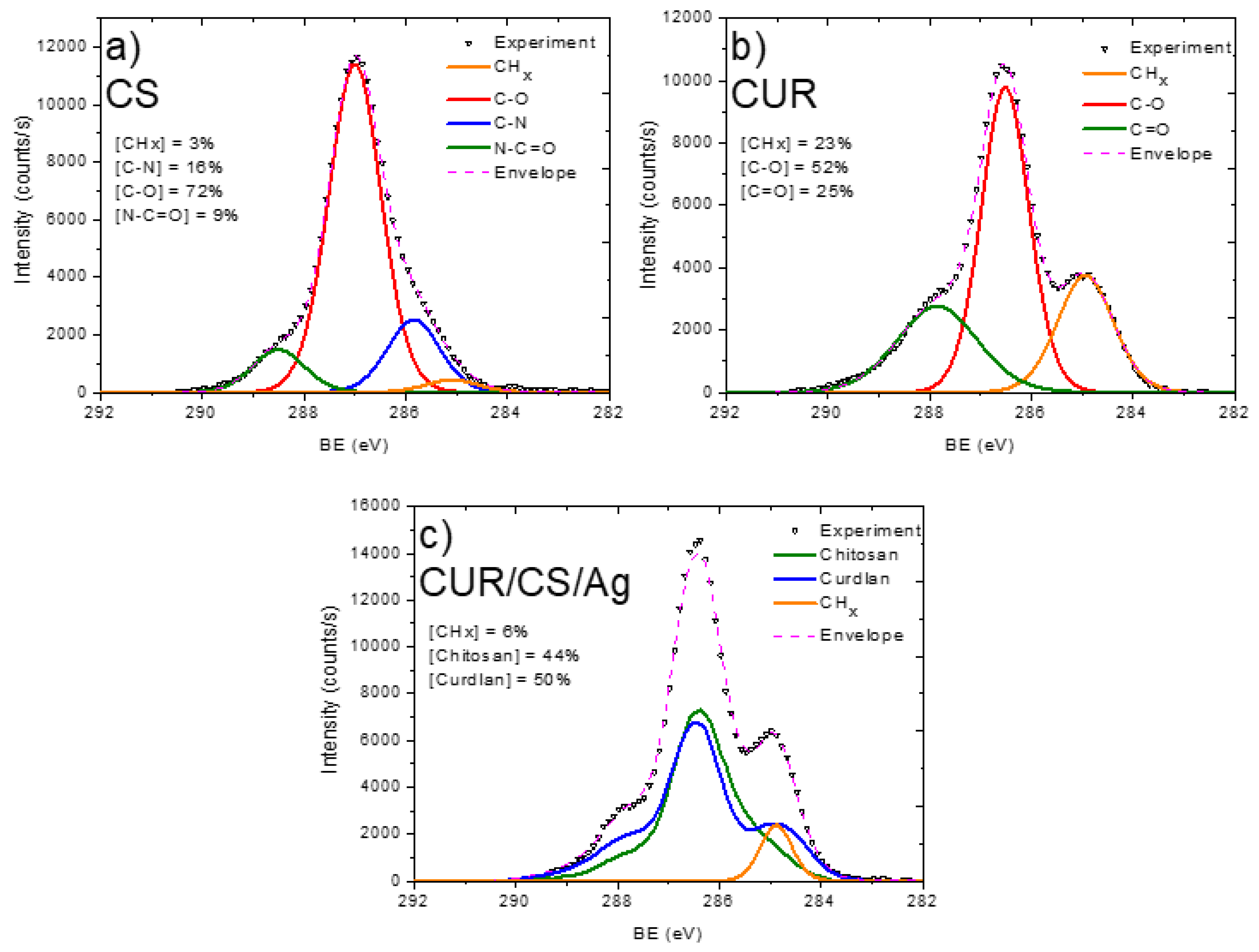
3.2. XPS Analysis and Modelling of CUR/CS and CUR/CS/Ag Biomaterials
3.3. FT-IR Analysis of CUR/CS and CUR/CS/Ag Biomaterials
3.4. PBS Absorbance Ability of CUR/CS and CUR/CS/Ag Biomaterials
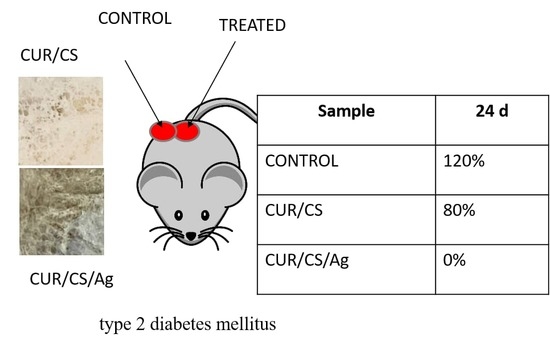
3.5. In Vivo Assay
4. Discussion and Final Remarks
| № | Dressings | Size of Wound | Observations In Vivo | Animal | Reference |
|---|---|---|---|---|---|
| 1 | Structure: nanofibers had a diameter between 200 to 300 nm, size of NPs 50–100 nm Composition: chitosan/polyvinyl alcohol/copper NPs | Wound: 1.5 cm × 1.5 cm Area: 225 mm2 | The wound closure rate of the negative control group was 18.46%, 59.89%, 62.42%, and 88.07%, and the wound closure rate of the positive control group was 25.33%, 72.85%, 95.32%, and 97.90% for 3, 7, 11, and 15 days, respectively | Rat | [42] |
| 2 | Structure: film and gel functionalized by NPs (11.5–18.71 nm) Composition: bacterial cellulose/ betulin diphosphate/ ZnO NPs | Burn rea: 1400 ± 50 mm2 Depth: 3–5 mm | On day 21, the wound area treated with BC-ZnO NPs-BDP films was reduced by 34.3%, while when treated with ZnO NPs-BDP oleogel, a large decrease of up to 40.6% was observed. In the untreated control, the closure rate was just 19.2% | Rat | [40] |
| 3 | Structure: electrospun fibers (648.1 ± 72.2 nm) with NPs Composition: PLA + Ca NPs | 12 mm square skin wounds Area: 452 mm2 | 80% contraction in wound area vs. 62% in the untreated control on 8 days | Diabetic mice | [44] |
| 4 | Structure: hydrogel with NPs (size 99.1 ± 2.3 nm) Composition: chitosan/PEG/Ag NPs | 20 mm square skin wounds Area: 1256 mm2 | A 47.7 ± 1.8% contraction in the wound area was recorded with the AgNPs impregnated chitosan-PEG hydrogel group, compared to 12.6 ± 1.3% in the negative control | Diabetic rabbit | [51] |
| 5 | Standard of care dressings impregnated with copper oxide microparticles (COD) | 9.26 ± 6.9 cm2 (range of 1.35–23.6 cm2) | Following 1 month of copper improved treatment, there was a clear reduction in the mean wound area (53.2%; p = 0.003), an increase in granulation tissue (43.37; p < 0.001), and a reduction in fibrins (47.8%; p = 0.002). In the control group, wound closure was less than 20% | Clinic diabetic foot ucler | [45] |
| 6 | Structure: hydrogel Composition: gelatin/hyaluronic acid/thrombomodulin | 8-mm diameter round-shaped wound Area: 201 mm2 | On day 10, wound closure was 80% for hydrogel with thrombomodulin vs. the 40% untreated control | Mice | [50] |
| 7 | Structure: electrospun fibers (90–120 nm) Composition:Enteromorpha polysaccharide and polyvinyl alcohol (PVA) | 10-mm diameter round-shaped wound Area: 314 mm2 | On day 9, the wound contraction rate for the PVA/EPP1 group reached nearly 72% vs. 54% for the control group | Diabetic mice | [52] |
| 8 | Structure: electrospun fibers (110 ± 74 nm) Composition: hydroxypropyl methylcellulose (HPMC)/polyethylene oxide (PEO)/ Beta-glucan | 1 cm × 1 cm Area: 100 mm2 | βG-nanofibers 95% healing vs. 40% healing of control in 24 days | Diabetic mice | [53] |
| 9 | Structure: hydrogel Composition: chitosan, heparin and poly (γ-glutamic acid) and loaded with superoxide dismutase | 10-mm diameter round-shaped wound Area: 314 mm2 | After 21 days, closure rate is 92.0% ± 3.7% compared with the control group (85.4% ± 2.4%) | Diabetic mice | [49] |
| 10 | Structure: hydrogel Composition: glycol chitosan, loaded by growth factors (VEGF and PDGF-BB) | 5-mm diameter round-shaped wound Area: 157 mm2 | On day 3, hydrogel dressing demonstrated 60% closure rate vs. less than 5% for the Duoderm dressing | Diabetic mice | [48] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential applications of nanomaterials and technology for diabetic wound healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Long, H.; Liang, M.; Chu, B.; Ren, Z.; Zhou, P.; Wu, C.; Liu, Z.; Wang, Y. Antifracture, Antibacterial, and Anti-inflammatory Hydrogels Consisting of Silver-Embedded Curdlan Nanofibrils. ACS Appl. Mater. Interfaces 2021, 13, 36747–36756. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Da, L.-C.; Huang, Y.-Z.; Xie, H.-Q.; Zheng, B.-H.; Huang, Y.-C.; Du, S.-R. Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing. Pharmaceutics 2021, 13, 1796. [Google Scholar] [CrossRef]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Saligan, L.N. Wound Pain and Wound Healing Biomarkers From Wound Exudate: A Scoping Review. J. Wound Ostomy Cont. 2020, 47, 559–568. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide based superabsorbent hydrogels and their methods of synthesis: A review. Carbohydr. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Choi, J.; Jeon, H. Manufacture and Characterization of Alginate-CMC-Dextran Hybrid Double Layer Superabsorbent Scaffolds. Appl. Sci. 2021, 11, 11573. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- Verma, D.K.; Niamah, A.K.; Patel, A.R.; Thakur, M.; Singh Sandhu, K.; Chávez-González, M.L.; Shah, N.; Noe Aguilar, C. Chemistry and microbial sources of curdlan with potential application and safety regulations as prebiotic in food and health. Food Res. Int. 2020, 133, 109136. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Pieta, I.S.; Nowakowski, R.; Pisarek, M.; Przekora, A. Effect of gelation temperature on the molecular structure and physicochemical properties of the curdlan matrix: Spectroscopic and microscopic analyses. Int. J. Mol. Sci. 2020, 21, 6154. [Google Scholar] [CrossRef] [PubMed]
- Dahri, S.H. Effect of Different Forms of Super Absorbent Polymers on Soil Physical & Chemical Properties in Orchard field. World Acad J. Res. Eng. Sci. 2019, 6, 12–20. [Google Scholar]
- Zaleski, R.; Stefaniak, W.; MacIejewska, M.; Goworek, J. Porosity of polymer materials by various techniques. J. Porous Mater. 2009, 16, 691–698. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, Y.; Lv, M.; Li, P.; Xu, H.; Wang, L. Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohydr. Polym. 2011, 84, 952–959. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent curdlan-based foam dressings with typical hydrocolloids properties for highly exuding wound management. Mater. Sci. Eng. C 2021, 124, 112068. [Google Scholar] [CrossRef]
- Toullec, C.; Le Bideau, J.; Geoffroy, V.; Halgand, B.; Buchtova, N.; Molina-peña, R.; Garcion, E.; Avril, S.; Sindji, L.; Dube, A.; et al. Curdlan–chitosan electrospun fibers as potential scaffolds for bone regeneration. Polymers 2021, 13, 526. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in nanotechnology towards development of silver nanoparticle-based wound-healing agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Moreno-Couranjou, M.; Choquet, P.; Boscher, N.D.; Pireaux, J.-J. Diene functionalisation of atmospheric plasma copolymer thin films. Surf. Coat. Technol. 2011, 205, S466–S469. [Google Scholar] [CrossRef]
- Stoica, A.; Manakhov, A.; Polčák, J.; Ondračka, P.; Buršíková, V.; Zajíčková, R.; Medalová, J.; Zajíčková, L. Cell proliferation on modified DLC thin films prepared by plasma enhanced chemical vapor deposition. Biointerphases 2015, 10, 029520. [Google Scholar] [CrossRef]
- Palumbo, F.; Camporeale, G.; Yang, Y.W.; Wu, J.S.; Sardella, E.; Dilecce, G.; Calvano, C.D.; Quintieri, L.; Caputo, L.; Baruzzi, F.; et al. Direct Plasma Deposition of Lysozyme-Embedded Bio-Composite Thin Films. Plasma Process. Polym. 2015, 12, 1302–1310. [Google Scholar] [CrossRef]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The effect of pH on cell viability, cell migration, cell proliferation, wound closure, and wound reepithelialization: In vitro and in vivo study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Caetano, P.C.; Strixino, J.F.; Raniero, L. Analysis of saliva by fourier transform infrared spectroscopy for diagnosis of physiological stress in athletes. Rev. Bras. Eng. Biomed. 2015, 31, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent applications of vibrational mid-infrared (IR) spectroscopy for studying soil components: A review. J. Cent. Eur. Agric. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef] [PubMed]
- Lekamge, S.; Miranda, A.F.; Abraham, A.; Li, V.; Shukla, R.; Bansal, V.; Nugegoda, D. The Toxicity of Silver Nanoparticles (AgNPs) to Three Freshwater Invertebrates With Different Life Strategies: Hydra vulgaris, Daphnia carinata, and Paratya australiensis. Front. Environ. Sci. 2018, 6, 152–165. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Boudreau, M.; Meng, J.; Yin, J.; Liu, J.; Xu, H. Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter- endothelial junction. Part. Fibre Toxicol. 2016, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Moon, T. Comparison of toxicity of uncoated and coated silver nanoparticles. J. Phys. Conf. Ser. 2013, 49, 012025. [Google Scholar] [CrossRef] [Green Version]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Mitra, C.; Gummadidala, P.M.; Afshinnia, K.; Corrin, R.; Baalousha, M.A.; Lead, J.R.; Chanda, A. Citrate-coated silver nanoparticles growth- independently inhibit aflatoxin synthesis in Aspergillus parasiticus. Environ. Sci. Technol 2017, 51, 8085–8093. [Google Scholar] [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Czupryn, M.; Antos-Bielska, M.; Szemraj, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Tannic acid-modified silver nanoparticles for wound healing: The importance of size. Int. J. Nanomed. 2018, 13, 991–1007. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Tang, H.; Liu, J.H.; Wang, H.; Liu, Y. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicol. Lett. 2013, 219, 42–48. [Google Scholar] [CrossRef]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Alexandra, C.; Rayyif, S.M.I.; Mohammed, H.B.; Curut, C.; Chifiriuc, M.C.; Mih, G.; Holban, A.M. ZnO Nanoparticles-Modified Dressings to Inhibit Wound Pathogens. Materials 2021, 14, 3084. [Google Scholar]
- Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar]
- Lou, Z.; Han, X.; Liu, J.; Ma, Q.; Yan, H.; Yuan, C.; Yang, L.; Han, H.; Weng, F.; Li, Y. Nano-Fe3O4/bamboo bundles/phenolic resin oriented recombination ternary composite with enhanced multiple functions. Compos. Part B 2021, 226, 109335. [Google Scholar] [CrossRef]
- Advances, T.; Perez-amodio, S.; Rubio, N.; Vila, O.F.; Navarro-requena, C.; Sanchez-ferrero, A.; Marti-munoz, J.; Blanco, J.; Engel, E.; Engineering, M.; et al. Polymeric Composite Dressings Containing Calcium-Releasing Nanoparticles Accelerate Wound Healing in Diabetic Mice. Adv. Wound Care 2020, 10, 1–39. [Google Scholar] [CrossRef]
- Melamed, E.; Rovitsky, A.; Roth, T.; Assa, L.; Borkow, G. Stimulation of Healing of Non-Infected Stagnated Diabetic Wounds by Copper Oxide-Impregnated Wound Dressings. Medicina 2021, 57, 1129. [Google Scholar] [CrossRef]
- Rodríguez-acosta, H.; Rivera, M.T.-; Guerrero-guzm, A.; García, J.J.O.G.-; Hern, E.; Pablo, E.P.; Ramírez, S.F.V.-; Anguiano, A.C.R.-; Vel, G.; Morales, S.G.-; et al. Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J. Tissue Viability 2022, 31, 173–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Zhang, Q.; Deng, T. Growth factors, as biological macromolecules in bioactivity enhancing of electrospun wound dressings for diabetic wound healing: A review. Int. J. Biol. Macromol. 2021, 193, 205–218. [Google Scholar] [CrossRef]
- Hyeok, D.; In, D.; Lee, D.; Ho, S.; Park, K.; Jang, G.; Ho, C.; Jae, H. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Hsu, Y.; Liu, K.; Yeh, H.; Lin, H.; Wu, H.; Tsai, J. Sustained release of recombinant thrombomodulin from cross-linked gelatin / hyaluronic acid hydrogels potentiate wound healing in diabetic mice. Eur. J. Pharm. Biopharm. 2019, 135, 61–71. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guan, N.; Miao, W.; Zhao, W.; Li, Q. An Electrospun Scaffold Loaded with an Enteromorpha Polysaccharide for Accelerated Wound Healing in Diabetic Mice. Mar. Drugs 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Grip, J.; Engstad, R.E.; Skjæveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvořáková, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polčak, J.; Zajíčková, L.; et al. Immobilization of Platelet-Rich Plasma onto COOH Plasma-Coated PCL Nanofibers Boost Viability and Proliferation of Human Mesenchymal Stem Cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Manakhov, A.; Permyakova, E.S.; Ershov, S.; Sheveyko, A.; Kovalskii, A.; Polčák, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Zajíčková, L.; Shtansky, D.V. Bioactive TiCaPCON-coated PCL nanofibers as a promising material for bone tissue engineering. Appl. Surf. Sci. 2019, 479, 796–802. [Google Scholar] [CrossRef]
- Manakhov, A.; Permyakova, E.; Ershov, S.; Miroshnichenko, S.; Pykhtina, M.; Beklemishev, A.; Kovalskii, A.; Solovieva, A. XPS Modeling of Immobilized Recombinant Angiogenin and Apoliprotein A1 on Biodegradable Nanofibers. Nanomaterials 2020, 10, 879. [Google Scholar] [CrossRef]


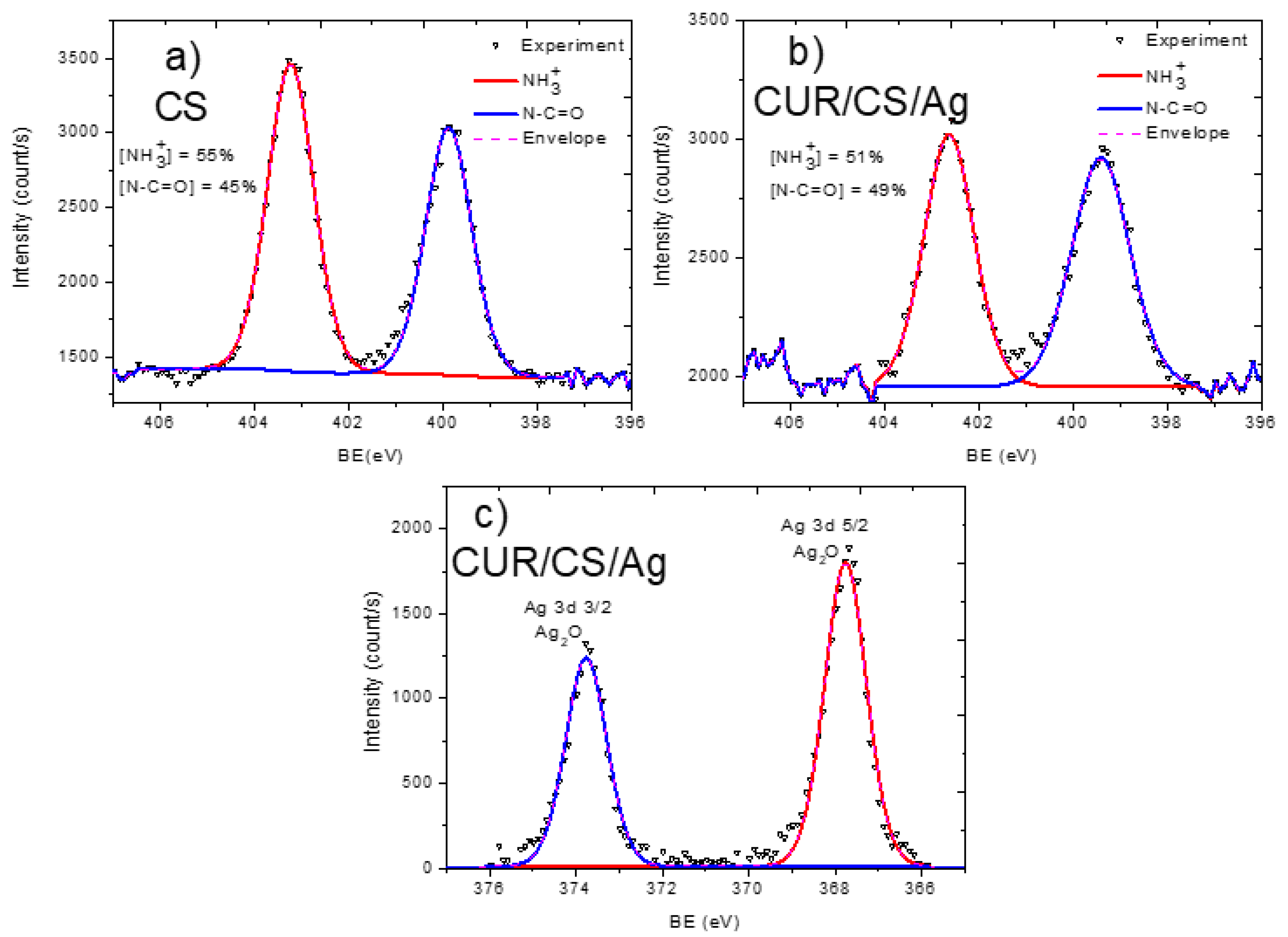
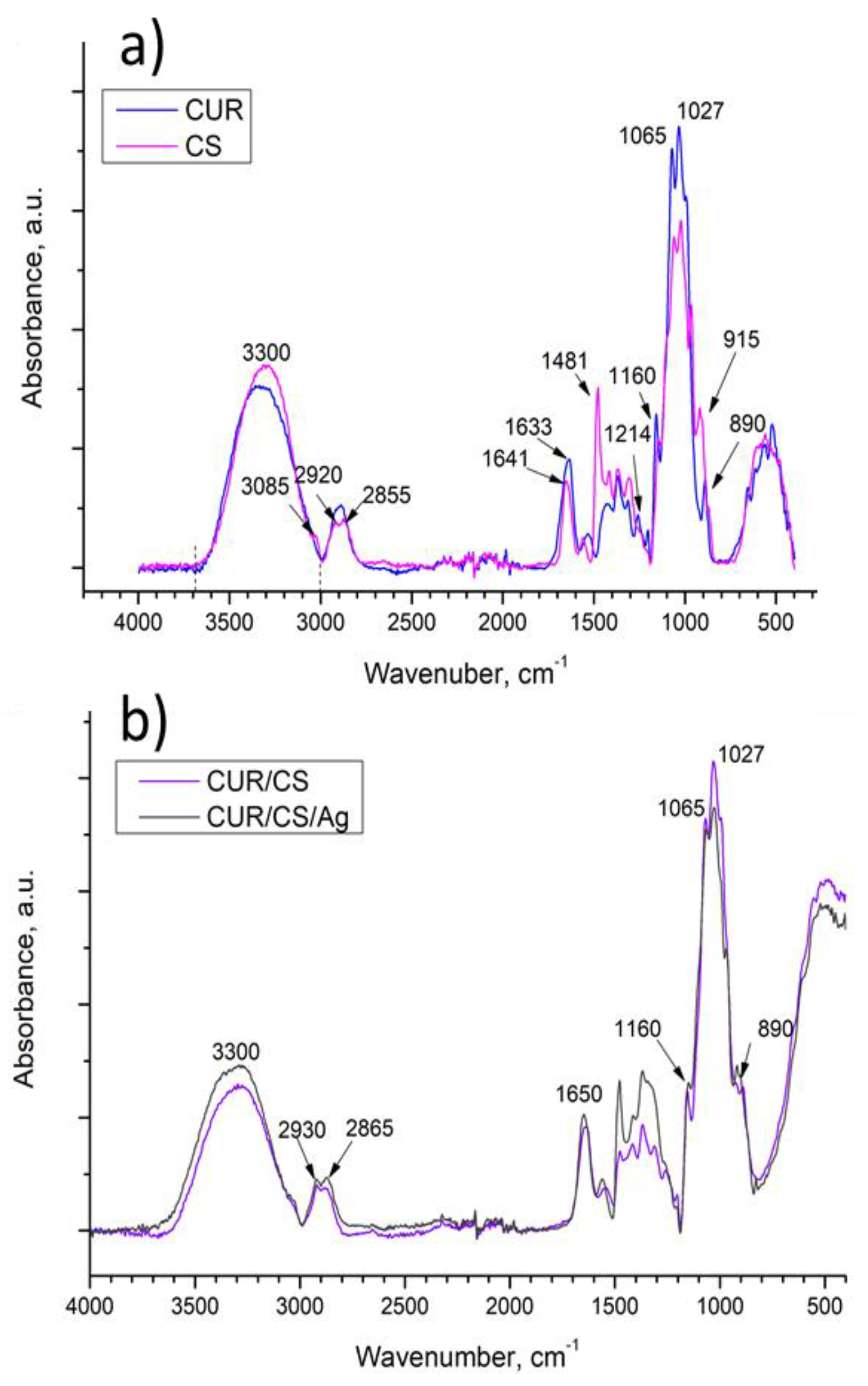
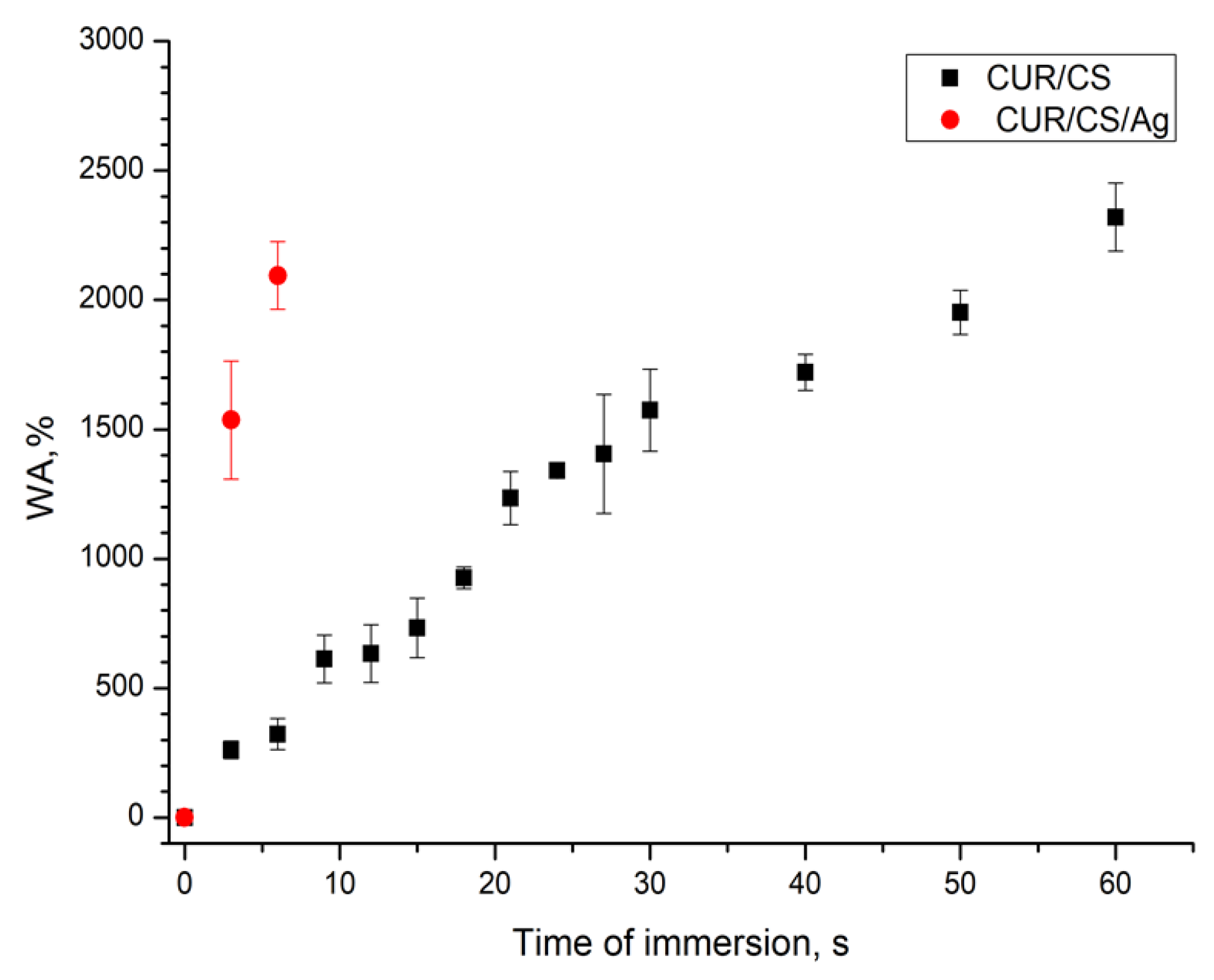



| Samples | Atomic Percentage (%) | ||||
|---|---|---|---|---|---|
| C | O | N | Ag | Pt | |
| CUR/CS | 53.0 | 41.0 | 5.8 | - | 0.2 |
| CUR/CS-Ag | 52.6 | 40.2 | 6.6 | 0.4 | 0.2 |
| Atomic Percentage (%) | Samples | |||
|---|---|---|---|---|
| CS | CUR | CUR/CS | CUR/CS/Ag | |
| C | 68.6 | 62.8 | 60.6 | 63.7 |
| O | 22.6 | 37.2 | 31.2 | 32.1 |
| N | 8.8 | 0.0 | 8.2 | 3.8 |
| Ag | 0.0 | 0.0 | 0.0 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, E.S.; Konopatsky, A.S.; Ershov, K.I.; Bakhareva, K.I.; Sitnikova, N.A.; Shtansky, D.V.; Solovieva, A.O.; Manakhov, A.M. Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model. Pharmaceutics 2022, 14, 724. https://doi.org/10.3390/pharmaceutics14040724
Permyakova ES, Konopatsky AS, Ershov KI, Bakhareva KI, Sitnikova NA, Shtansky DV, Solovieva AO, Manakhov AM. Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model. Pharmaceutics. 2022; 14(4):724. https://doi.org/10.3390/pharmaceutics14040724
Chicago/Turabian StylePermyakova, Elizaveta S., Anton S. Konopatsky, Konstantin I. Ershov, Ksenia I. Bakhareva, Natalya A. Sitnikova, Dmitry V. Shtansky, Anastasiya O. Solovieva, and Anton M. Manakhov. 2022. "Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model" Pharmaceutics 14, no. 4: 724. https://doi.org/10.3390/pharmaceutics14040724
APA StylePermyakova, E. S., Konopatsky, A. S., Ershov, K. I., Bakhareva, K. I., Sitnikova, N. A., Shtansky, D. V., Solovieva, A. O., & Manakhov, A. M. (2022). Ag-Contained Superabsorbent Curdlan–Chitosan Foams for Healing Wounds in a Type-2 Diabetic Mice Model. Pharmaceutics, 14(4), 724. https://doi.org/10.3390/pharmaceutics14040724