RETRACTED: Poly (N-vinylcaprolactam-grafted-sodium alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chemically Grafted Thermoresponsive Poly (N-Vinylcaprolactam-Graft-Sodium Alginate) In Situ Depot Gels
2.3. Clarity of the In Situ Gel Formulations
2.4. Solid State Characterization of Gels
2.4.1. 1H Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
2.4.2. FTIR Analysis
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. Scanning Electron Microscopy (SEM)
2.5. Cloud Point Determination by Titling Method (Tsol-Gel)
2.6. Rheological Determination
2.7. Optical Transmittance Measurement
2.8. Swelling Experiments
2.8.1. On–Off Switching Behavior
2.8.2. Solvent Diffusion Coefficient
2.8.3. Molecular Weight between Cross-Links (Mc)
2.8.4. Volume Fraction of Polymers
2.8.5. Solvent Interaction Parameters (χ)
2.9. Percent Crosslinking Measurements
2.10. Drug Contents Determination
2.11. Grafting Efficiency Measurement
2.12. In Vitro Drug Release
2.13. Drug Release Kinetics
2.13.1. Zero Order Kinetic Model
2.13.2. First Order Kinetic Model
2.13.3. Higuchi Model
2.13.4. Korsmeyer–Peppas Model
2.14. In Vitro Degradation
2.15. Cell Lines and Culture Conditions
2.16. In Vivo Analysis in Rabbits
2.16.1. High Performance Liquid Chromatography Analysis
2.16.2. Animal Handling
2.16.3. Drug Administration and Sampling
2.16.4. 5-FU Plasma Concentration Quantification and Pharmacokinetic Profiling
2.16.5. Preliminary Safety and Histopathological Evaluation via Injectable Route
2.17. Statistical Analysis
3. Results and Discussion
3.1. Clarity of Formulations
3.2. Solid State Characterization
3.2.1. NMR Spectroscopic Analysis
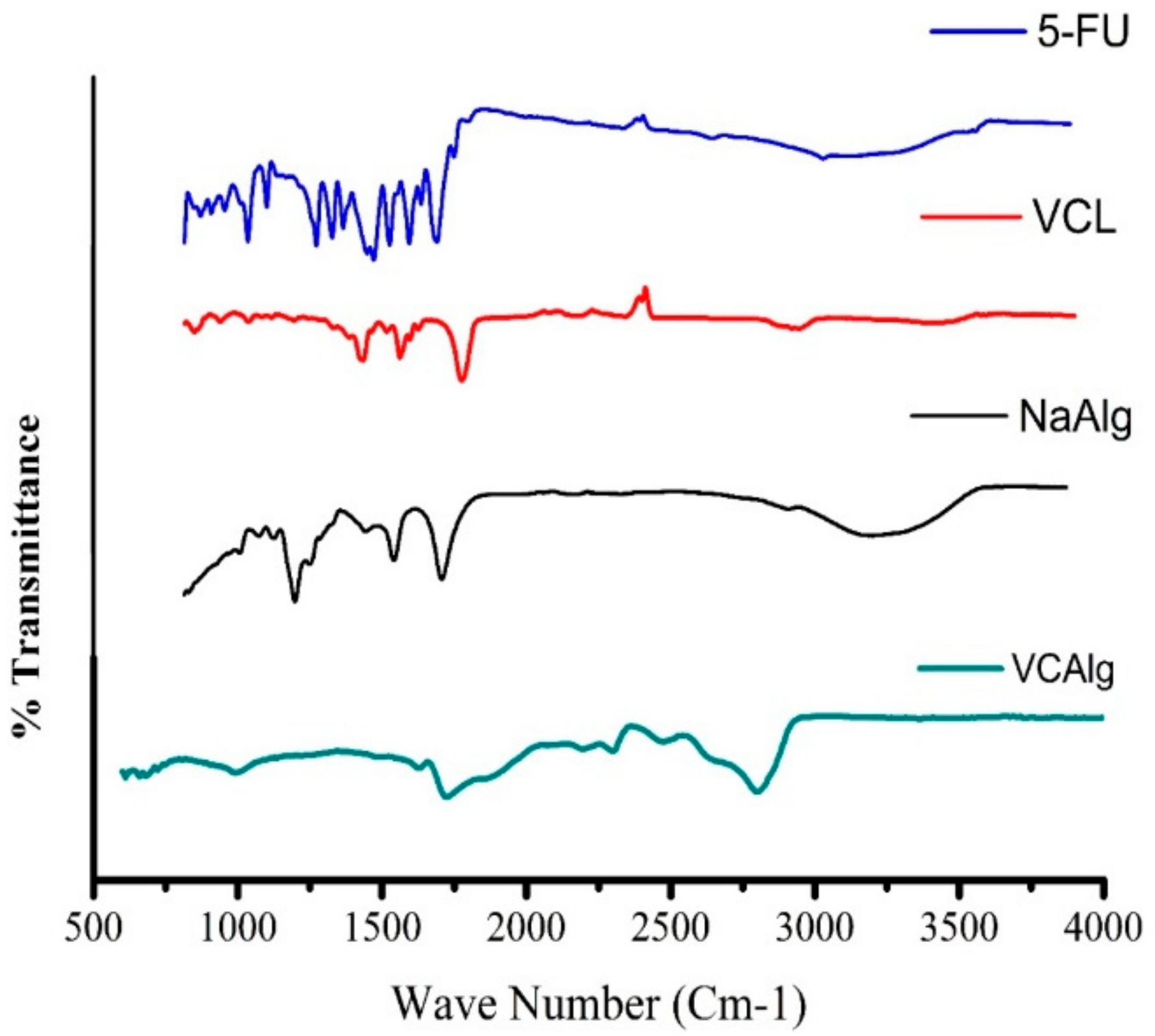
3.2.2. FTIR Analysis
3.2.3. DSC Analysis
3.2.4. TG Analysis
3.2.5. SEM Analysis
3.3. Phase Diagram Measurement and Mechanism of Gelation
3.4. Rheological Analysis
3.4.1. Time Sweep Test
3.4.2. Temperature Sweep Test
3.4.3. Frequency Sweep Test
3.4.4. Continuous Ramp Test
3.5. Grafting Efficiency of Poly (NVCL-g-NaAlg) In Situ Depot Gels
3.6. Transparency Assessment of Formulations
3.7. Percent Crosslinking Determinations
3.8. In Vitro Degradation
3.9. Swelling Experiments
3.9.1. pH Sensitivity of the Chemically Grafted Poly (NVCL-g-NaAlg) Depot Gels
3.9.2. Temperature Sweep Swelling Experiments
3.9.3. Effect of MBA Concentration on Equilibrium Swelling Ratio (ESR)
3.9.4. On–Off Switching Behavior
3.10. Drug Contents
3.11. Drug Release Study from In Situ Depot Gels
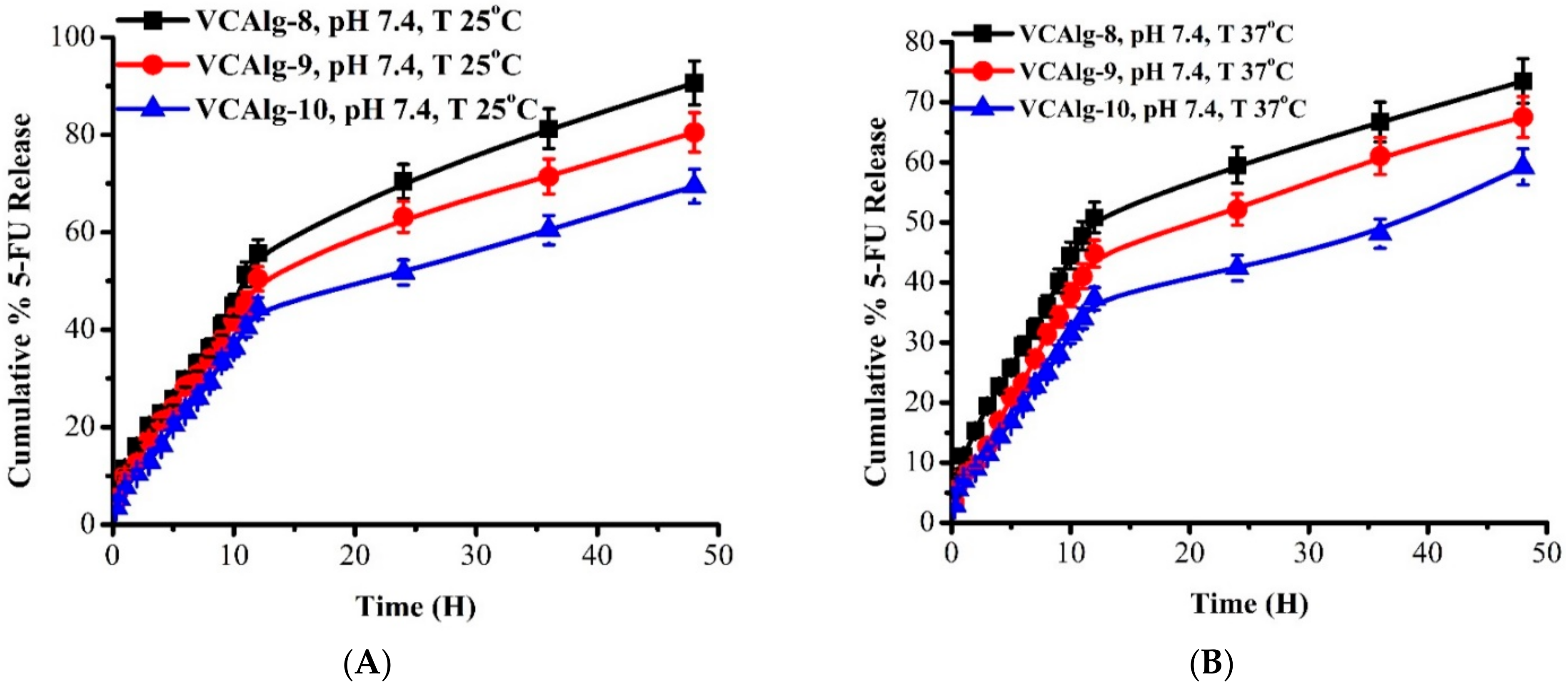
3.11.1. Effect of pH and Temperature on 5-FU Release
3.11.2. Effect of NaAlg Contents on 5-FU Release
3.11.3. Effect of Degree of Crosslinking on 5-FU Release
3.11.4. Effect of N-Vinylcaprolactam (NVCL) Contents on Drug Release
3.12. Networking Parameters of Poly (NVCL-g-NaAlg) In Situ Depot Gels
3.12.1. Diffusion Coefficient (D)
3.12.2. Molecular Weight between Crosslinks (Mc) and Solvent Interaction Parameters (χ)
3.12.3. Polymer Volume Fraction
3.13. Drug Release Kinetics
3.14. In Vitro Cytocompatibility Study of In Situ Depot Hydrogels
3.15. In Vitro Anticancer Activity of In Situ Depot Hydrogels
3.16. IC50 Values Evaluation
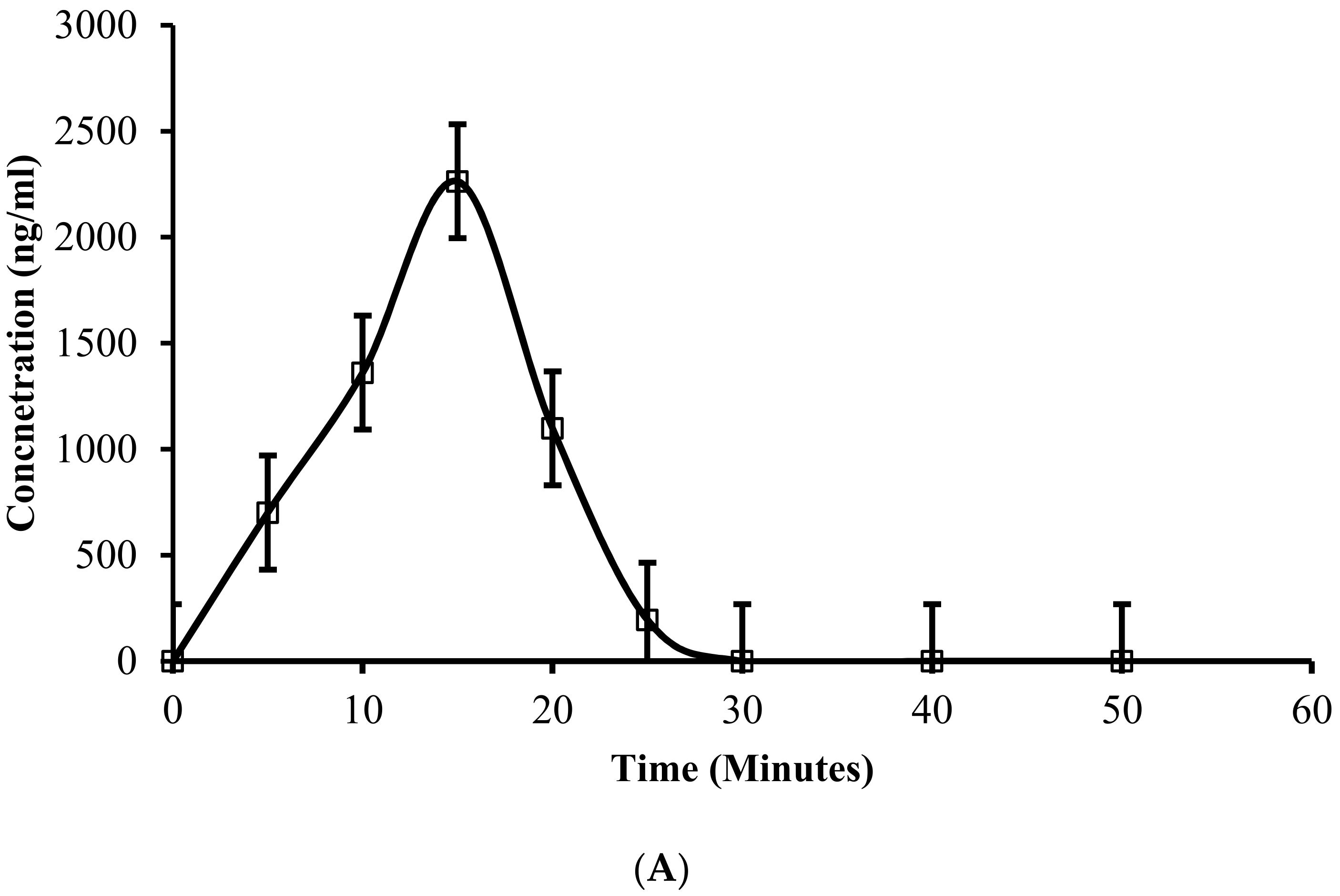
3.17. In Vivo Absorption and Pharmacokinetic Profiling in Rabbits
3.18. Tolerability and Preliminary Safety Evaluation
3.18.1. General Conditions
3.18.2. Maximal Tolerance Dose (MTD)
3.18.3. Histopathological Examination
3.18.4. Major Organs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, K.M.; Rao, K.S.V.K.; Ha, C.S. Stimuli Responsive Poly (Vinyl Caprolactam) Gels for Biomedical Applications. Gels 2016, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery and bionanotechnology. AIChE J 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Rao, K.M.; Rao, K.S.V.K.; Sudhakar, P.; Rao, K.C.; Subha, M.C.S. Synthesis and Characterization of biodegradable Poly (Vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 061–069. [Google Scholar]
- Enas, M.A. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res 2015, 6, 105–121. [Google Scholar]
- Kulkarni, R.V.; Sa, B. Polyacrylamide-grafted alginate- based pH-sensitive hydrogel beads for delivery of ketoprofen to the intestine: In vitro and in vivo evaluation. J. Biomater. Sci. 2009, 20, 235–251. [Google Scholar] [CrossRef]
- Mather, P.T. Responsive materials: Soft answers for hard problems. Nat. Mater. 2007, 6, 93–94. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.I.; Reilly, R.K. To aggregate, or not to aggregate? Considerations in the design and application of polymeric thermally-responsive nanoparticles. Chem. Soc. Rev. 2013, 42, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Mancuso, A.; Giuliano, E.; Cosco, D.; Paolino, D.; Fresta, M. EtoGel for Intra- Articular Drug Delivery: A New Challenge for Joint Diseases Treatment. J. Funct. Biomater. 2021, 12, 34. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Zhang, M.; Lv, X.; Li, Z.; Mohammadniaei, M.; Zhou, N.; Sun, Y. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr. Polym. 2021, 265, 118065. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y. Temperature-responsive biodegradable injectable polymer systems with conveniently controllable properties. Polym. J. 2019, 51, 997–1005. [Google Scholar] [CrossRef]
- Chassenieux, C.; Tsitsilianis, C. Recent trends on pH/thermo-responsive self-assembling hydrogels from polyions to peptide-based polymeric gelators. Soft. Mater. 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Amer. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Babu, V.R.; Sairam, M.; Hosamani, K.M.; Aminabhavi, T.M. Preparations of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr. Polym. 2007, 69, 241–250. [Google Scholar] [CrossRef]
- Yassin, A.E.B.; Alrra, I.A.; Al-Mohizea, A.M. Chitosan beads as a new gastroretentive system of verapamil. Sci. Pharma. 2006, 74, 175–188. [Google Scholar] [CrossRef]
- Halder, A.; Mukherjee, S.B. Development and evaluation of polyethyleneimine-treated calcium alginate beads for sustained release of diltiazem. J. Microencapsul. 2005, 22, 67–80. [Google Scholar] [CrossRef]
- Swamy, B.Y.; Chang, J.H.; Ahn, H.; Lee, W.K.; Chung, I. Thermoresponsive N-vinyl caprolactam grafted sodium alginate hydrogel beads for the controlled release of an anticancer drug. Cellulose 2013, 20, 1261–1273. [Google Scholar] [CrossRef]
- Sanli, O.; Ay, N.; Isiklan, N. Release characteristics of diclofenac sodium form Poly (vinyl alcohol)/sodium alginate and Poly (vinyl alcohol)-grafted-Poly (acrylamide)/ Sodium alginate blend beads. Eur. J. Pharm. Biopharm. 2007, 65, 204–214. [Google Scholar] [CrossRef]
- Deng, K.L.; Gou, Y.B.; Dong, L.R.; Li, Q.; Bai, L.B.; Gao, T.; Huang, C.Y.; Wang, S.L. Drug release behaviors of a pH/temperature sensitive core-shelled bead with alginate and poly(N-acryloyl glycinates). Front. Mater. Sci. China 2010, 4, 353–358. [Google Scholar] [CrossRef]
- Montes, J.A.N.; Ortega, A.; Burillo, G. Dual-stimuli responsive copolymers based on N-vinylcaprolactam/chitosan. J. Radioanal. Nucl. Chem. 2015, 303, 2143–2150. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Radecki, M.; Zhigunov, A.; Šťastná, J.; Valentová, H.; Sedláková, Z. Structures and interactions in collapsed hydrogels of thermoresponsive interpenetrating polymer networks. Colloids Polym. Sci. 2015, 293, 709–720. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Rangaswamy, V.; Aminabhavi, T.M. A novel method to prepare 5-fluorouracil, an anti-cancer drug, loaded microspheres from poly(N-vinylcaprolactam-co-acrylamide) and controlled release studies. Des. Mono. Polym. 2010, 13, 325–336. [Google Scholar] [CrossRef]
- Fernández-Quiroz, D.; González-Gómez, A.; Lizardi-Mendoza, J.; Vázquez-Lasa, B.; Goycoolea, F.M.; Román, J.S.; Argüelles-Monal, W.M. Conformational study on the thermal transition of chitosan-g-poly(N-vinylcaprolactam) in aqueous solution. Colloids Polym. Sci. 2016, 294, 555–563. [Google Scholar] [CrossRef]
- Spěváček, J.; Dybal, J.; Starovoytova, L.; Zhigunov, A.; Sedláková, Z. Temperature-induced phase separation and hydration in poly(N-vinylcaprolactam) aqueous solutions: A study by NMR and IR spectroscopy, SAXS, and quantum-chemical calculations. Soft. Mater. 2012, 8, 6110–6119. [Google Scholar] [CrossRef]
- Li, R.; Shu, C.; Wang, W.; Wang, X.; Li, H.; Xu, D.; Zhong, W. Encapsulation of 10-Hydroxy Camptothecin in Supramolecular Hydrogel as an Injectable Drug Delivery System. J. Pharma. Sci. 2015, 104, 2266–2275. [Google Scholar] [CrossRef]
- Sheua, M.T.; Jhana, H.J.; Sua, C.Y.; Chena, L.C.; Chang, C.E.; Liuc, D.Z.; Hoa, H.O. Co-delivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointer. 2016, 143, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Xiao, B.L.; Zheng, J.W.; Chen, H.B.; Zou, S.Q. Effect of targeted magnetic nanoparticles containing 5-FU on expression of bcl-2, bax and caspase 3 in nude mice with transplanted human liver cancer. World. J. Gastroenterol. 2007, 3, 3171–3175. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, P.; Cheng, M.; Zhang, G.; Zhang, F. 5-Fluorouracilloaded self-assembled pH-sensitive nanoparticles as novel drug carrier for treatment of malignant tumors. Chin. J. Chem. Eng. 2006, 14, 377. [Google Scholar] [CrossRef]
- Jae, H.P.; Seunglee, K.; Juock, N.; Woon, P.R. Self-assembled nanoparticles based on glycol chitosan bearing 5B-cholanic acid for RGD peptide delivery. J. Control Rel. 2004, 95, 579. [Google Scholar]
- Kumar, S.S.D.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and characterization of curcumin loaded polymer/lipid based nanoparticles and evaluation of their antitumor effects on MCF-7 cells. Biochim. Biophys. Acta 2014, 840, 61913–61922. [Google Scholar] [CrossRef]
- Nasir, F.; Iqbal, Z.; Khan, J.A.; Khan, A.; Khuda, F.; Ahmad, L.; Khan, A.; Khan, A.; Dayoo, A.; Roohullah. Development and evaluation of diclofenac sodium thermorevesible subcutaneous drug delivery system. Int. J. Pharm. 2012, 439, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O’Doherty, M.; Chalanqui, M.; Kelly, D.J.; Donahue, T.H.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Macromol. Biosci. 2017, 1700118. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Chen, J.P.; Leu, Y.L.; Hu, J.W. The Delivery of Platinum Drugs from Thermosensitive Hydrogels Containing Different Ratios of Chitosan. Drug. Deliv. 2008, 15, 235–243. [Google Scholar] [CrossRef]
- Shah, H.S.; Al-Oweini, R.; Haider, A.; Kortz, U.; Iqbal, J. Cytotoxicity and enzyme inhibition studies of polyoxometalates and their chitosan nanoassemblies. Toxicol. Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, N. Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation. Carbohydr. Polym. 2021, 257, 117617. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly(N-Isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS Pharm. Sci. Technol. 2019, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Akhtar, N.; Minhas, M.U. Fabrication, rheological analysis, and in vitro characterization of in situ chemically cross-linkable thermogels as controlled and prolonged drug depot for localized and systemic delivery. Polym. Adv. Technol. 2018, 1–17. [Google Scholar] [CrossRef]
- Qu, Y.; Chu, B.Y.; Peng, J.R.; Liao, J.F.; Qi, T.T.; Shi, K.; Zhang, X.N.; Wei, Y.Q.; Qian, Z.Y. A biodegradable thermo-responsive hybrid hydrogel: Therapeutic applications in preventing the post-operative recurrence of breast cancer. NPG. Asian Mater. 2015, 7, 207. [Google Scholar] [CrossRef]
- JagadeeshBabu, P.E.; Kumar, R.S.; Maheswari, B. Synthesis and characterization of temperature sensitive PNIPAM macro/micro hydrogels. Colloids Surf A Physicochem. Eng. Asp. 2011, 384, 466–472. [Google Scholar] [CrossRef]
- Guo, B.L.; Gao, Q.Y. Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly(N-isopropylacrylamide)semi-IPN hydrogel for oral delivery of drugs. Carbohydr. Res. 2007, 342, 2416–2422. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Jankaew, R.; Rodkate, N.; Lamlertthon, S.; Rutnakornpituk, B.; Wichai, U.; Ross, G.; Rutnakornpituk, M. “Smart” carboxymethylchitosan hydrogels crosslinked with poly(N-isopropylacrylamide) and poly(acrylic acid) for controlled drug release. Polym. Test 2015, 42, 26–36. [Google Scholar] [CrossRef]
- Ruan, H.; Yu, Y.; Liu, Y.; Ding, X.; Guo, X.; Jiang, Q. Preparation and characteristics of thermoresponsive gel of minocycline hydrochloride and evaluation of its effect on experimental periodontitis models. Drug. Deliv. 2016, 23, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Guo, B.; Ma, P.X. Cytocompatible injectable carboxymethyl chitosan/N-isopropylacrylamide hydrogels for localized drug delivery. Carbohydr. Polym. 2014, 103, 110–118. [Google Scholar] [CrossRef] [PubMed]
















| Formulation Codes | NVCL (g) | NaAlg (g) | MBA (g) | APS (g) | Tsol-gel (°C) | Gelation Time (Tg) (Minute’s) | 5-FU (mg) | Distilled Water (g) |
|---|---|---|---|---|---|---|---|---|
| VCAlg-1 | 1 | 0.100 | 0.075 | 0.150 | 35 ± 0.50 | 9 | 100 | 10 g |
| VCAlg-2 | 1 | 0.100 | 0.100 | 0.150 | 35 ± 0.60 | 8 | 100 | 10 g |
| VCAlg-3 | 1 | 0.100 | 0.125 | 0.150 | 35 ± 0.40 | 7.5 | 100 | 10 g |
| VCAlg-4 | 1 | 0.100 | 0.150 | 0.150 | 35 ± 0.50 | 6.5 | 100 | 10 g |
| VCAlg-5 | 1 | 0.125 | 0.100 | 0.150 | 35 ± 0.90 | 8 | 100 | 10 g |
| VCAlg-6 | 1 | 0.150 | 0.100 | 0.150 | 36 ± 0.20 | 9 | 100 | 10 g |
| VCAlg-7 | 1 | 0.175 | 0.100 | 0.150 | 36 ± 0.70 | 8.5 | 100 | 10 g |
| VCAlg-8 | 1.50 | 0.150 | 0.100 | 0.150 | 34 ± 0.70 | 6 | 100 | 10 g |
| VCAlg-9 | 2 | 0.150 | 0.100 | 0.150 | 33 ± 0.10 | 5 | 100 | 10 g |
| VCAlg-10 | 2.50 | 0.150 | 0.100 | 0.150 | 31 ± 0.90 | 5 | 100 | 10 g |
| Formulation Codes | Clarity of Formulations | % Crosslinking | Drug Contents % | % Grafting Efficiency |
|---|---|---|---|---|
| VCAlg-1 | +++++ | 90.23 | - | 176 |
| VCAlg-2 | +++++ | 92.44 | 90 ± 0.38 | 189 |
| VCAlg-3 | +++++ | 95.69 | 87 ± 0.63 | 197 |
| VCAlg-4 | +++++ | 98.22 | 83 ± 0.22 | 205 |
| VCAlg-5 | +++++ | 88.84 | 92 ± 0.17 | 224 |
| VCAlg-6 | +++++ | 91.45 | 94 ± 0.12 | 237 |
| VCAlg-7 | +++++ | 93.72 | 95 ± 0.53 | 235 |
| VCAlg-8 | +++++ | 92.62 | 84 ± 0.18 | 193 |
| VCAlg-9 | ++++ | 95.61 | 83 ± 0.33 | 228 |
| VCAlg-10 | ++++ | 97.33 | 78 ± 0.79 | 208 |
| Formulation Codes | V2,s | χ | Mc | D 10−6 (cm2/s) |
|---|---|---|---|---|
| VCAlg-1 | 0.012 | 0.503 | 202,426.34 | 0.136 |
| VCAlg-2 | 0.011 | 0.511 | 670,58.44 | 0.161 |
| VCAlg-3 | 0.007 | 0.517 | 638,10.36 | 0.229 |
| VCAlg-4 | 0.009 | 0.524 | 778,04.02 | 0.593 |
| VCAlg-5 | 0.010 | 0.507 | 101,281.24 | 0.419 |
| VCAlg-6 | 0.011 | 0.501 | 126,178.69 | 0.289 |
| VCAlg-7 | 0.063 | 0.522 | 3592.22 | 0.416 |
| VCAlg-8 | 0.092 | 0.533 | 1526.30 | 0.738 |
| VCAlg-9 | 0.102 | 0.537 | 1230.47 | 0.815 |
| Sample Codes | pH | Zero Order Kinetics | First Order Kinetics | Higuchi Model | Korsmeyer–Peppas Model | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ko (h−1) | R2 | K1(h−1) | R2 | K2 (h−1) | R2 | n | R2 | ||
| VCAlg-1 | DW | 3.840 | 0.994 | 0.0557 | 0.996 | 15.54 | 0.980 | 0.573 | 0.989 |
| 7.4 | 3.108 | 0.985 | 0.0425 | 0.995 | 12.69 | 0.990 | 0.508 | 0.993 | |
| VCAlg-2 | DW | 3.673 | 0.990 | 0.0515 | 0.995 | 14.88 | 0.980 | 0.636 | 0.980 |
| 7.4 | 3.901 | 0.998 | 0.0541 | 0.986 | 15.54 | 0.955 | 0.701 | 0.988 | |
| VCAlg-3 | DW | 3.025 | 0.979 | 0.0399 | 0.992 | 12.41 | 0.994 | 0.599 | 0.993 |
| 7.4 | 3.325 | 0.996 | 0.0438 | 0.988 | 13.26 | 0.954 | 0.687 | 0.980 | |
| VCAlg-5 | 1.2 | 1.835 | 0.996 | 0.0213 | 0.998 | 7.395 | 0.974 | 0.591 | 0.983 |
| 7.4 | 3.855 | 0.995 | 0.0557 | 0.984 | 15.42 | 0.961 | 0.574 | 0.984 | |
| VCAlg-6 | 1.2 | 1.700 | 0.966 | 0.0198 | 0.996 | 6.82 | 0.967 | 0.507 | 0.977 |
| 7.4 | 3.969 | 0.991 | 0.0586 | 0.974 | 15.89 | 0.958 | 0.592 | 0.988 | |
| VCAlg-7 | 1.2 | 2.001 | 0.995 | 0.0241 | 0.992 | 8.081 | 0.963 | 0.482 | 0.973 |
| 7.4 | 4.375 | 0.996 | 0.0671 | 0.991 | 17.63 | 0.975 | 0.579 | 0.988 | |
| VCAlg-8 | 1.2 | 1.694 | 0.994 | 0.0195 | 0.991 | 6.750 | 0.951 | 0.572 | 0.977 |
| 7.4 | 3.681 | 0.996 | 0.0515 | 0.989 | 14.78 | 0.968 | 0.606 | 0.988 | |
| VCAlg-9 | 1.2 | 1.602 | 0.997 | 0.0182 | 0.996 | 6.419 | 0.965 | 0.593 | 0.985 |
| 7.4 | 3.350 | 0.998 | 0.0443 | 0.989 | 13.34 | 0.954 | 0.645 | 0.981 | |
| Sample Codes | aIC50 (µg/mL) against HeLa Cells | aIC50 (µg/mL) against MCF-7 Cells | % Inhibition in Vero Cells |
|---|---|---|---|
| Triton X100 | - | - | 81 ± 0.33 |
| 5-FU | 50±0.52 | 53 ± 0.58 | - |
| VCAlg-6 | 39±0.91 | 46 ± 0.82 | 6 ± 0.22 |
| VCAlg-10 | 49±0.60 | 26 ± 0.70 | 9 ± 0.31 |
| S. No. | Pharmacokinetic Parameters | Subcutaneous Pure 5-FU Solution (Mean ± SD) | 5-FU Loaded Injectable Hydrogel (Mean ± SD) |
|---|---|---|---|
| 1. | Cmax (ng/mL) | 2263.31 ± 13.36 | 1433.59 ± 45.09 |
| 2. | Tmax (min) | 15.00 ± 0.00 | 36.00 ± 0.00 (h) |
| 3. | AUCtot (ng/mL × h) | 26,630.03 ± 259.55 | 69,904.17 ± 1208.75 |
| 4. | AUMCtot (ng·h2/mL) | 365,928.17 ± 3384.46 | 2.86 ± 0.07 |
| 5. | Kel (hr−1) | 0.07 ± 0.00 | 0.09 ± 0.00 |
| 6. | t1/2 (min, h) | 9.60 ± 0.00 | 12.50 ± 0.74 (h) |
| 7. | MRT (min, h) | 14.19 ± 0.09 | 42.28 ± 0.68 (h) |
| 8. | Clearance (L/min) | 0.73 ± 0.01 | 0.69 ± 1.42 |
| 9. | Vd (L) | 2.97 ± 0.07 | 5.06 ± 0.29 |
| 10. | Vss (L) | 10.34 ± 0.13 | 11.87 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Minhas, M.U.; Aqeel, M.T.; Shah, I.; Khan, S.; Kazi, M.; Warnken, Z.N. RETRACTED: Poly (N-vinylcaprolactam-grafted-sodium alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation. Pharmaceutics 2022, 14, 1050. https://doi.org/10.3390/pharmaceutics14051050
Khan S, Minhas MU, Aqeel MT, Shah I, Khan S, Kazi M, Warnken ZN. RETRACTED: Poly (N-vinylcaprolactam-grafted-sodium alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation. Pharmaceutics. 2022; 14(5):1050. https://doi.org/10.3390/pharmaceutics14051050
Chicago/Turabian StyleKhan, Samiullah, Muhammad Usman Minhas, Muhammad Tahir Aqeel, Ihsan Shah, Shahzeb Khan, Mohsin Kazi, and Zachary N. Warnken. 2022. "RETRACTED: Poly (N-vinylcaprolactam-grafted-sodium alginate) Based Injectable pH/Thermo Responsive In Situ Forming Depot Hydrogels for Prolonged Controlled Anticancer Drug Delivery; In Vitro, In Vivo Characterization and Toxicity Evaluation" Pharmaceutics 14, no. 5: 1050. https://doi.org/10.3390/pharmaceutics14051050








