Preparation of Aditoprim Injection against Streptococcus suis in Pigs and a Dose Regimen Based on Pharmacokinetic-Pharmacodynamic Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Bacteria
2.3. Animals
2.4. Preparation of ADP Injection
2.5. Quality Evaluation
2.6. Pharmacodynamic Study
2.6.1. Determination of MIC, MBC, MPC and PAE
2.6.2. Establishment of In Vitro and Ex Vivo Growth Inhibition Curves
2.7. Pharmacokinetic Study
2.8. PK-PD Modeling and Dosage Regimen
3. Results
3.1. Pharmacodynamic Study
3.1.1. The MIC, MBC, MPC and PAE of ADP against S. suis CVCC 607
3.1.2. The In Vitro and Ex Vivo Antimicrobial Activity
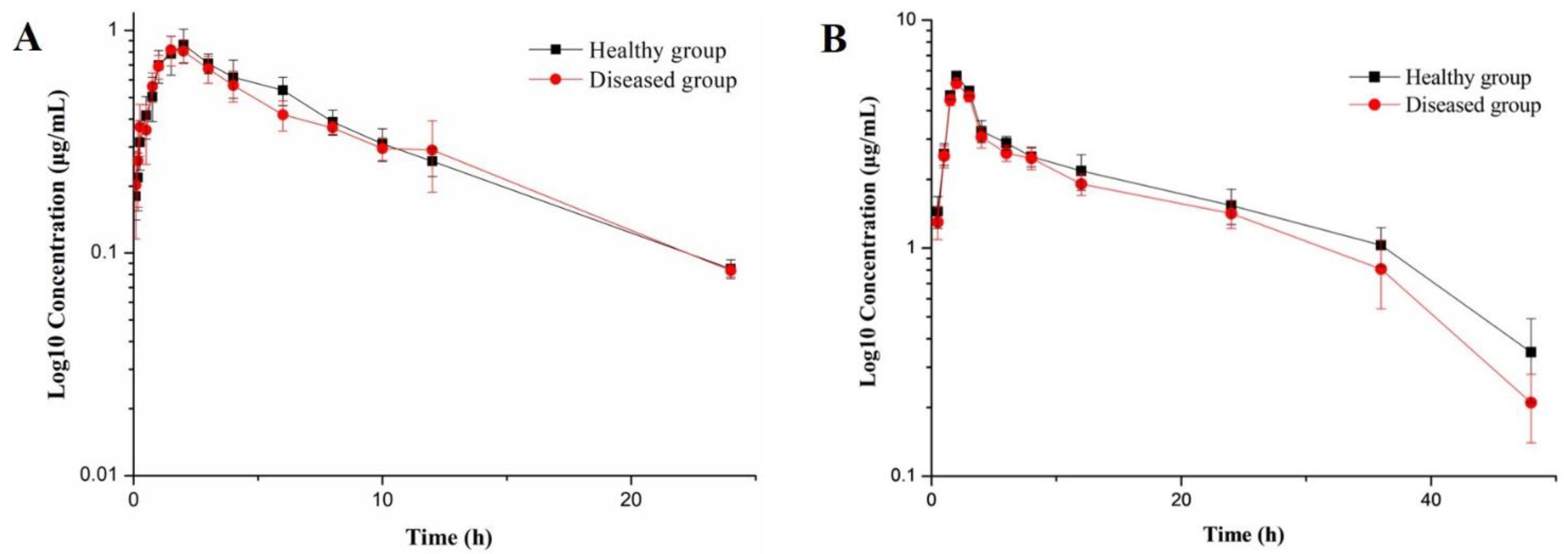
3.2. Pharmacokinetic Study
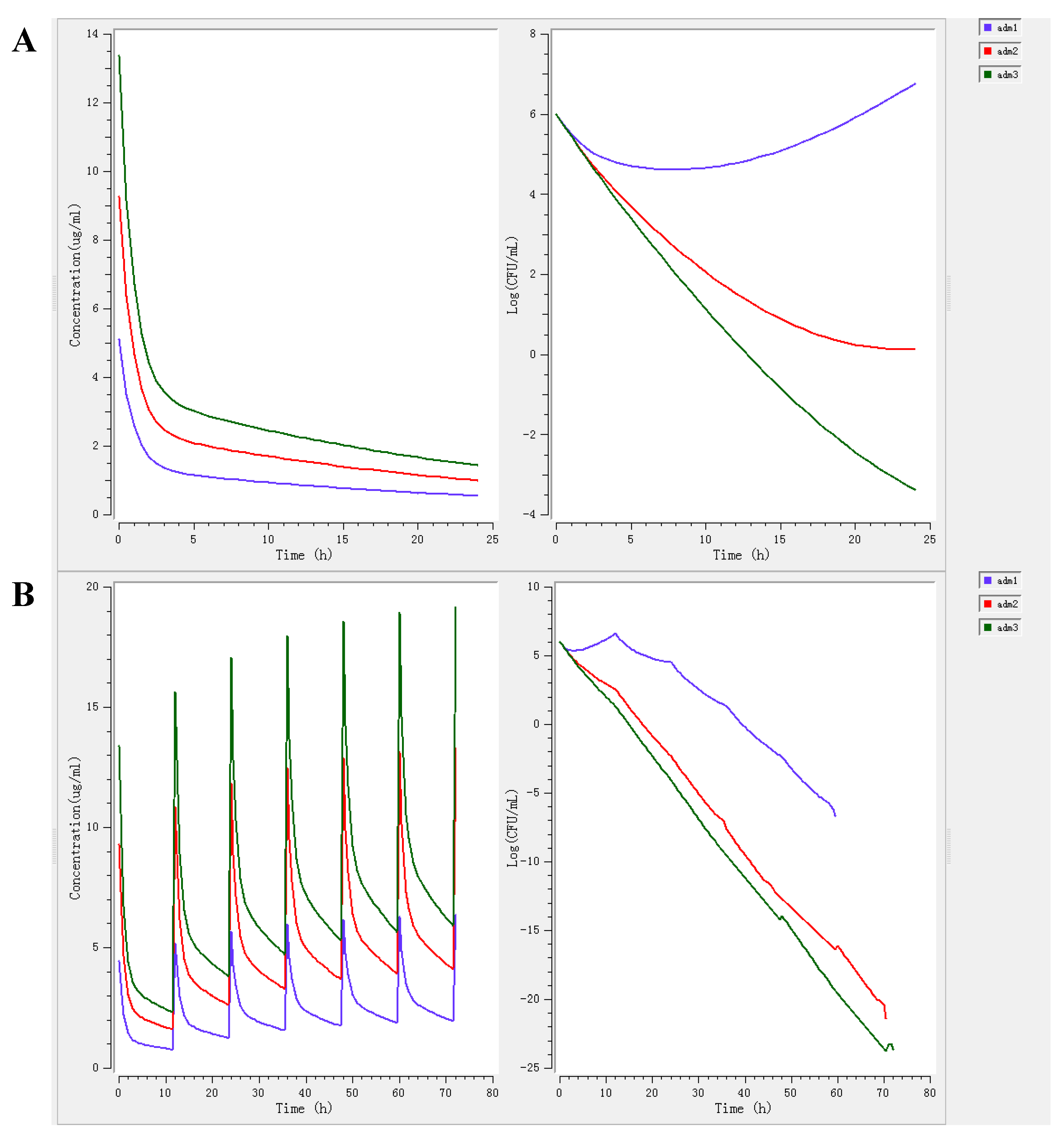
3.3. PK-PD Modeling and Dosage Regimen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Quality Evaluation of ADP Injection
Appendix A.1. Appearance, Specifications, pH Value and Content Determination
Appendix A.2. Hemolysis and Injection Site Irritation
| Tube Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2% erythrocyte suspension (mL) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Physiological saline (mL) | 2.4 | 2.3 | 2.2 | 2.1 | 2.0 | 2.5 | 0 |
| Distilled water (mL) | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 |
| ADP Injection (mL) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0 | 0 |

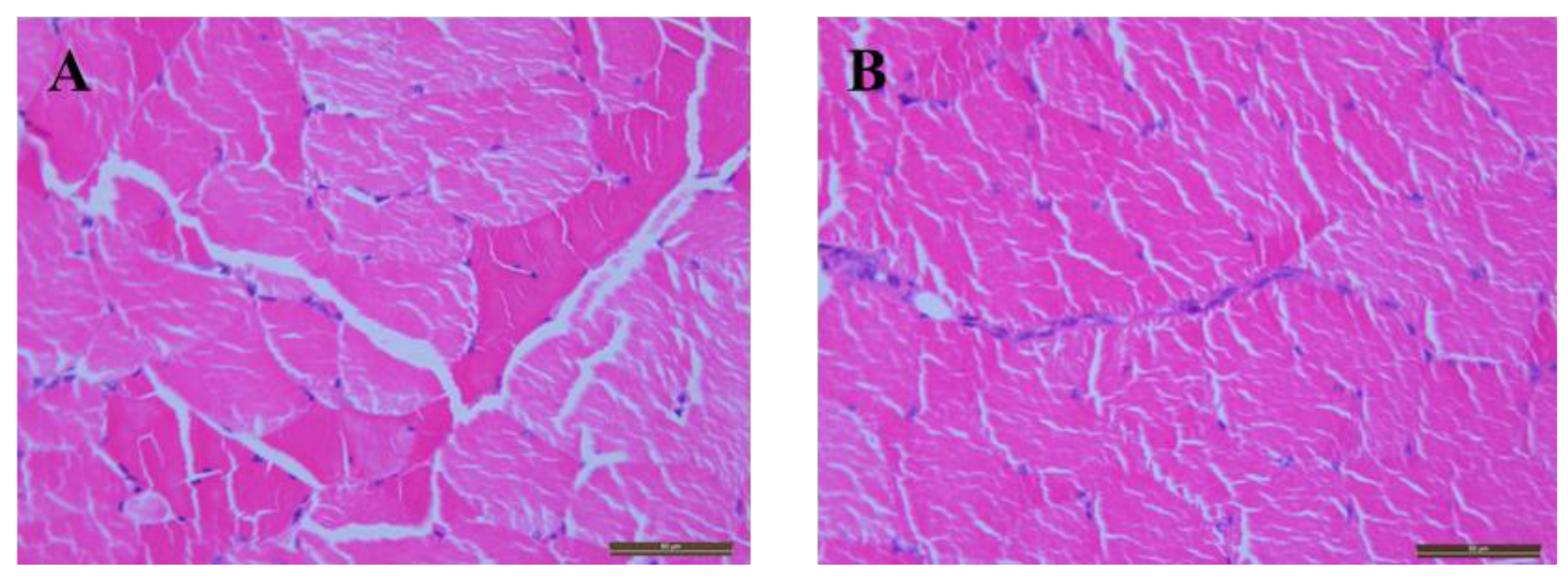
Appendix A.3. Stability Studies
| Projects | Time | Color | Content (%) | pH | Visible Particles |
|---|---|---|---|---|---|
| Accelerated test | 0 month | yellow | 99.77 ± 0.56 | 6.21 ± 0.04 | None |
| 1 month | yellow | 99.64 ± 0.51 | 6.23 ± 0.04 | None | |
| 2 months | yellow | 99.48 ± 0.50 | 6.22 ± 0.04 | None | |
| 3 months | yellow | 99.45 ± 0.62 | 6.25 ± 0.03 | None | |
| 6 months | yellow | 99.16 ± 0.71 | 6.23 ± 0.03 | None | |
| Long-term test | 0 month | yellow | 99.77 ± 0.56 | 6.21 ± 0.04 | None |
| 3 months | yellow | 99.67 ± 0.61 | 6.21 ± 0.04 | None | |
| 6 months | yellow | 99.62 ± 0.59 | 6.21 ± 0.05 | None | |
| 9 months | yellow | 99.63 ± 0.60 | 6.21 ± 0.04 | None | |
| 12 months | yellow | 99.56 ± 0.60 | 6.22 ± 0.01 | None |
Appendix B. Determination the Free Fraction of ADP in Alveolar Fluid
References
- Pu, J.H.; Mao, Z.W.; Wang, Q. Research progress on porcine Streptococcosis suis. Shanghai J. Ani. Husb. Vet. Med. 2016, 4, 13–15. [Google Scholar]
- Wisselink, H.J.; Veldman, K.T.; Van, D.E.C.; Salmon, S.A.; Mevius, D.J. Quantitative susceptibility of Streptococcus suis strains isolated from diseased pigs in seven European countries to antimicrobial agents licensed in veterinary medicine. Vet. Microbiol. 2006, 113, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.R. Comprehensive prevention and control of porcine respiratory disease. Chin. J. Ani. Husb. Vet. Med. 2014, 9, 28–29. [Google Scholar]
- Vela, A.I.; Moreno, M.A.; Cebolla, J.A.; González, S.; Latre, M.V.; Domínguez, L. Antimicrobial susceptibility of clinical strains of Streptococcus suis isolated from pigs in Spain. Vet. Microbiol. 2005, 105, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Riond, J.L.; Muller, P.; Wanner, M. The influence of age on the Pharmacokinetics of aditoprim in pigs after intravenous and oral administration. Vet. Res. Commun. 1992, 16, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.Y.; Xu, Y.M.; Zhu, X.D.; Xie, S.Y.; Wang, L.Y.; Huang, L.L. The antibacterial activities of aditoprim and its efficacy in the treatment of swine streptococcosis. Sci. Rep. 2017, 7, 41370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Y.; Huang, L.L.; Pan, Y.H.; Kuča, K.; Klímová, B.; Wu, Q. Metabolism and disposition of aditoprim in swine, broilers, carp and rats. Sci. Rep. 2016, 6, 20370. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Huang, L.L.; Pan, Y.H.; Wu, Q.; Xie, S.Y.; Yuan, Z.H. Simultaneous determination of aditoprim and its three major metabolites in pigs, broilers and carp tissues, and its application in tissue distribution and depletion studies. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1299–1311. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Z.Q.; Pan, Y.H.; Ihsan, A.; Liu, Q.; Huang, L.L.; Yuan, Z.H. Safety assessment of aditoprim acute, subchronic toxicity and mutagenicity studies. J. Appl. Toxicol. 2015, 35, 1415–1426. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Z.Q.; Cheng, G.Y.; Awais, I.; Huang, L.L.; Yuan, Z.H. Two-generation reproduction and teratology studies of feeding aditoprim in Wistar rats. J. Appl. Toxicol. 2015, 35, 1531–1538. [Google Scholar] [CrossRef]
- Nouws, J.F.M.; Smulders, A.; Rappalini, M. A comparative study on irritation and residue aspects of five oxytetracycline formulations administered intramuscularly to calves, pigs and sheep. Vet. Q. 1990, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.; Landoni, M.; Aliabadi, F.; Lees, P. PK-PD integration and modeling of marbofloxacin in sheep. Res. Vet. Sci. 2010, 88, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, F.S.; Ali, B.H.; Landoni, M.F.; Lees, P. Pharmacokinetics and PK-PD modelling of danofloxacin in camel serum and tissue cage fluids. Vet. J. 2003, 165, 104–118. [Google Scholar] [CrossRef]
- Menge, M.; Rose, M.; Bohland, C.; Zschiesche, E.; Kilp, S.; Metz, W. Pharmacokinetics of tildipirosin in bovine plasma, lung tissue, and bronchial fluid (from live, nonanesthetized cattle). J. Vet. Pharmacol. Ther. 2012, 35, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Giguère, S.; Tessman, R.K. Rational dosing of antimicrobial agents for bovine respiratory disease: The use of plasma versus tissue concentrations in predicting efficacy. Int. J. Appl. Res. Vet. Med. 2011, 9, 342–355. [Google Scholar]
- Foster, D.M.; Martin, L.G.; Papich, M.G. Comparison of Active Drug Concentrations in the Pulmonary Epithelial Lining Fluid and Interstitial Fluid of Calves Injected with Enrofloxacin, Florfenicol, Ceftiofur, or Tulathromycin. PLoS ONE 2016, 11, e0149100. [Google Scholar] [CrossRef]
- Cakir, E.; Kut, A.; Ozkaya, E.; Gedik, A.H.; Midyat, L.; Nursoy, M. Bronchoscopic evaluation in childhood pulmonary tubercμLosis: Risk factors of airway involvement and contribution to the bacteriologic diagnosis. Pediatr. Infect. Dis. J. 2013, 32, 921–923. [Google Scholar] [CrossRef]
- Goussard, P.; Gie, R. The role of bronchoscopy in the diagnosis and management of pediatric pulmonary tuberculosis. Expert Rev. Respir. Med. 2014, 8, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Hasselt, J.G.; Rizk, M.L.; Lala, M.; Chavez, E.C.; Visser, S.A.; Kerbusch, T. Pooled population Pharmacokinetic model of imipenem in plasma and the lung epithelial lining fluid. Br. J. Clin. Pharmacol. 2016, 81, 1113–1123. [Google Scholar] [CrossRef] [Green Version]
- Hennig, P.I.; Bremerich, S.; Nienhoff, H.; Schröder, C.; Ganter, M.; Blecha, F. Comparison of three common methods of lung lavage in healthy pigs. J. Vet. Med. A 2007, 54, 428–433. [Google Scholar] [CrossRef]
- Donin, N.M.; Duarte, S.; Lenis, A.T.; Caliliw, R.; Torres, C.; Smithson, A.; Strauss-Ayali, D.; Agmon-Gerstein, Y.; Malchi, N.; Said, J.; et al. Sustained-release Formulation of Mitomycin C to the Upper Urinary Tract Using a Thermosensitive Polymer: A Preclinical Study. Urology 2017, 99, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.J.; Liaubet, L.; Concordet, D.; SanCristobal, M.; Uro-Coste, E.; Tosser-Klopp, G.; Bonnet, A.; Toutain, P.L.; Hatey, F.; Lefebvre, H.P. Longitudinal analysis of gene expression in porcine skeletal muscle after post-injection local injury. Pharm. Res. 2007, 24, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Zhang, H.Y.; Li, M.; Ahmad, I.; Wang, Y.L.; Yuan, Z.H. Pharmacokinetic-pharmacodynamic modeling of tylosin against Streptococcus suis in pigs. BMC Vet. Res. 2018, 14, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maan, M.K.; Sattar, A.; Mi, K.; Bakr Shabbir, M.A.; Xie, S.Y.; Xin, L.; Ahmed, S.; Algharib, S.A.; Huang, L.L.; Yuan, Z.H. Integration of PK/PD for dose optimization of aditoprim against Trueperella pyogenes causing endometritis in bovines. Microb. Pathog. 2020, 142, 104097. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Luo, W.H.; Pan, Y.H.; Qu, W.; Xie, S.Y.; Huang, L.L.; Wang, Y.L. Antibacterial activity of combined aditoprim and sulfamethoxazole against Escherichia coli from swine and a dose regimen based on pharmacokinetic–pharmacodynamic modeling. J. Vet. Pharmacol. Ther. 2022, 45, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C. Encyclopedia of Toxicology, 3rd ed.; Academic Press: Waltham, MA, USA, 2014; pp. 881–882. [Google Scholar]
- Smith, H.E.; Veenbergen, V.; Van, D.V.J.; Damman, M.; Wisselink, H.J.; Smits, M.A. The cps genes of Streptococcus suis serotypes 1, 2, and 9: Development of rapid serotype-specific PCR assays. J. Clin. Microbiol. 1999, 37, 3146–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marois, C.; Bougeard, S.; Gottschalk, M.; Kobisch, M. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J. Clin. Microbiol. 2004, 42, 3169–3175. [Google Scholar] [CrossRef] [Green Version]
- Somogyi, Z.; Mag, P.; Kovacs, D.; Kerek, A.; Szabo, P.; Makrai, L.; Jerzsele, A. Synovial and Systemic Pharmacokinetics of Florfenicol and PK/PD Integration against Streptococcus suis in Pigs. Pharmaceutics 2022, 14, 109. [Google Scholar] [CrossRef]
- Toutain, P.L.; Pelligand, L.; Lees, P.; Bousquet-Melou, A.; Ferran, A.A.; Turnidge, J.D. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 2021, 44, 172–200. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Hope, W.W.; Boyd, S.E. Considerations for effect site pharmacokinetics to estimate drug exposure: Concentrations of antibiotics in the lung. Curr. Opin. Pharmacol. 2017, 36, 114–123. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Noreddin, A.M. Pharmacokinetics and Pharmacodynamics of the new fluoroquinolones: Focus on respiratory infections. Curr. Opin. Pharmacol. 2001, 1, 459–463. [Google Scholar] [CrossRef]
- Booker, B.M.; Smith, P.F.; Forrest, A.; Bullock, J.; Kelchlin, P.; Bhavnani, S.M. Application of an in vitro infection model and simulation for reevaluation of fluoroquinolone breakpoints for Salmonella enterica serotype typhi. Antimicrob. Agents Chemother. 2005, 49, 1775–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carral, N.; Lukas, J.C.; Oteo, I.; Suarez, E. Impact of poor compliance with levofloxacin and moxifloxacin on respiratory tract infection antimicrobial efficacy: A Pharmacokinetic/Pharmacodynamic simulation study. Int. J. Antimicrob. Agents 2015, 45, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Craig, W.A. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 2002, 46, 1665–1670. [Google Scholar] [CrossRef] [Green Version]
- Mouton, J.W.; Theuretzbacher, U.; Craig, W.A.; Tulkens, P.M.; Derendorf, H.; Cars, O. Tissue concentrations: Do we ever learn? J. Antimicrob. Chemother. 2008, 61, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKellar, Q.; Gibson, I.; Monteiro, A.; Bregante, M. Pharmacokinetics of enrofloxacin and danofloxacin in plasma, inflammatory exudate, and bronchial secretions of calves following subcutaneous administration. Antimicrob. Agents Chemother. 1999, 43, 1988–1992. [Google Scholar] [CrossRef] [Green Version]
- Terhune, T.N.; Skogerboe, T.L.; Shostrom, V.K.; Weigel, D.J. Comparison of Pharmacokinetics of danofloxacin and enrofloxacin in calves challenged with Mannheimia haemolytica. Am. J. Vet. Res. 2005, 66, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Messenger, K.M.; Papich, M.G.; Blikslager, A.T. Distribution of enrofloxacin and its active metabolite, using an in vivo ultrafiltration sampling technique after the injection of enrofloxacin to pigs. J. Vet. Pharmacol. Ther. 2012, 35, 452–459. [Google Scholar] [CrossRef]
- Rottbøll, L.A.H.; Friis, C. Penetration of antimicrobials to pulmonary epithelial lining fluid and muscle and impact of drug physicochemical properties determined by microdialysis. J. Pharmacol. Toxicol. Methods 2016, 78, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K. The mutant selection window and antimicrobial resistance. J. Antimicrob. Chemother. 2003, 52, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.M.; Johnson, W.; Rodriguez, L.; Gounder, M.; Hall, G.S.; Buckley, B. A study of the interactions between carboplatin and blood plasma proteins using size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2007, 387, 2815–2822. [Google Scholar] [CrossRef] [PubMed]

| Concentration (μg/mL) | PAE (h) | |
|---|---|---|
| Exposed for 1 h | Exposed for 2 h | |
| 1 MIC | 0.25 | 0.53 |
| 2 MIC | 0.71 | 0.93 |
| 4 MIC | 1.09 | 1.28 |
| Parameters | Units | Healthy Group | Infected Group |
|---|---|---|---|
| Emax | Log10 CFU | 2.17 | 2.11 |
| E0 | Log10 CFU | −5.10 | −4.93 |
| Emax-E0 | Log10 CFU | 7.27 | 7.04 |
| EC50 | h | 25.00 | 24.00 |
| N | - | 2.24 | 2.48 |
| Bacteriostatic (E = 0) | h | 17.07 | 17.04 |
| Bactericidal (E = −3) | h | 37.38 | 35.54 |
| Eradication (E = −4) | h | 53.98 | 51.27 |
| Parameters | Healthy Group | Infected Group | ||
|---|---|---|---|---|
| Plasma | Alveolar Fluid | Plasma | Alveolar Fluid | |
| AUC (h·μg/mL) | 8.82 ± 0.94 | 95.38 ± 11.74 | 9.33 ± 1.52 | 86.75 ± 8.26 |
| CL/F (mL/h) | 573.07 ± 71.81 | 52.42 ± 3.03 | 545.99 ± 72.59 | 57.64 ± 2.56 |
| Tmax (h) | 1.96 ± 0.34 | 1.96 ± 0.14 | 1.75 ± 0.29 | 1.95 ± 0.15 |
| Cmax (μg/mL) | 0.80 ± 0.11 | 5.45 ± 0.47 | 0.77 ± 0.07 | 5.10 ± 0.17 |
| A (μg/mL) | −30.49 ± 54.07 | 1.08 ± 0.01 | −49.80 ± 150.66 | 1.10 ± 0.03 |
| Β (μg/mL) | 0.72 ± 0.14 | 0.03 ± 0.01 | 0.54 ± 0.18 | 0.04 ± 0.02 |
| T1/2α (h) | 1.25 ± 0.63 | 0.64 ± 0.15 | 1.46 ± 0.97 | 0.62 ± 0.09 |
| T1/2β (h) | 7.95 ± 0.81 | 5.32 ± 0.74 | 11.09 ± 2.78 | 5.33 ± 0.49 |
| Effect (E) | Healthy Group | Infected Group |
|---|---|---|
| Bacteriostatic action (E = 0) | 1.79 mg/kg b.w. | 1.96 mg/kg b.w. |
| Bactericidal action (E = −3) | 3.92 mg/kg b.w. | 4.10 mg/kg b.w. |
| Bacterial eradication (E = −4) | 5.66 mg/kg b.w. | 5.91 mg/kg b.w. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, W.; Dong, M.; Pan, Y.; Xie, S.; Yuan, Z.; Huang, L. Preparation of Aditoprim Injection against Streptococcus suis in Pigs and a Dose Regimen Based on Pharmacokinetic-Pharmacodynamic Modeling. Pharmaceutics 2022, 14, 730. https://doi.org/10.3390/pharmaceutics14040730
Qu W, Dong M, Pan Y, Xie S, Yuan Z, Huang L. Preparation of Aditoprim Injection against Streptococcus suis in Pigs and a Dose Regimen Based on Pharmacokinetic-Pharmacodynamic Modeling. Pharmaceutics. 2022; 14(4):730. https://doi.org/10.3390/pharmaceutics14040730
Chicago/Turabian StyleQu, Wei, Mengxiao Dong, Yuanhu Pan, Shuyu Xie, Zonghui Yuan, and Lingli Huang. 2022. "Preparation of Aditoprim Injection against Streptococcus suis in Pigs and a Dose Regimen Based on Pharmacokinetic-Pharmacodynamic Modeling" Pharmaceutics 14, no. 4: 730. https://doi.org/10.3390/pharmaceutics14040730
APA StyleQu, W., Dong, M., Pan, Y., Xie, S., Yuan, Z., & Huang, L. (2022). Preparation of Aditoprim Injection against Streptococcus suis in Pigs and a Dose Regimen Based on Pharmacokinetic-Pharmacodynamic Modeling. Pharmaceutics, 14(4), 730. https://doi.org/10.3390/pharmaceutics14040730





