Comparison of the Effects of Three Dual-Nucleos(t)ide Reverse Transcriptase Inhibitor Backbones on Placenta Mitochondria Toxicity and Oxidative Stress Using a Mouse Pregnancy Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Protocols
2.2. Quantification of mtDNA Copy Number
2.3. RNA Extraction and Real-Time qPCR
2.4. Lipid Peroxidation (TBARS Assay)
2.5. Statistical Analysis
3. Results
3.1. Pregnancy Outcomes
3.2. Placental mtDNA Content and Expression of POLG, COX-II, and Citrate Synthase Are Highest in Pregnant Mice on TDF/FTC
3.3. Placental Levels of MDA Were Highest in AZT/3TC-Exposed Pregnant Mice
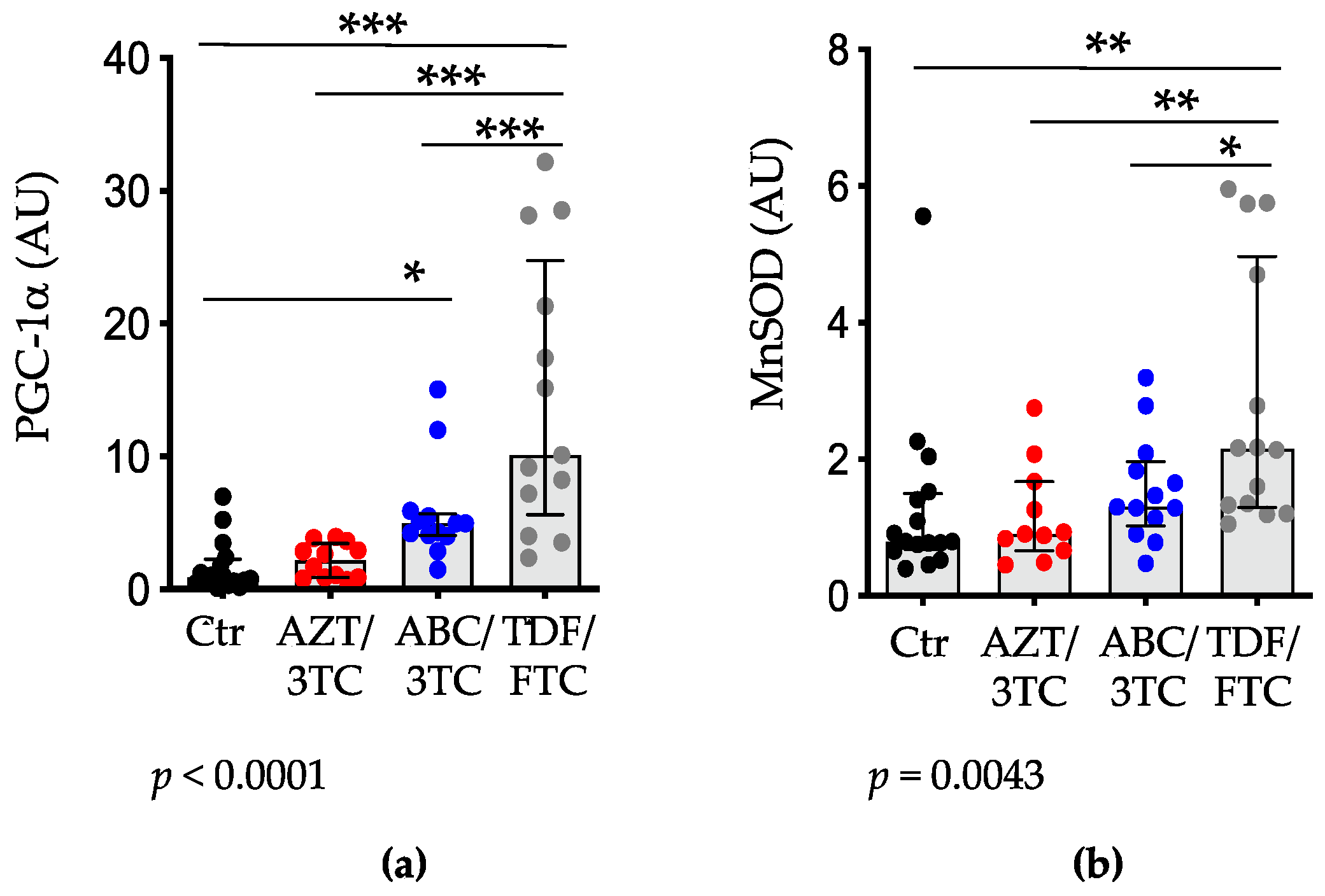
3.4. Placental Expression of PGC-1α and MnSOD Are Highest in Pregnant Mice on TDF/FTC
3.5. Correlations with Fetal Weight and Resorption Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidari, S.; Mofenson, L.M.; Bekker, L.-G. Realization of an AIDS-Free Generation: Ensuring Sustainable Treatment for Children. JAMA 2014, 312, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Govender, T.; Coovadia, H. Eliminating Mother to Child Transmission of HIV-1 and Keeping Mothers Alive: Recent Progress. J. Infect. 2014, 68 (Suppl. 1), S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A Review of the Toxicity of HIV Medications. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B. Mitochondrial off Targets of Drug Therapy. Trends Pharmacol. Sci. 2008, 29, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Blanche, S.; Tardieu, M.; Benhammou, V.; Warszawski, J.; Rustin, P. Mitochondrial Dysfunction Following Perinatal Exposure to Nucleoside Analogues. AIDS Lond. Engl. 2006, 20, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Blanche, S.; Tardieu, M.; Rustin, P.; Slama, A.; Barret, B.; Firtion, G.; Ciraru-Vigneron, N.; Lacroix, C.; Rouzioux, C.; Mandelbrot, L.; et al. Persistent Mitochondrial Dysfunction and Perinatal Exposure to Antiretroviral Nucleoside Analogues. Lancet Lond. Engl. 1999, 354, 1084–1089. [Google Scholar] [CrossRef]
- Lewis, W. Nucleoside Reverse Transcriptase Inhibitors, Mitochondrial DNA and AIDS Therapy. Antivir. Ther. 2005, 10 (Suppl. 2), 13–27. [Google Scholar] [CrossRef]
- Kohler, J.J.; Lewis, W. A Brief Overview of Mechanisms of Mitochondrial Toxicity from NRTIs. Environ. Mol. Mutagen. 2007, 48, 166–172. [Google Scholar] [CrossRef]
- de la Asunción, J.G.; del Olmo, M.L.; Sastre, J.; Pallardó, F.V.; Viña, J. Zidovudine (AZT) Causes an Oxidation of Mitochondrial DNA in Mouse Liver. Hepatology 1999, 29, 985–987. [Google Scholar] [CrossRef]
- de la Asunción, J.G.; del Olmo, M.L.; Sastre, J.; Millán, A.; Pellín, A.; Pallardó, F.V.; Viña, J. AZT Treatment Induces Molecular and Ultrastructural Oxidative Damage to Muscle Mitochondria. Prevention by Antioxidant Vitamins. J. Clin. Investig. 1998, 102, 4–9. [Google Scholar] [CrossRef]
- Dyall, S.D.; Brown, M.T.; Johnson, P.J. Ancient Invasions: From Endosymbionts to Organelles. Science 2004, 304, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Graziewicz, M.A.; Longley, M.J.; Copeland, W.C. DNA Polymerase Gamma in Mitochondrial DNA Replication and Repair. Chem. Rev. 2006, 106, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, K.; ter Hofstede, H.J.; Burger, D.M.; Smeitink, J.A.; Koopmans, P.P. Adverse Effects of Reverse Transcriptase Inhibitors: Mitochondrial Toxicity as Common Pathway. AIDS Lond. Engl. 1998, 12, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.; Day, B.J.; Copeland, W.C. Mitochondrial Toxicity of NRTI Antiviral Drugs: An Integrated Cellular Perspective. Nat. Rev. Drug Discov. 2003, 2, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.C.; Olivero, O.A.; Walker, D.M.; Walker, V.E. Perinatal Genotoxicity and Carcinogenicity of Anti-Retroviral Nucleoside Analog Drugs. Toxicol. Appl. Pharmacol. 2004, 199, 151–161. [Google Scholar] [CrossRef]
- Anderson, P.L.; Kakuda, T.N.; Lichtenstein, K.A. The Cellular Pharmacology of Nucleoside- and Nucleotide-Analogue Reverse-Transcriptase Inhibitors and Its Relationship to Clinical Toxicities. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 38, 743–753. [Google Scholar] [CrossRef]
- Côté, H.C.F. Possible Ways Nucleoside Analogues Can Affect Mitochondrial DNA Content and Gene Expression during HIV Therapy. Antivir. Ther. 2005, 10 (Suppl. 2), 3–11. [Google Scholar] [CrossRef]
- Koczor, C.A.; Lewis, W. Nucleoside Reverse Transcriptase Inhibitor Toxicity and Mitochondrial DNA. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1493–1504. [Google Scholar] [CrossRef]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J. Cell. Biochem. 2015, 116, 1939–1946. [Google Scholar] [CrossRef]
- Mallon, P.W.G.; Unemori, P.; Sedwell, R.; Morey, A.; Rafferty, M.; Williams, K.; Chisholm, D.; Samaras, K.; Emery, S.; Kelleher, A.; et al. In Vivo, Nucleoside Reverse-Transcriptase Inhibitors Alter Expression of Both Mitochondrial and Lipid Metabolism Genes in the Absence of Depletion of Mitochondrial DNA. J. Infect. Dis. 2005, 191, 1686–1696. [Google Scholar] [CrossRef]
- McCormack, S.A.; Best, B.M. Protecting the Fetus against HIV Infection: A Systematic Review of Placental Transfer of Antiretrovirals. Clin. Pharmacokinet. 2014, 53, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Morén, C.; Hernández, S.; Guitart-Mampel, M.; Garrabou, G. Mitochondrial Toxicity in Human Pregnancy: An Update on Clinical and Experimental Approaches in the Last 10 Years. Int. J. Environ. Res. Public Health 2014, 11, 9897–9918. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Leong, T.; Avery, A.; Castillo-Duran, M.; Bonilla, H.; Lebrecht, D.; Walker, U.A.; Storer, N.; Labbato, D.; Khaitan, A.; et al. Effects of in Utero Antiretroviral Exposure on Mitochondrial DNA Levels, Mitochondrial Function and Oxidative Stress. HIV Med. 2012, 13, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Jao, J.; Powis, K.M.; Kirmse, B.; Yu, C.; Epie, F.; Nshom, E.; Abrams, E.J.; Sperling, R.S.; Leroith, D.; Geffner, M.E.; et al. Lower Mitochondrial DNA and Altered Mitochondrial Fuel Metabolism in HIV-Exposed Uninfected Infants in Cameroon. AIDS Lond. Engl. 2017, 31, 2475–2481. [Google Scholar] [CrossRef]
- Jao, J.; Abrams, E.J. Metabolic Complications of in Utero Maternal HIV and Antiretroviral Exposure in HIV-Exposed Infants. Pediatr. Infect. Dis. J. 2014, 33, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Gerschenson, M.; Poirier, M.C. Fetal Patas Monkeys Sustain Mitochondrial Toxicity as a Result of in Utero Zidovudine Exposure. Ann. N. Y. Acad. Sci. 2000, 918, 269–281. [Google Scholar] [CrossRef]
- Ewings, E.L.; Gerschenson, M.; St Claire, M.C.; Nagashima, K.; Skopets, B.; Harbaugh, S.W.; Harbaugh, J.W.; Poirier, M.C. Genotoxic and Functional Consequences of Transplacental Zidovudine Exposure in Fetal Monkey Brain Mitochondria. J. Acquir. Immune Defic. Syndr. 1999 2000, 24, 100–105. [Google Scholar] [CrossRef]
- Barret, B.; Tardieu, M.; Rustin, P.; Lacroix, C.; Chabrol, B.; Desguerre, I.; Dollfus, C.; Mayaux, M.-J.; Blanche, S.; French Perinatal Cohort Study Group. Persistent Mitochondrial Dysfunction in HIV-1-Exposed but Uninfected Infants: Clinical Screening in a Large Prospective Cohort. AIDS Lond. Engl. 2003, 17, 1769–1785. [Google Scholar] [CrossRef]
- Brogly, S.B.; Ylitalo, N.; Mofenson, L.M.; Oleske, J.; Van Dyke, R.; Crain, M.J.; Abzug, M.J.; Brady, M.; Jean-Philippe, P.; Hughes, M.D.; et al. In Utero Nucleoside Reverse Transcriptase Inhibitor Exposure and Signs of Possible Mitochondrial Dysfunction in HIV-Uninfected Children. AIDS Lond. Engl. 2007, 21, 929–938. [Google Scholar] [CrossRef]
- Divi, R.L.; Walker, V.E.; Wade, N.A.; Nagashima, K.; Seilkop, S.K.; Adams, M.E.; Nesel, C.J.; O’Neill, J.P.; Abrams, E.J.; Poirier, M.C. Mitochondrial Damage and DNA Depletion in Cord Blood and Umbilical Cord from Infants Exposed in Utero to Combivir. AIDS Lond. Engl. 2004, 18, 1013–1021. [Google Scholar] [CrossRef]
- Aldrovandi, G.M.; Chu, C.; Shearer, W.T.; Li, D.; Walter, J.; Thompson, B.; McIntosh, K.; Foca, M.; Meyer, W.A.; Ha, B.F.; et al. Antiretroviral Exposure and Lymphocyte MtDNA Content among Uninfected Infants of HIV-1-Infected Women. Pediatrics 2009, 124, e1189–e1197. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.C.; Divi, R.L.; Al-Harthi, L.; Olivero, O.A.; Nguyen, V.; Walker, B.; Landay, A.L.; Walker, V.E.; Charurat, M.; Blattner, W.A.; et al. Long-Term Mitochondrial Toxicity in HIV-Uninfected Infants Born to HIV-Infected Mothers. J. Acquir. Immune Defic. Syndr. 1999 2003, 33, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Shiramizu, B.; Shikuma, K.M.; Kamemoto, L.; Gerschenson, M.; Erdem, G.; Pinti, M.; Cossarizza, A.; Shikuma, C. Placenta and Cord Blood Mitochondrial DNA Toxicity in HIV-Infected Women Receiving Nucleoside Reverse Transcriptase Inhibitors during Pregnancy. J. Acquir. Immune Defic. Syndr. 1999 2003, 32, 370–374. [Google Scholar] [CrossRef]
- Foster, C.; Lyall, H. HIV and Mitochondrial Toxicity in Children. J. Antimicrob. Chemother. 2008, 61, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Papp, E.; Mohammadi, H.; Loutfy, M.R.; Yudin, M.H.; Murphy, K.E.; Walmsley, S.L.; Shah, R.; MacGillivray, J.; Silverman, M.; Serghides, L. HIV Protease Inhibitor Use during Pregnancy Is Associated with Decreased Progesterone Levels, Suggesting a Potential Mechanism Contributing to Fetal Growth Restriction. J. Infect. Dis. 2015, 211, 10–18. [Google Scholar] [CrossRef]
- Kala, S.; Watson, B.; Zhang, J.G.; Papp, E.; Guzman Lenis, M.; Dennehy, M.; Cameron, D.W.; Harrigan, P.R.; Serghides, L. Improving the Clinical Relevance of a Mouse Pregnancy Model of Antiretroviral Toxicity; a Pharmacokinetic Dosing-Optimization Study of Current HIV Antiretroviral Regimens. Antivir. Res. 2018, 159, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.C.; Zhang, G.; Cameron, D.W.; Serghides, L.; Bendayan, R. Impact of in-utero antiretroviral drug exposure on expression of membrane-associated transporters in mouse placenta and fetal brain. AIDS 2021, 35, 2249–2258. [Google Scholar] [CrossRef]
- Quiros, P.M.; Goyal, A.; Jha, P.; Auwerx, J. Analysis of MtDNA/NDNA Ratio in Mice. Curr. Protoc. Mouse Biol. 2017, 7, 47–54. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Copeland, W.C. Defects in Mitochondrial DNA Replication and Human Disease. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Birkus, G.; Hitchcock, M.J.M.; Cihlar, T. Assessment of Mitochondrial Toxicity in Human Cells Treated with Tenofovir: Comparison with Other Nucleoside Reverse Transcriptase Inhibitors. Antimicrob. Agents Chemother. 2002, 46, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O.; Oscai, L.B.; Don, I.J.; Molé, P.A. Mitochondrial Citric Acid Cycle and Related Enzymes: Adaptive Response to Exercise. Biochem. Biophys. Res. Commun. 1970, 40, 1368–1373. [Google Scholar] [CrossRef]
- Williams, R.S.; Salmons, S.; Newsholme, E.A.; Kaufman, R.E.; Mellor, J. Regulation of Nuclear and Mitochondrial Gene Expression by Contractile Activity in Skeletal Muscle. J. Biol. Chem. 1986, 261, 376–380. [Google Scholar] [CrossRef]
- Hood, D.A.; Zak, R.; Pette, D. Chronic Stimulation of Rat Skeletal Muscle Induces Coordinate Increases in Mitochondrial and Nuclear MRNAs of Cytochrome-c-Oxidase Subunits. Eur. J. Biochem. 1989, 179, 275–280. [Google Scholar] [CrossRef]
- Turrens, J.F.; Boveris, A. Generation of Superoxide Anion by the NADH Dehydrogenase of Bovine Heart Mitochondria. Biochem. J. 1980, 191, 421–427. [Google Scholar] [CrossRef]
- Hernández, S.; Catalán-García, M.; Morén, C.; García-Otero, L.; López, M.; Guitart-Mampel, M.; Milisenda, J.; Coll, O.; Cardellach, F.; Gratacós, E.; et al. Placental Mitochondrial Toxicity, Oxidative Stress, Apoptosis, and Adverse Perinatal Outcomes in HIV Pregnancies Under Antiretroviral Treatment Containing Zidovudine. J. Acquir. Immune Defic. Syndr. 1999 2017, 75, e113–e119. [Google Scholar] [CrossRef]
- Liu, Y.; Shim, E.; Crespo-Mejias, Y.; Nguyen, P.; Gibbons, A.; Liu, D.; Shide, E.; Poirier, M.C. Cardiomyocytes Are Protected from Antiretroviral Nucleoside Analog-Induced Mitochondrial Toxicity by Overexpression of PGC-1α. Cardiovasc. Toxicol. 2015, 15, 224–231. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Hu, X.; Fassett, J.; Zhu, G.; Tao, Y.; Li, J.; Huang, Y.; Zhang, P.; Zhao, B.; et al. PGC-1 Alpha Regulates Expression of Myocardial Mitochondrial Antioxidants and Myocardial Oxidative Stress after Chronic Systolic Overload. Antioxid. Redox Signal. 2010, 13, 1011–1022. [Google Scholar] [CrossRef]
- Schilling, J.; Kelly, D.P. The PGC-1 Cascade as a Therapeutic Target for Heart Failure. J. Mol. Cell. Cardiol. 2011, 51, 578–583. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Pardi, G.; Marconi, A.M.; Cetin, I. Placental-Fetal Interrelationship in IUGR Fetuses—A Review. Placenta 2002, 23 (Suppl. A), S136–S141. [Google Scholar] [CrossRef]
- Mayeur, S.; Lancel, S.; Theys, N.; Lukaszewski, M.-A.; Duban-Deweer, S.; Bastide, B.; Hachani, J.; Cecchelli, R.; Breton, C.; Gabory, A.; et al. Maternal Calorie Restriction Modulates Placental Mitochondrial Biogenesis and Bioenergetic Efficiency: Putative Involvement in Fetoplacental Growth Defects in Rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E14–E22. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Igosheva, N.; Mistry, H.D.; Seed, P.T.; Shennan, A.H.; Rana, S.; Karumanchi, S.A.; Chappell, L.C. Role of Oxidative Stress and Antioxidant Supplementation in Pregnancy Disorders. Am. J. Clin. Nutr. 2011, 94 (Suppl. 6), 1980S–1985S. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Disturbances in Lipid Metabolism in Diabetic Pregnancy - Are These the Cause of the Problem? Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.; Zash, R.; Rasi, V.; Thorne, C. HIV Treatment in Pregnancy. Lancet HIV 2018, 5, e457–e467. [Google Scholar] [CrossRef]
- Tshivuila-Matala, C.O.O.; Honeyman, S.; Nesbitt, C.; Kirtley, S.; Kennedy, S.H.; Hemelaar, J. Adverse Perinatal Outcomes Associated with Antiretroviral Therapy Regimens: Systematic Review and Network Meta-Analysis. AIDS Lond. Engl. 2020, 34, 1643–1656. [Google Scholar] [CrossRef]
- Holland, O.J.; Hickey, A.J.R.; Alvsaker, A.; Moran, S.; Hedges, C.; Chamley, L.W.; Perkins, A.V. Changes in Mitochondrial Respiration in the Human Placenta over Gestation. Placenta 2017, 57, 102–112. [Google Scholar] [CrossRef]
- Wakefield, S.L.; Lane, M.; Mitchell, M. Impaired Mitochondrial Function in the Preimplantation Embryo Perturbs Fetal and Placental Development in the Mouse. Biol. Reprod. 2011, 84, 572–580. [Google Scholar] [CrossRef]
- Jiang, B.; Hebert, V.Y.; Li, Y.; Mathis, J.M.; Alexander, J.S.; Dugas, T.R. HIV Antiretroviral Drug Combination Induces Endothelial Mitochondrial Dysfunction and Reactive Oxygen Species Production, but Not Apoptosis. Toxicol. Appl. Pharmacol. 2007, 224, 60–71. [Google Scholar] [CrossRef]
- Sutliff, R.L.; Dikalov, S.; Weiss, D.; Parker, J.; Raidel, S.; Racine, A.K.; Russ, R.; Haase, C.P.; Taylor, W.R.; Lewis, W. Nucleoside Reverse Transcriptase Inhibitors Impair Endothelium-Dependent Relaxation by Increasing Superoxide. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2363–H2370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Hebert, V.Y.; Zavecz, J.H.; Dugas, T.R. Antiretrovirals Induce Direct Endothelial Dysfunction in Vivo. J. Acquir. Immune Defic. Syndr. 1999 2006, 42, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Komarov, A.M.; Hall, J.M.; Weglicki, W.B. Azidothymidine Promotes Free Radical Generation by Activated Macrophages and Hydrogen Peroxide-Iron-Mediated Oxidation in a Cell-Free System. Biochim. Biophys. Acta 2004, 1688, 257–264. [Google Scholar] [CrossRef][Green Version]
- de la Asunción, J.G.; Del Olmo, M.L.; Gómez-Cambronero, L.G.; Sastre, J.; Pallardó, F.V.; Viña, J. AZT Induces Oxidative Damage to Cardiac Mitochondria: Protective Effect of Vitamins C and E. Life Sci. 2004, 76, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Nedelec, L.F.; Weglicki, W.B. Pro-Oxidant Properties and Cytotoxicity of AZT-Monophosphate and AZT. Cardiovasc. Toxicol. 2004, 4, 109–115. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Katoh, I.; Kurata, S. Azidothymidine Causes Functional and Structural Destruction of Mitochondria, Glutathione Deficiency and HIV-1 Promoter Sensitization. Eur. J. Biochem. 2002, 269, 2782–2788. [Google Scholar] [CrossRef]
- Glover, M.; Hebert, V.Y.; Nichols, K.; Xue, S.Y.; Thibeaux, T.M.; Zavecz, J.A.; Dugas, T.R. Overexpression of Mitochondrial Antioxidant Manganese Superoxide Dismutase (MnSOD) Provides Protection against AZT- or 3TC-Induced Endothelial Dysfunction. Antivir. Res. 2014, 111, 136–142. [Google Scholar] [CrossRef]




| Gene 1 | Primer (Sense) | Primer (Anti-Sense) |
|---|---|---|
| ND1 | CTAGCAGAAACAAACCGGGC | CCGGCTGCGTATTCTACGTT |
| 16S rRNA | CCGCAAGGGAAAGATGAAAGAC | TCGTTTGGTTTCGGGGTTTC |
| HK2 | GCCAGCCTCTCCTGATTTTAGTGT | GGGAACACAAAAGACCTCTTCTGG |
| POLG | GCAGGATGGGCAGGAACA | GCATCCGGGAGTCCTGAA |
| CS | CAGCAGTATCGGAGCCATTGA | GGGTCGGTGTAGCCTAACAT |
| PGC-1α | GCCGTGTGATTTACGTTGGTAA | AAAACTTCAAAGCGGTCTCTCAA |
| MnSOD | CTGGAGCCACACATTAACGC | CGGTGGCGTTGAGATTGTTC |
| COX-II | AACCGAGTCGTTCTGCCAAT | CTAGGGAGGGGACTGCTCAT |
| COX-IV | TTCACTGCGCTCGTTCTGAT | CACCCAGTCACGATCGAAAGTA |
| HPRT | AGCGTCGTGATTAGCGATGA | ACACTTTTTCCAAATCCTCGGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogun, K.; Serghides, L. Comparison of the Effects of Three Dual-Nucleos(t)ide Reverse Transcriptase Inhibitor Backbones on Placenta Mitochondria Toxicity and Oxidative Stress Using a Mouse Pregnancy Model. Pharmaceutics 2022, 14, 1063. https://doi.org/10.3390/pharmaceutics14051063
Balogun K, Serghides L. Comparison of the Effects of Three Dual-Nucleos(t)ide Reverse Transcriptase Inhibitor Backbones on Placenta Mitochondria Toxicity and Oxidative Stress Using a Mouse Pregnancy Model. Pharmaceutics. 2022; 14(5):1063. https://doi.org/10.3390/pharmaceutics14051063
Chicago/Turabian StyleBalogun, Kayode, and Lena Serghides. 2022. "Comparison of the Effects of Three Dual-Nucleos(t)ide Reverse Transcriptase Inhibitor Backbones on Placenta Mitochondria Toxicity and Oxidative Stress Using a Mouse Pregnancy Model" Pharmaceutics 14, no. 5: 1063. https://doi.org/10.3390/pharmaceutics14051063
APA StyleBalogun, K., & Serghides, L. (2022). Comparison of the Effects of Three Dual-Nucleos(t)ide Reverse Transcriptase Inhibitor Backbones on Placenta Mitochondria Toxicity and Oxidative Stress Using a Mouse Pregnancy Model. Pharmaceutics, 14(5), 1063. https://doi.org/10.3390/pharmaceutics14051063






