Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Hydrogel Base
2.2.1. Synthesis of the Host Molecules
2.2.2. Synthesis of the Guest Part
2.3. Construction of the Hydrogel Base Loaded with 5-FU/MTX Mixture
2.4. Rheological Studies
2.5. In Vitro Release Studies of the Medicated Hydrogel Samples
2.6. Syringeability and Injectability Study
2.7. In Vitro Antitumor Activity and Cell Viability
2.8. In Vivo Studies
2.8.1. Animal Preparation
2.8.2. Plasma Samples Pretreatment
2.8.3. Chromatographic Analysis of the Drugs’ Mixture in Plasma
2.8.4. Pharmacokinetic Study
2.9. Histopathological Appraisals and Traits
2.10. Effect of the Proposed Hydrogel System on the Tumor Growth
2.11. Detection of the Tumor Markers in Rats’ Blood
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterizations of the Hydrogel Base
3.2. Assembling the Hydrogel Base and Loading It with 5-FU/MTX Mixture
3.3. Physiochemical Characterizations of the Constructed Medicated Hydrogel System
3.3.1. Visual Appearance, pH, and Individual Drug Content Uniformity Measurements
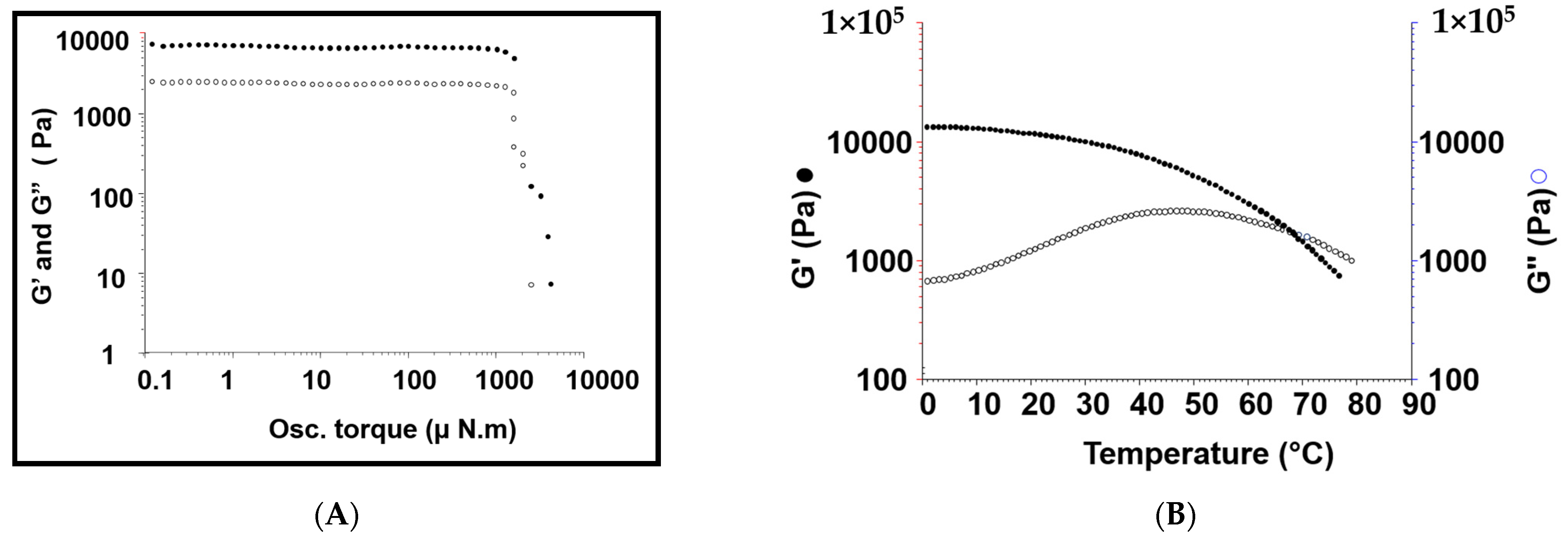
3.3.2. Rheological Studies
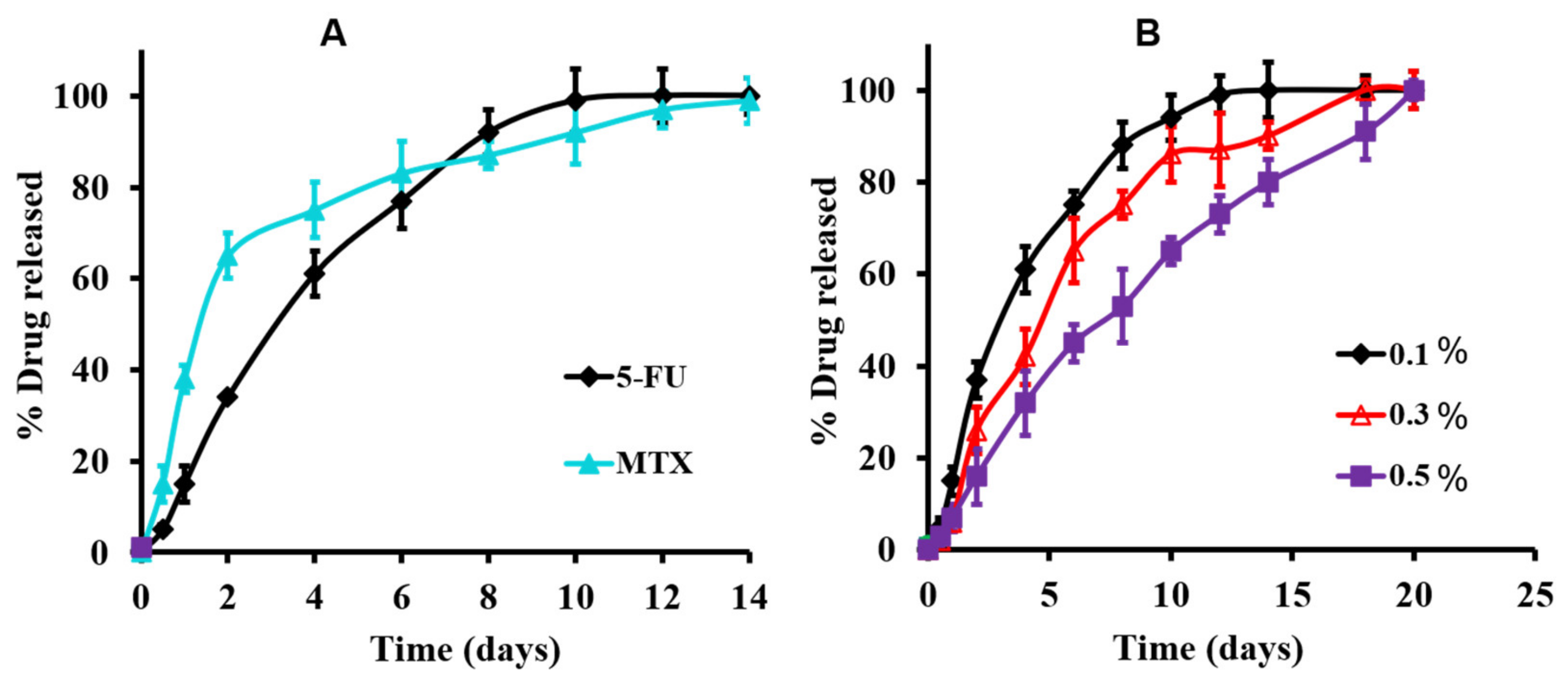
3.3.3. In Vitro Release Studies of the Medicated Hydrogel
3.3.4. Injectability Studies
3.4. Cell Viability Studies
3.5. In Vivo Studies
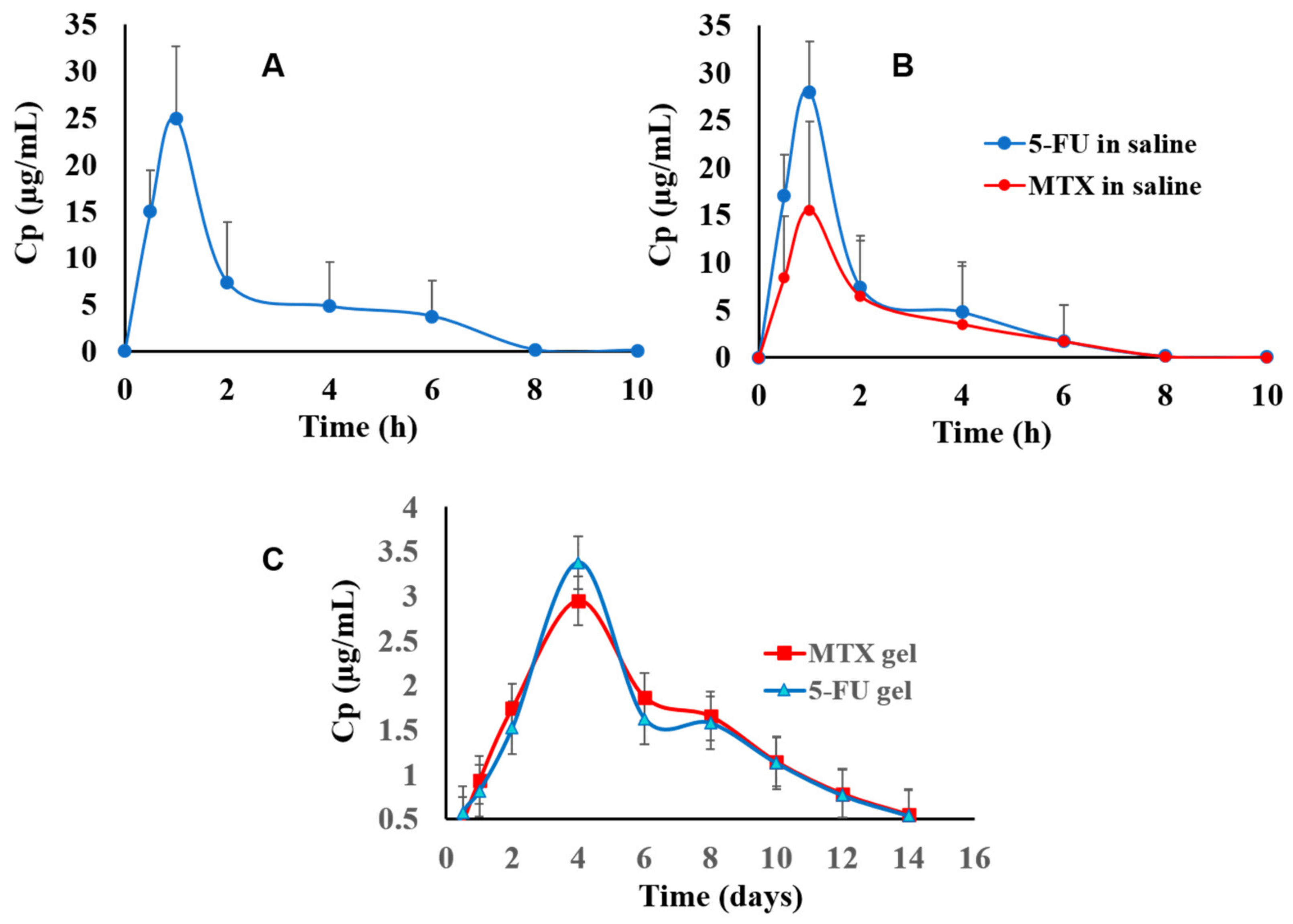
3.5.1. Chromatographic Data Analysis and Pharmacokinetics’ Parameters
3.5.2. Histopathological Appraisals and Traits
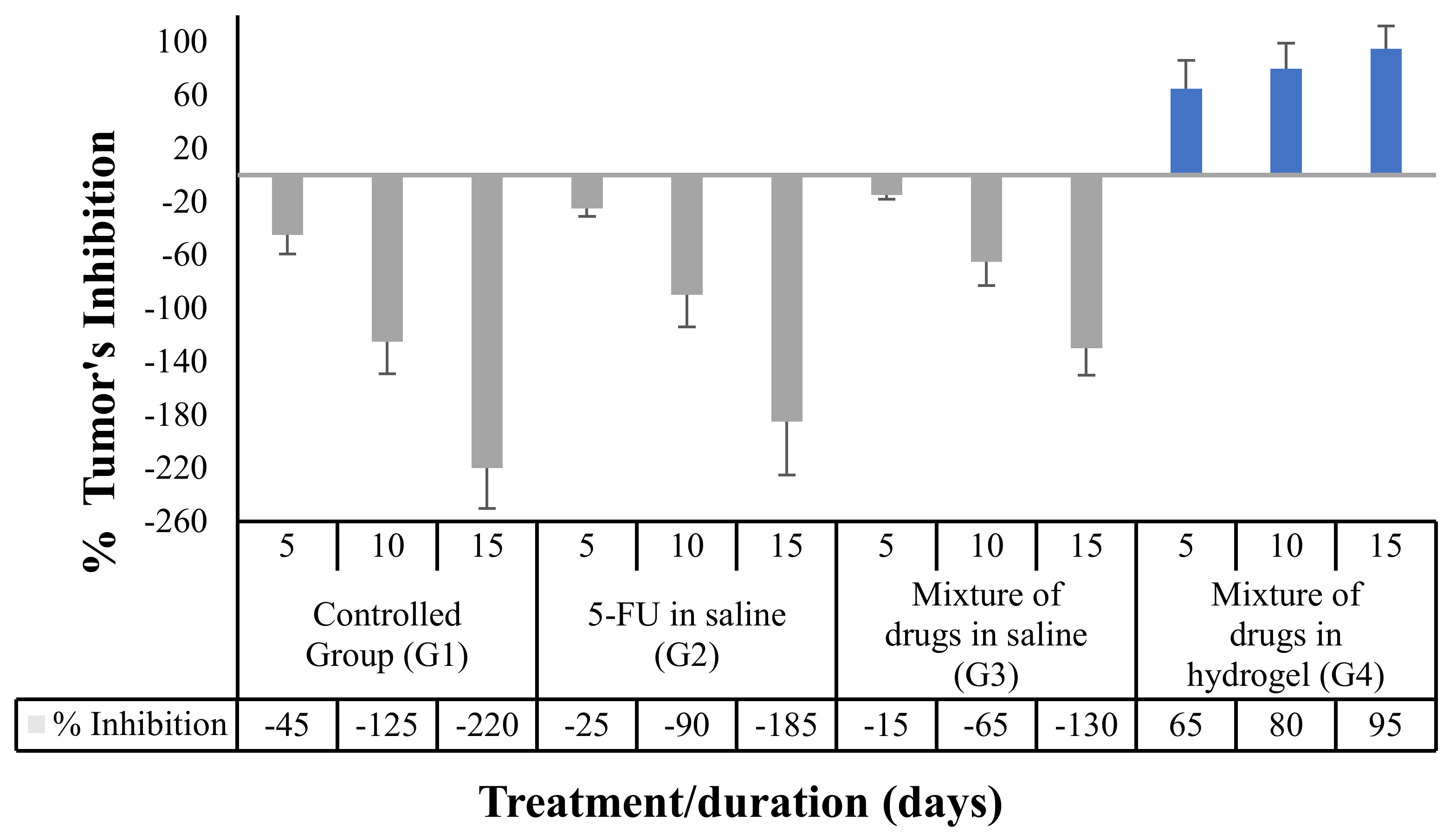
3.5.3. Effect of the Proposed Hydrogel System on the Tumor Growth
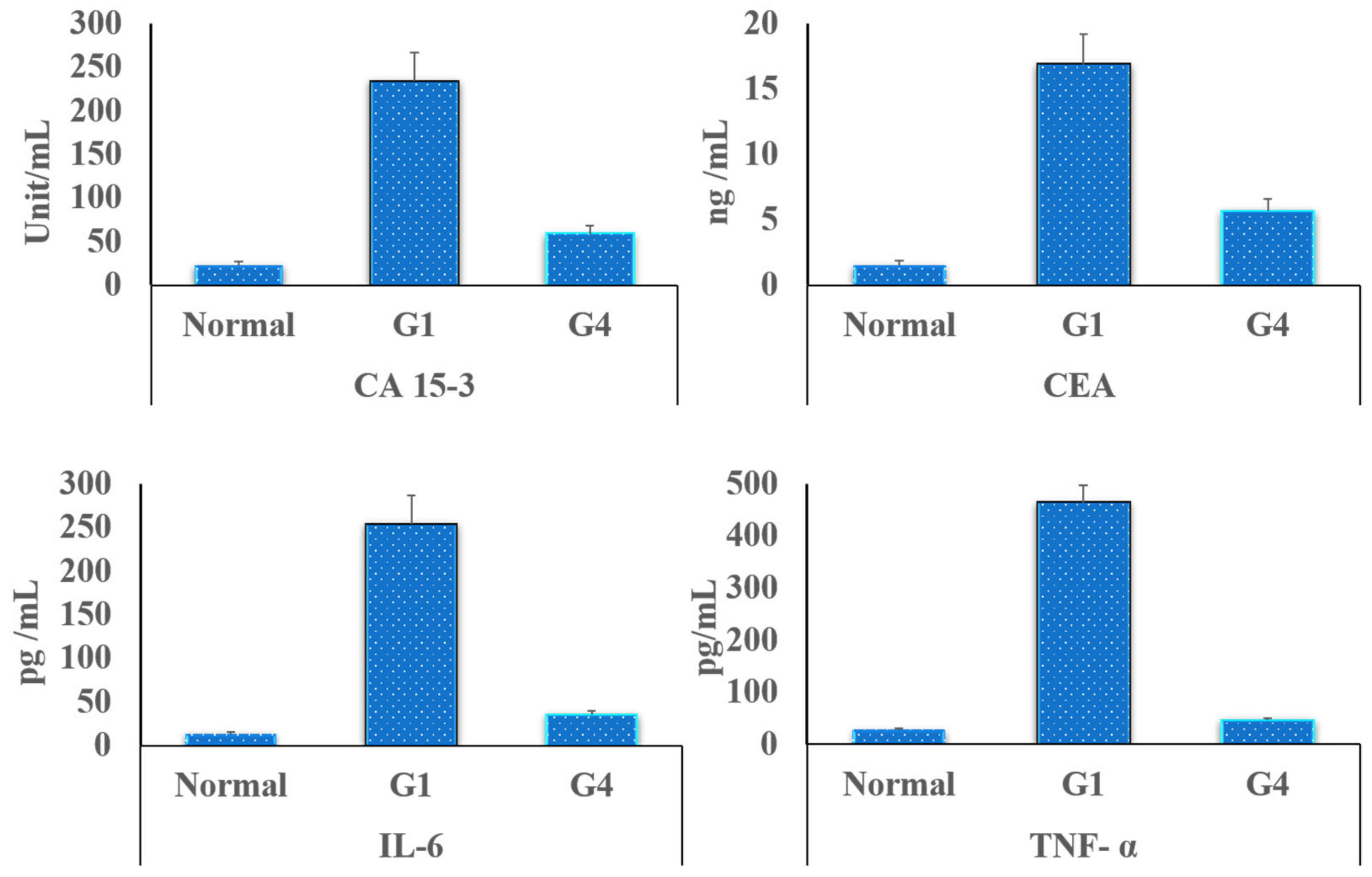
3.5.4. Tumor Markers Detection in the Rats’ Blood
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.; Ciszewski, T.; Łopacka-Szatan, K.; Miotła, P.; Starosławska, E. Breast cancer risk factors. Prz. Menopauzalny 2015, 14, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Bonadonna, G.; Brusamolino, E.; Valagussa, P.; Rossi, A.; Brugnatelli, L.; Brambilla, C.; De Lena, M.; Tancini, G.; Bajetta, E.; Musumeci, R.; et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N. Engl. J. Med. 1976, 294, 915–924. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Fournier, E.; Passirani, C.; Colin, N.; Breton, P.; Sagodira, S.; Benoit, J.-P. Development of novel 5-FU-loaded poly(methylidene malonate 2.1.2)-based microspheres for the treatment of brain cancers. Eur. J. Pharm. Biopharm. 2004, 57, 189–197. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable chitosan hydrogels for localised cancer therapy. J. Control. Release 2008, 126, 205–216. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Islami, F.; Guerra, C.E.; Minihan, A.; Yabroff, K.R.; Fedewa, S.A.; Sloan, K.; Wiedt, T.L.; Thomson, B.; Siegel, R.L.; Nargis, N.; et al. American Cancer Society’s report on the status of cancer disparities in the United States. CA Cancer J. Clin. 2021, 72, 112–143. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Gref, R.; Amiel, C.; Molinard, K.; Daoud-Mahammed, S.; Sébille, B.; Gillet, B.; Beloeil, J.C.; Ringard, C.; Rosilio, V.; Poupaert, J.; et al. New self-assembled nanogels based on host-guest interactions: Characterization and drug loading. J. Control. Release 2006, 111, 316–324. [Google Scholar] [CrossRef]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Nostro, P.L.; Baglioni, P. Self-assembly of β-cyclodextrin in water. Part 1: Cryo-TEM and dynamic and static light scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef]
- Layre, A.M.; Volet, G.; Wintgens, V.; Amiel, C. Associative network based on cyclodextrin polymer: A model system for drug delivery. Biomacromolecules 2009, 10, 3283–3289. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Steffensen, K.; Larsen, K.L. Self-assembling microparticles with controllable disruption properties based on cyclodextrin interactions. Colloids Surf. B Biointerfaces 2009, 73, 267–275. [Google Scholar] [CrossRef]
- Koopmans, C.; Ritter, H. Formation of physical hydrogels via host-guest interactions of β-cyclodextrin polymers and copolymers bearing adamantyl groups. Macromolecules 2008, 41, 7418–7422. [Google Scholar] [CrossRef]
- Osman, S.K.; Brandl, F.P.; Zayed, G.M.; Teßmar, J.K.; Göpferich, A.M. Cyclodextrin based hydrogels: Inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer 2011, 52, 4806–4812. [Google Scholar] [CrossRef]
- Osman, S.K.; Soliman, G.M.; Abd El Rasoul, S. Physically cross-linked hydrogels of β -cyclodextrin polymer and poly(ethylene glycol)-cholesterol as delivery systems for macromolecules and small drug molecules. Curr. Drug Deliv. 2015, 12, 415–424. [Google Scholar] [CrossRef]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Osman, S.K.; Saleh, K.I.; Samy, A.M. In vitro release of 5-fluorouracil and methotrexate from different thermosensitive chitosan hydrogel systems. AAPS PharmSciTech 2020, 21, 131. [Google Scholar] [CrossRef]
- Kasinski, A.; Zielinska-Pisklak, M.; Oledzka, E.; Nałecz-Jawecki, G.; Drobniewska, A.; Sobczak, M. Hydrogels based on poly (ether-ester)s as highly and characterization. Materials 2021, 14, 98. [Google Scholar] [CrossRef]
- Tibbetts, G.G. Growing carbon fibers with a linearly increasing temperature sweep: Experiments and modeling. Carbon 1992, 30, 399–406. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tafaghodi, M.; Ganji, F.; Abnoos, K.; Naghizadeh, H. In vitro insulin release from thermosensitive chitosan hydrogel. AAPS PharmSciTech 2012, 13, 460–466. [Google Scholar] [CrossRef]
- Arregui, J.R.; Kovvasu, S.P.; Betageri, G.V. Daptomycin proliposomes for oral delivery: Formulation, characterization, and in vivo pharmacokinetics. AAPS PharmSciTech 2018, 19, 1802–1809. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Adami, M.; Bertoni, E.; Lauria, S.; Montanari, L. Injectability evaluation: An open issue. AAPS PharmSciTech 2011, 12, 604–609. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Di Martino, A.; Kucharczyk, P.; Capakova, Z.; Humpolicek, P.; Sedlarik, V. Chitosan-based nanocomplexes for simultaneous loading, burst reduction and controlled release of doxorubicin and 5-fluorouracil. Int. J. Biol. Macromol. 2017, 102, 613–624. [Google Scholar] [CrossRef]
- Dalwadi, C.; Patel, G. Thermosensitive nanohydrogel of 5-fluorouracil for head and neck cancer: Preparation, characterization and cytotoxicity assay. Int. J. Nanomed. 2018, 13, 31–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, H.; Cho, H.-J.; Cho, S.-M.; Lee, D.-G.; Abd El-Aty, A.M.; Yoon, S.-J.; Bae, G.-W.; Nho, K.; Kim, B.; Lee, C.-H.; et al. Pharmacokinetic properties and antitumor efficacy of the 5-fluorouracil loaded PEG-hydrogel. BMC Cancer 2010, 10, 211. [Google Scholar] [CrossRef]
- Ige, S.F.; Adeniyi, M.J.; Joanna, A.O.; Oluwaseyi, D.A. Allium cepa juice prevented oxidative stress-mediated metabolic disorder following chronic lead acetate exposure in male rats. Albanian J. Med. Health Sci. 2019, 50, 11. [Google Scholar]
- Lin, Q.; Cai, Y.; Yuan, M.; Ma, L.; Qiu, M.; Su, J. Development of a 5-fluorouracil-loaded PLGA microsphere delivery system by a solid-in-oil-in-hydrophilic oil (S/O/hO) novel method for the treatment of tumors. Oncol. Rep. 2014, 32, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P.J. Basic Pharmacokinetics, 1st ed.; Pharmaceutical Press: London, UK, 2009; ISBN 2013206534. [Google Scholar]
- Huang, M.; Zong, S.L.; Zhang, Q.-Y. The effect of food intake on the PK of rhein released from diacerein. Braz. J. Pharm. Sci. 2020, 56, 1–6. [Google Scholar] [CrossRef]
- Suvarna, J.D.; Layton, J.K.; Bancroft, C. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2013. [Google Scholar]
- Abba, M.C.; Zhong, Y.; Lee, J.; Kil, H.; Lu, Y.; Takata, Y.; Simper, M.S.; Gaddis, S.; Shen, J.; Marcelo Aldaz, C. DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations. Oncotarget 2016, 7, 64289–64299. [Google Scholar] [CrossRef]
- Habib, F.S.; Hassan, M.A.A.; Abou El Ela, A.E.S.F.; Abdel Raheem, E.S.R. Different topical formulations of ketorolac tromethamine for anti-inflammatory application and clinical efficacy. Dig. J. Nanomater. Biostruct. 2014, 9, 705–719. [Google Scholar]
- Khan, A.; Ali, Z. Normal ranges for acute phase reactants (interleukin-6, tumour necrosis factor-alpha and C-reactive protein) in umbilical cord blood of healthy term neonates at the Mount Hope Women’s Hospital, Trinidad. West Indian Med. J. 2014, 63, 465–469. [Google Scholar] [CrossRef][Green Version]
- Dytham, C. Choosíng and Using Statístícs, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bibby, D.C.; Davies, N.M.; Tucker, I.G. Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int. J. Pharm. 2000, 197, 1–11. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A.A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Kost, B.; Brzezinski, M.; Socka, M.; Basko, M.; Biela, T. Biocompatible polymers combined with cyclodextrins: Fascinating materials for drug delivery applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef]
- Van de Manakker, F. Self-Assembled Hydrogels Based on B-Cyclodextrin/Cholesterol Inclusion Complexes: Properties and Pharmaceutical Applications. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2009. [Google Scholar]
- Cadman, E.; Heimer, R.; Davis, L. Enhanced 5-Fluorouracil nucleotide formation After methotrexate administration: Explanation for drug synergism. Science 1979, 205, 1135–1137. [Google Scholar] [CrossRef]
- Blanco, M.D.; Garcia, O.; Gomez, C.; Sastre, R.L.; Teijon, J.M. In-vivo drug delivery of 5-Fluorouracil using Poly (2-hydroxyethyl methacrylate-co-acrylamide) hydrogels. J. Pharmacol. 2000, 52, 1319–1325. [Google Scholar] [CrossRef]


| Polymer | Degree of Substitution (%) | Molecular Weight (kDa) | The Technique(s) Used for Mw Determination | Yield (%) |
|---|---|---|---|---|
| pβ-CD | 62 | 106 | DLS | 62–65 |
| 8armPEG20-(Chol)7 | 87 | 22.7 | NMR, MALDI | 85–90 |
| Drug Content | pH | Tgel (°C) | Cross-Over (Hz) | Gel Stability (μN·m) | Viscosity (Pa) | G* (Pa) | |
|---|---|---|---|---|---|---|---|
| 5-FU | MTX | ||||||
| 105 ± 2.0 | 99.5 ± 0.5 | 7.4 ± 0.1 | 68 | 0.1 ± 0.03 | 1258 ± 30.2 | 6940 ± 70.2 | 6950 ± 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almawash, S.; El Hamd, M.A.; Osman, S.K. Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations. Pharmaceutics 2022, 14, 817. https://doi.org/10.3390/pharmaceutics14040817
Almawash S, El Hamd MA, Osman SK. Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations. Pharmaceutics. 2022; 14(4):817. https://doi.org/10.3390/pharmaceutics14040817
Chicago/Turabian StyleAlmawash, Saud, Mohamed A. El Hamd, and Shaaban K. Osman. 2022. "Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations" Pharmaceutics 14, no. 4: 817. https://doi.org/10.3390/pharmaceutics14040817
APA StyleAlmawash, S., El Hamd, M. A., & Osman, S. K. (2022). Polymerized β-Cyclodextrin-Based Injectable Hydrogel for Sustained Release of 5-Fluorouracil/Methotrexate Mixture in Breast Cancer Management: In Vitro and In Vivo Analytical Validations. Pharmaceutics, 14(4), 817. https://doi.org/10.3390/pharmaceutics14040817







