Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges
Abstract
:1. Introduction
1.1. Immunological Aspects of COVID-19 Medicaments
1.2. Structural Considerations of Coronavirus in Vaccine Development
1.3. Distribution Concerns of COVID-19 Vaccines
1.4. The Need for Novel Vaccine Delivery System
1.5. Microneedles in Transdermal Drug and Vaccine Delivery
2. Dissolving Microneedles in Immunization
2.1. Fabrication of Dissolving Microneedles
2.2. Biodegradation Kinetics of Dissolvable Microneedles
2.3. Loading Capacity of Dissolvable Microneedles
2.4. Significance of Novel Transdermal Vaccination
2.5. Mathematical Modeling of Microneedles
2.5.1. MN Delivery Mechanisms
2.5.2. Effect of Polymer Type
2.5.3. Effect of Microneedle Array Geometric Parameters
2.5.4. Effect of Skin Properties
3. Dissolving Microneedles: Some Satisfactory Aspects
3.1. Patient Compliance
3.2. Overall Vaccination Cost Reduction
4. Preclinical and Stability Studies of MN Vaccination
4.1. Stability Studies of MN Vaccination
4.2. MN Patch Packaging and Storage
4.3. Preclinical Studies of Vaccine MN Array Patch
5. Microneedle Array Patch Vaccination: Clinical Trials and Human Studies
5.1. Microneedle Vaccination Clinical Trials
5.2. Vaccine Coated MN Array for Human Studies
5.3. Vaccine Dissolvable MAP for Human Studies
5.4. Concerns about Vaccination via MAP
5.4.1. Commercialized MAP
5.4.2. Manufacturing Issues
5.4.3. Regulatory Issues
6. Challenges in Ensuring Global Access to COVID-19 Vaccines and Socio-Economic Factors
6.1. Vaccine Hesitancy
6.2. Needle Phobia
6.3. Availability
Available Vaccines and Variants of Concern
6.4. Affordability
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Der Li, Y.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus Vaccine Development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Gaziano, L.; Giambartolomei, C.; Pereira, A.C.; Gaulton, A.; Posner, D.C.; Swanson, S.A.; Ho, Y.L.; Iyengar, S.K.; Kosik, N.M.; Vujkovic, M.; et al. Actionable Druggable Genome-Wide Mendelian Randomization Identifies Repurposing Opportunities for COVID-19. Nat. Med. 2021, 27, 668–676. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence That D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Roberts, C.A.K.; Pryce, G.; Kang, A.S.; Marta, M.; Reyes, S.; Schmierer, K.; Giovannoni, G.; Amor, S. COVID-19 Vaccine-Readiness for Anti-CD20-Depleting Therapy in Autoimmune Diseases. Clin. Exp. Immunol. 2020, 202, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lin, D.; Operario, D. Interest in COVID-19 Vaccine Trials Participation among Young Adults in China: Willingness, Reasons for Hesitancy, and Demographic and Psychosocial Determinants. Prev. Med. Rep. 2021, 22, 101350. [Google Scholar] [CrossRef] [PubMed]
- Redhead, M.A.; Owen, C.D.; Brewitz, L.; Collette, A.H.; Lukacik, P.; Strain-Damerell, C.; Robinson, S.W.; Collins, P.M.; Schäfer, P.; Swindells, M.; et al. Bispecific Repurposed Medicines Targeting the Viral and Immunological Arms of COVID-19. Sci. Rep. 2021, 11, 13208. [Google Scholar] [CrossRef]
- Jeong, H.J.; Min, S.; Chae, H.; Kim, S.; Lee, G.; Namgoong, S.K.; Jeong, K. Signal Amplification by Reversible Exchange for COVID-19 Antiviral Drug Candidates. Sci. Rep. 2020, 10, 14290. [Google Scholar] [CrossRef]
- Güven, M.; Gültekin, H. The Effect of High-Dose Parenteral Vitamin D3 on COVID-19-Related Inhospital Mortality in Critical COVID-19 Patients during Intensive Care Unit Admission: An Observational Cohort Study. Eur. J. Clin. Nutr. 2021, 75, 1383–1388. [Google Scholar] [CrossRef]
- Yang, B.; Fan, J.; Huang, J.; Guo, E.; Fu, Y.; Liu, S.; Xiao, R.; Liu, C.; Lu, F.; Qin, T.; et al. Clinical and Molecular Characteristics of COVID-19 Patients with Persistent SARS-CoV-2 Infection. Nat. Commun. 2021, 12, 3501. [Google Scholar] [CrossRef]
- Ita, K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef]
- Cantudo-Cuenca, M.D.; Gutiérrez-Pizarraya, A.; Pinilla-Fernández, A.; Contreras-Macías, E.; Fernández-Fuertes, M.; Lao-Domínguez, F.A.; Rincón, P.; Pineda, J.A.; Macías, J.; Morillo-Verdugo, R. Drug–Drug Interactions between Treatment Specific Pharmacotherapy and Concomitant Medication in Patients with COVID-19 in the First Wave in Spain. Sci. Rep. 2021, 11, 12414. [Google Scholar] [CrossRef]
- Prieto Curiel, R.; González Ramírez, H. Vaccination Strategies against COVID-19 and the Diffusion of Anti-Vaccination Views. Sci. Rep. 2021, 11, 6626. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O., III. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Korkmaz, E.; Balmert, S.C.; Sumpter, T.L.; Carey, C.D.; Erdos, G.; Falo, L.D. Microarray Patches Enable the Development of Skin-Targeted Vaccines against COVID-19. Adv. Drug Deliv. Rev. 2021, 171, 164–186. [Google Scholar] [CrossRef]
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Blakney, A.K.; Wright, D.; Sharpe, H.R.; et al. Heterologous Vaccination Regimens with Self-Amplifying RNA and Adenoviral COVID Vaccines Induce Robust Immune Responses in Mice. Nat. Commun. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Brady, E.; Oertelt-prigione, S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat. Commun. 2021, 12, 4015. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bodrud-Doza, M.; Griffiths, M.D.; Mamun, M.A. Biomedical Waste amid COVID-19: Perspectives from Bangladesh. Lancet Glob. Health 2020, 8, e1262. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Ding, Y.; Luo, S.; Zheng, X.; Wu, X.; Liu, Z.; Ilyas, I.; Chen, S.; Han, S. The Zinc Fi Nger Transcription Factor, KLF2, Protects against COVID-19 Associated Endothelial Dysfunction. Signal Transduct. Target. Ther. 2021, 6, 266. [Google Scholar] [CrossRef]
- World Bank Fast-Tracks $100 Million COVID-19 (Coronavirus) Support for Bangladesh. Available online: https://www.worldbank.org/en/news/press-release/2020/04/03/world-bank-fast-tracks-100-million-covid-19-coronavirus-support-for-bangladesh (accessed on 18 July 2021).
- Watson, O.J.; Alhaffar, M.; Mehchy, Z.; Whittaker, C.; Akil, Z.; Brazeau, N.F.; Cuomo-Dannenburg, G.; Hamlet, A.; Thompson, H.A.; Baguelin, M.; et al. Leveraging Community Mortality Indicators to Infer COVID-19 Mortality and Transmission Dynamics in Damascus, Syria. Nat. Commun. 2021, 12, 2394. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 Vaccine Phase 3 Trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Le, B.L.; Andreoletti, G.; Oskotsky, T.; Vallejo-Gracia, A.; Rosales, R.; Yu, K.; Kosti, I.; Leon, K.E.; Bunis, D.G.; Li, C.; et al. Transcriptomics-Based Drug Repositioning Pipeline Identifies Therapeutic Candidates for COVID-19. Sci. Rep. 2021, 11, 12310. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Zhan, M.; Jiang, J.; Yin, H.; Dauphars, D.J.; Li, S.Y.; Li, Y.; He, Y.W. Antibody Response and Therapy in COVID-19 Patients: What Can Be Learned for Vaccine Development? Sci. China Life Sci. 2020, 63, 1833–1849. [Google Scholar] [CrossRef] [PubMed]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in Symptomatic COVID-19 Is Persistent and Critical for Survival. Nat. Commun. 2021, 12, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. Coming to Terms with COVID-19 Personally and Professionally in Bangladesh. Lancet Glob. Health 2021, 9, e1471–e1473. [Google Scholar] [CrossRef]
- DiPiazza, A.T.; Leist, S.R.; Abiona, O.M.; Moliva, J.I.; Werner, A.; Minai, M.; Nagata, B.M.; Bock, K.W.; Phung, E.; Schäfer, A.; et al. COVID-19 Vaccine MRNA-1273 Elicits a Protective Immune Profile in Mice That Is Not Associated with Vaccine-Enhanced Disease upon SARS-CoV-2 Challenge. Immunity 2021, 54, 1869–1882.e6. [Google Scholar] [CrossRef]
- Rahi, M.; Sharma, A. Mass Vaccination against COVID-19 May Require Replays of the Polio Vaccination Drives. EClinicalMedicine 2020, 25, 100501. [Google Scholar] [CrossRef] [PubMed]
- Usherwood, T.; LaJoie, Z.; Srivastava, V. A Model and Predictions for COVID-19 Considering Population Behavior and Vaccination. Sci. Rep. 2021, 11, 12051. [Google Scholar] [CrossRef]
- Yang, J.; Marziano, V.; Deng, X.; Guzzetta, G.; Zhang, J.; Trentini, F.; Cai, J.; Poletti, P.; Zheng, W.; Wang, W.; et al. Despite Vaccination, China Needs Non-Pharmaceutical Interventions to Prevent Widespread Outbreaks of COVID-19 in 2021. Nat. Hum. Behav. 2021, 5, 1009–1020. [Google Scholar] [CrossRef]
- Amani, H.; Shahbazi, M.A.; D’Amico, C.; Fontana, F.; Abbaszadeh, S.; Santos, H.A. Microneedles for Painless Transdermal Immunotherapeutic Applications. J. Control. Release 2021, 330, 185–217. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Vassilieva, E.V.; Stavropoulou, A.; Zarnitsyn, V.G.; Esser, E.S.; Taherbhai, M.T.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Delivery of Subunit Influenza Vaccine to Skin with Microneedles Improves Immunogenicity and Long-Lived Protection. Sci. Rep. 2012, 2, 357. [Google Scholar] [CrossRef] [Green Version]
- Vrdoljak, A.; Allen, E.A.; Ferrara, F.; Temperton, N.; Crean, A.; Moore, A.C. Induction of Broad Immunity by Thermostabilised Vaccines Incorporated in Dissolvable Microneedles Using Novel Fabrication Methods. J. Control. Release 2016, 225, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.; Sharma, P.K.; McCann, T.; Bloomekatz, J.; Repka, M.A.; Murthy, S.N. Fabrication and Development of Controlled Release PLGA Microneedles for Macromolecular Delivery Using FITC-Dextran as Model Molecule. J. Drug Deliv. Sci. Technol. 2021, 68, 102712. [Google Scholar] [CrossRef]
- Koh, K.J.; Liu, Y.; Lim, S.H.; Loh, X.J.; Kang, L.; Lim, C.Y.; Phua, K.K.L. Formulation, Characterization and Evaluation of MRNA-Loaded Dissolvable Polymeric Microneedles (RNApatch). Sci. Rep. 2018, 8, 11842. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.Y.; Park, J.H.; Lee, Y.S.; Kim, Y.S.; Park, J.Y.; Kim, S.Y. The Current Status of Clinical Research Involving Microneedles: A Systematic Review. Pharmaceutics 2020, 12, 1113. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of Dissolvable Microneedles Using an Atomised Spray Process: Effect of Microneedle Composition on Skin Penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka Honey Microneedles for Enhanced Wound Healing and the Prevention and/or Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA) Surgical Site Infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef]
- Schipper, P.; van der Maaden, K.; Groeneveld, V.; Ruigrok, M.; Romeijn, S.; Uleman, S.; Oomens, C.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Diphtheria Toxoid and N-Trimethyl Chitosan Layer-by-Layer Coated PH-Sensitive Microneedles Induce Potent Immune Responses upon Dermal Vaccination in Mice. J. Control. Release 2017, 262, 28–36. [Google Scholar] [CrossRef]
- Choi, H.J.; Yoo, D.G.; Bondy, B.J.; Quan, F.S.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Stability of Influenza Vaccine Coated onto Microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Rao, F.; Wu, D.; Huang, Y.; Xu, H.; Gao, W.; Zhang, J.; Sun, J. Study on the Fabrication and Characterization of Tip-Loaded Dissolving Microneedles for Transdermal Drug Delivery. Eur. J. Pharm. Biopharm. 2020, 157, 66–73. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; McLoughlin, P. Formulation and Characterisation of Dissolving Microneedles for the Transdermal Delivery of Therapeutic Peptides. Int. J. Pharm. 2017, 526, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Pramanick, B.; Maiti, T.K.; Bhattacharyya, T.K. Glassy Carbon Microneedles—New Transdermal Drug Delivery Device Derived from a Scalable C-MEMS Process. Microsyst. Nanoeng. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, M.; Priester, M.I.; Romeijn, S.; Nejadnik, M.R.; Mönkäre, J.; O’Mahony, C.; Jiskoot, W.; Kersten, G.; Bouwstra, J.A. Hyaluronan-Based Dissolving Microneedles with High Antigen Content for Intradermal Vaccination: Formulation, Physicochemical Characterization and Immunogenicity Assessment. Eur. J. Pharm. Biopharm. 2019, 134, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Kim, S.Y.; Lee, J.E.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Kim, E.S.; Duong, H.T.T.; Kim, H.K.; Kim, S.; et al. Enhanced Cancer Vaccination by in Situ Nanomicelle-Generating Dissolving Microneedles. ACS Nano 2018, 12, 9702–9713. [Google Scholar] [CrossRef]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, R.; Wang, S.; Yang, X.; Ling, G.; Zhang, P. Fabrication, Evaluation and Applications of Dissolving Microneedles. Int. J. Pharm. 2021, 604, 120749. [Google Scholar] [CrossRef]
- Steinbach, S.; Jalili-Firoozinezhad, S.; Srinivasan, S.; Melo, M.B.; Middleton, S.; Konold, T.; Coad, M.; Hammond, P.T.; Irvine, D.J.; Vordermeier, M.; et al. Temporal Dynamics of Intradermal Cytokine Response to Tuberculin in Mycobacterium Bovis BCG-Vaccinated Cattle Using Sampling Microneedles. Sci. Rep. 2021, 11, 7074. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Quan, F.-S.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Formulation and Coating of Microneedles with Inactivated Influenza Virus to Improve Vaccine Stability and Immunogenicity. J. Control. Release 2010, 142, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.T.M.; Gavitt, T.D.; Farrell, N.J.; Curry, E.J.; Mara, A.B.; Patel, A.; Brown, L.; Kilpatrick, S.; Piotrowska, R.; Mishra, N.; et al. Transdermal Microneedles for the Programmable Burst Release of Multiple Vaccine Payloads. Nat. Biomed. Eng. 2020, 5, 998–1007. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lai, K.Y.; Chiu, Y.H.; Wu, Y.W.; Shiau, A.L.; Chen, M.C. Implantable Microneedles with an Immune-Boosting Function for Effective Intradermal Influenza Vaccination. Acta Biomater. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Ita, K. Dissolving Microneedles for Transdermal Drug Delivery: Advances and Challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef]
- Zhu, Z.; Ye, X.; Ku, Z.; Liu, Q.; Shen, C.; Luo, H.; Luan, H.; Zhang, C.; Tian, S.; Lim, C.Y.; et al. Transcutaneous Immunization via Rapidly Dissolvable Microneedles Protects against Hand-Foot-and-Mouth Disease Caused by Enterovirus 71. J. Control. Release 2016, 243, 291–302. [Google Scholar] [CrossRef]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current Advances in the Fabrication of Microneedles for Transdermal Delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for Drug and Vaccine Delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Chen, J.; Zhao, Y.; Yan, X.; Zhang, L.; Choy, K.; Hu, J.; Sant, H.J.; Gale, B.K.; Tang, T. Transdermal Delivery of SiRNA through Microneedle Array. Sci. Rep. 2016, 6, 21422. [Google Scholar] [CrossRef]
- Martin, A.; McConville, A.; Anderson, A.; McLister, A.; Davis, J. Microneedle Manufacture: Assessing Hazards and Control Measures. Safety 2017, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.B.; Vrdoljak, A.; O’Mahony, C.; Hill, A.V.S.; Draper, S.J.; Moore, A.C. Microneedle-Mediated Immunization of an Adenovirus-Based Malaria Vaccine Enhances Antigen-Specific Antibody Immunity and Reduces Anti-Vector Responses Compared to the Intradermal Route. Sci. Rep. 2014, 4, 6154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle Patches: Usability and Acceptability for Self-Vaccination against Influenza. Vaccine 2014, 32, 1856. [Google Scholar] [CrossRef] [Green Version]
- Than, A.; Liu, C.; Chang, H.; Duong, P.K.; Cheung, C.M.G.; Xu, C.; Wang, X.; Chen, P. Self-Implantable Double-Layered Micro-Drug-Reservoirs for Efficient and Controlled Ocular Drug Delivery. Nat. Commun. 2018, 9, 4433. [Google Scholar] [CrossRef] [Green Version]
- Van Der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle Technologies for (Trans) Dermal Drug and Vaccine Delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef]
- Chang, H.; Chew, S.W.T.; Zheng, M.; Lio, D.C.S.; Wiraja, C.; Mei, Y.; Ning, X.; Cui, M.; Than, A.; Shi, P.; et al. Cryomicroneedles for Transdermal Cell Delivery. Nat. Biomed. Eng. 2021, 5, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Oh, Y.; Kim, Y.; Shin, Y.; Baek, S.K.; Park, J.H. Progress in Microneedle Array Patch (MAP) for Vaccine Delivery. Hum. Vaccines Immunother. 2020, 17, 316–327. [Google Scholar] [CrossRef]
- Rodgers, A.M.; McCrudden, M.T.C.; Vincente-Perez, E.M.; Dubois, A.V.; Ingram, R.J.; Larrañeta, E.; Kissenpfennig, A.; Donnelly, R.F. Design and Characterisation of a Dissolving Microneedle Patch for Intradermal Vaccination with Heat-Inactivated Bacteria: A Proof of Concept Study. Int. J. Pharm. 2018, 549, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA Vaccination for Cervical Cancer; a Novel Technology Platform of RALA Mediated Gene Delivery via Polymeric Microneedles. Nanomedicine 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsukasa, A.; Kuruma, K.; Okamatsu, M.; Hiono, T.; Suzuki, M.; Matsuno, K.; Kida, H.; Oyamada, T.; Sakoda, Y. Potency of Whole Virus Particle and Split Virion Vaccines Using Dissolving Microneedle against Challenges of H1N1 and H5N1 Influenza Viruses in Mice. Vaccine 2017, 35, 2855–2861. [Google Scholar] [CrossRef]
- Resch, T.K.; Wang, Y.; Moon, S.S.; Joyce, J.; Li, S.; Prausnitz, M.; Jiang, B. Inactivated Rotavirus Vaccine by Parenteral Administration Induces Mucosal Immunity in Mice. Sci. Rep. 2018, 8, 561. [Google Scholar] [CrossRef] [Green Version]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle Arrays Combined with Nanomedicine Approaches for Transdermal Delivery of Therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Koutsonanos, D.G.; Del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving Polymer Microneedle Patches for Influenza Vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Trautmann, A.; Roth, G.L.; Nujiqi, B.; Walther, T.; Hellmann, R. Towards a Versatile Point-of-Care System Combining Femtosecond Laser Generated Microfluidic Channels and Direct Laser Written Microneedle Arrays. Microsyst. Nanoeng. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Silveira, M.M.; Moreira, G.M.S.G.; Mendonça, M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021, 267, 118919. [Google Scholar] [CrossRef]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-Dose ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic and Hemorrhagic Events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef]
- Yokoyama, M.; Chihara, N.; Tanaka, A.; Katayama, Y.; Taruya, A.; Ishida, Y.; Yuzaki, M.; Honda, K.; Nishimura, Y.; Kondo, T.; et al. A Biodegradable Microneedle Sheet for Intracorporeal Topical Hemostasis. Sci. Rep. 2020, 10, 18831. [Google Scholar] [CrossRef]
- Cardozo, T.; Veazey, R. Informed Consent Disclosure to Vaccine Trial Subjects of Risk of COVID-19 Vaccines Worsening Clinical Disease. Int. J. Clin. Pract. 2021, 75, e13795. [Google Scholar] [CrossRef]
- Florindo, H.F.; Kleiner, R.; Vaskovich-Koubi, D.; Acúrcio, R.C.; Carreira, B.; Yeini, E.; Tiram, G.; Liubomirski, Y.; Satchi-Fainaro, R. Immune-Mediated Approaches against COVID-19. Nat. Nanotechnol. 2020, 15, 630–645. [Google Scholar] [CrossRef]
- Rysz, S.; Al-Saadi, J.; Sjöström, A.; Farm, M.; Campoccia Jalde, F.; Plattén, M.; Eriksson, H.; Klein, M.; Vargas-Paris, R.; Nyrén, S.; et al. COVID-19 Pathophysiology May Be Driven by an Imbalance in the Renin-Angiotensin-Aldosterone System. Nat. Commun. 2021, 12, 2417. [Google Scholar] [CrossRef]
- Yang, R.; Deng, Y.; Huang, B.; Huang, L.; Lin, A.; Li, Y.; Wang, W.; Liu, J.; Lu, S.; Zhan, Z.; et al. A Core-Shell Structured COVID-19 MRNA Vaccine with Favorable Biodistribution Pattern and Promising Immunity. Signal Transduct. Target. Ther. 2021, 6, 213. [Google Scholar] [CrossRef]
- Neurath, M.F. COVID-19: Biologic and Immunosuppressive Therapy in Gastroenterology and Hepatology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 705–715. [Google Scholar] [CrossRef]
- Cho, J.; Lee, Y.J.; Kim, J.H.; Kim, S.S.; Choi, B.S.; Choi, J.H. Antiviral Activity of Digoxin and Ouabain against SARS-CoV-2 Infection and Its Implication for COVID-19. Sci. Rep. 2020, 10, 16200. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 2, 2637–2646. [Google Scholar] [CrossRef]
- Batty, G.D.; Deary, I.J.; Fawns-Ritchie, C.; Gale, C.R.; Altschul, D. Pre-Pandemic Cognitive Function and COVID-19 Vaccine Hesitancy: Cohort Study. Brain. Behav. Immun. 2021, 96, 100–105. [Google Scholar] [CrossRef]
- Robertson, E.; Reeve, K.S.; Niedzwiedz, C.L.; Moore, J.; Blake, M.; Green, M.; Katikireddi, S.V.; Benzeval, M.J. Predictors of COVID-19 Vaccine Hesitancy in the UK Household Longitudinal Study. Brain. Behav. Immun. 2021, 94, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Amani, B.; Khanijahani, A.; Amani, B. Hydroxychloroquine plus Standard of Care Compared with Standard of Care Alone in COVID-19: A Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2021, 11, 11974. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 MRNA Vaccine and Early Clinical Outcomes in Patients with Haematological Malignancies in Lithuania: A National Prospective Cohort Study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Dashtbali, M.; Mirzaie, M. A Compartmental Model That Predicts the Effect of Social Distancing and Vaccination on Controlling COVID-19. Sci. Rep. 2021, 11, 8191. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Filho, W.L.; Voronova, V.; Kloga, M.; Paço, A.; Minhas, A.; Salvia, A.L.; Ferreira, C.D.; Sivapalan, S. COVID-19 and Waste Production in Households: A Trend Analysis. Sci. Total Environ. 2021, 777, 145997. [Google Scholar] [CrossRef]
- Kreps, S.; Dasgupta, N.; Brownstein, J.S.; Hswen, Y.; Kriner, D.L. Public Attitudes toward COVID-19 Vaccination: The Role of Vaccine Attributes, Incentives, and Misinformation. NPJ Vaccines 2021, 6, 73. [Google Scholar] [CrossRef]
- Dzieciolowska, S.; Hamel, D.; Gadio, S.; Dionne, M.; Gagnon, D.; Robitaille, L.; Cook, E.; Caron, I.; Talib, A.; Parkes, L.; et al. COVID-19 Vaccine Acceptance, Hesitancy, and Refusal among Canadian Healthcare Workers: A Multicenter Survey. Am. J. Infect. Control 2021, 49, 1152–1157. [Google Scholar] [CrossRef]
- Habibi, M.; Taheri, G.; Aghdam, R. A SARS-CoV-2 (COVID-19) Biological Network to Find Targets for Drug Repurposing. Sci. Rep. 2021, 11, 9378. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, L.; Lian, R.; Song, Z.; Tian, J. COVID-19 Vaccine Research Focusses on Safety, Efficacy, Immunoinformatics, and Vaccine Production and Delivery: A Bibliometric Analysis Based on VOSviewer. Biosci. Trends 2021, 15, 64–73. [Google Scholar] [CrossRef]
- Kaur, U.; Ojha, B.; Pathak, B.K.; Singh, A.; Giri, K.R.; Singh, A.; Das, A.; Misra, A.; Yadav, A.K.; Chakrabarti, S.S.; et al. A Prospective Observational Safety Study on ChAdOx1 NCoV-19 Corona Virus Vaccine (Recombinant) Use in Healthcare Workers- First Results from India. eClinicalMedicine 2021, 38, 101038. [Google Scholar] [CrossRef]
- Moazzam, M.; Sajid, M.I.; Shahid, H.; Butt, J.; Bashir, I.; Jamshaid, M.; Shirazi, A.N.; Tiwari, R.K. Understanding COVID-19: From Origin to Potential Therapeutics. Int. J. Environ. Res. Public Health 2020, 17, 5904. [Google Scholar] [CrossRef]
- Orlando, V.; Coscioni, E.; Guarino, I.; Mucherino, S.; Perrella, A.; Trama, U.; Limongelli, G.; Menditto, E. Drug-Utilisation Profiles and COVID-19. Sci. Rep. 2021, 11, 8913. [Google Scholar] [CrossRef]
- Pagliusi, S.; Jarrett, S.; Hayman, B.; Kreysa, U.; Prasad, S.D.; Reers, M.; Hong Thai, P.; Wu, K.; Zhang, Y.T.; Baek, Y.O.; et al. Emerging Manufacturers Engagements in the COVID−19 Vaccine Research, Development and Supply. Vaccine 2020, 38, 5418–5423. [Google Scholar] [CrossRef]
- Arce, J.S.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; Mcmurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Zafar, B.; et al. COVID-19 Vaccine Acceptance and Hesitancy in Low- and Middle-Income Countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- van Riel, D.; de Wit, E. Next-Generation Vaccine Platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An Adenovirus-Vectored COVID-19 Vaccine Confers Protection from SARS-CoV-2 Challenge in Rhesus Macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Aygün, İ.; Kaya, M.; Alhajj, R. Identifying Side Effects of Commonly Used Drugs in the Treatment of COVID-19. Sci. Rep. 2020, 10, 21508. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Dinh, P.U.C.; Zhu, D.; Popowski, K.D.; Lutz, H.; Hu, S.; Lewis, M.G.; Cook, A.; Andersen, H.; et al. Cell-Mimicking Nanodecoys Neutralize SARS-CoV-2 and Mitigate Lung Injury in a Non-Human Primate Model of COVID-19. Nat. Nanotechnol. 2021, 16, 942–951. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Varghese, A.; Kolamban, S.; Sherimon, V.; Lacap, E.M.; Ahmed, S.S.; Sreedhar, J.P.; Al Harthi, H.; Al Shuaily, H.S. SEAMHCRD Deterministic Compartmental Model Based on Clinical Stages of Infection for COVID-19 Pandemic in Sultanate of Oman. Sci. Rep. 2021, 11, 11984. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 Vaccine Coverage in Health-Care Workers in England and Effectiveness of BNT162b2 MRNA Vaccine against Infection (SIREN): A Prospective, Multicentre, Cohort Study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef]
- Buckland, M.S.; Galloway, J.B.; Fhogartaigh, C.N.; Meredith, L.; Provine, N.M.; Bloor, S.; Ogbe, A.; Zelek, W.M.; Smielewska, A.; Yakovleva, A.; et al. Treatment of COVID-19 with Remdesivir in the Absence of Humoral Immunity: A Case Report. Nat. Commun. 2020, 11, 6385. [Google Scholar] [CrossRef]
- Dogra, P.; Koay, E.J.; Wang, Z.; Vahidy, F.S.; Ferrari, M.; Pasqualini, R.; Arap, W.; Boom, M.L.; Dirk Sostman, H.; Cristini, V. Is the Worst of the COVID-19 Global Pandemic yet to Come? Application of Financial Mathematics as Candidate Predictive Tools. Transl. Psychiatry 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Ho, P.C.; Liu, C.L.; Tzeng, K.T.; Nayeem, N.; Moore, J.S.; Wang, L.S.; Chou, S.Y. Reconcile the Debate over Protective Effects of BCG Vaccine against COVID-19. Sci. Rep. 2021, 11, 8356. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H. A Review of Vaccine Effects on Women in Light of the COVID-19 Pandemic. Taiwan. J. Obstet. Gynecol. 2020, 59, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rai, S.N.; Singh, V.; Singh, M.P. Molecular characterization, pathogen-host interaction pathway and in silico approaches for vaccine design against COVID-19. J. Chem. Neuroanat. 2020, 110, 101874. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, X.; Jiang, G.; Tang, H.; Ming, S.; Tang, L.; Lu, J. Altered Oral and Gut Microbiota and Its Association with SARS-CoV-2 Viral Load in COVID-19 Patients during Hospitalization. NPJ Biofilms Microbiomes. 2021, 7, 61. [Google Scholar] [CrossRef]
- Hillis, S.D.; Unwin, H.J.T.; Chen, Y.; Cluver, L.; Sherr, L.; Goldman, P.S.; Ratmann, O.; Donnelly, C.A.; Bhatt, S.; Villaveces, A.; et al. Global Minimum Estimates of Children Affected by COVID-19-Associated Orphanhood and Deaths of Caregivers: A Modelling Study. Lancet 2021, 398, 391–402. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef]
- Pushparajah, D.; Jimenez, S.; Wong, S.; Alattas, H.; Nafissi, N.; Slavcev, R.A. Advances in gene-based vaccine platforms to address the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 170, 113–141. [Google Scholar] [CrossRef]
- Dal-Ré, R.; Launay, O. Public Trust on Regulatory Decisions: The European Medicines Agency and the AstraZeneca COVID-19 Vaccine Label. Vaccine 2021, 39, 4029–4031. [Google Scholar] [CrossRef]
- Xie, X.; Muruato, A.E.; Zhang, X.; Lokugamage, K.G.; Fontes-Garfias, C.R.; Zou, J.; Liu, J.; Ren, P.; Balakrishnan, M.; Cihlar, T.; et al. A Nanoluciferase SARS-CoV-2 for Rapid Neutralization Testing and Screening of Anti-Infective Drugs for COVID-19. Nat. Commun. 2020, 11, 5214. [Google Scholar] [CrossRef]
- Tan, T.K.; Rijal, P.; Rahikainen, R.; Keeble, A.H.; Schimanski, L.; Hussain, S.; Harvey, R.; Hayes, J.W.P.; Edwards, J.C.; McLean, R.K.; et al. A COVID-19 Vaccine Candidate Using SpyCatcher Multimerization of the SARS-CoV-2 Spike Protein Receptor-Binding Domain Induces Potent Neutralising Antibody Responses. Nat. Commun. 2021, 12, 542. [Google Scholar] [CrossRef]
- Biswas, M.; Kali, M.S.K. Association of Angiotensin-Converting Enzyme Inhibitors and Angiotensin-Receptor Blockers with Risk of Mortality, Severity or SARS-CoV-2 Test Positivity in COVID-19 Patients: Meta-Analysis. Sci. Rep. 2021, 11, 5012. [Google Scholar] [CrossRef]
- Cot, C.; Cacciapaglia, G.; Islind, A.S.; Óskarsdóttir, M.; Sannino, F. Impact of US Vaccination Strategy on COVID-19 Wave Dynamics. Sci. Rep. 2021, 11, 10960. [Google Scholar] [CrossRef]
- Dutta, A.K. Vaccine Against COVID-19 Disease—Present Status of Development. Indian J. Pediatr. 2020, 87, 810–816. [Google Scholar] [CrossRef]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Koirala, A.; Joo, Y.J.; Khatami, A.; Chiu, C.; Britton, P.N. Vaccines for COVID-19: The Current State of Play. Paediatr. Respir. Rev. 2020, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Burn, E.; You, S.C.; Sena, A.G.; Kostka, K.; Abedtash, H.; Abrahão, M.T.F.; Alberga, A.; Alghoul, H.; Alser, O.; Alshammari, T.M.; et al. Deep Phenotyping of 34,128 Adult Patients Hospitalised with COVID-19 in an International Network Study. Nat. Commun. 2020, 11, 5009. [Google Scholar] [CrossRef]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Macías, J.; Pinilla, A.; Lao-Dominguez, F.A.; Corma, A.; Contreras-Macias, E.; González-Serna, A.; Gutierrez-Pizarraya, A.; Fernández-Fuertes, M.; Morillo-Verdugo, R.; Trigo, M.; et al. High Rate of Major Drug–Drug Interactions of Lopinavir–Ritonavir for COVID-19 Treatment. Sci. Rep. 2020, 10, 20958. [Google Scholar] [CrossRef]
- Bolcato, M.; Rodriguez, D.; Feola, A.; Mizio, G.D.; Bonsignore, A.; Ciliberti, R.; Tettamanti, C.; Aurilio, M.T.; Aprile, A. COVID-19 Pandemic and Equal Access to Vaccines. Vaccines 2021, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.A.; Puri, A.; Garg, S.; Nag, S.; Corbo, J.; Turabi, A.E.; Kaka, N.; Zemmel, R.W.; Hegarty, P.K.; Kamat, A.M. The Association of Coronavirus Disease-19 Mortality and Prior Bacille Calmette-Guerin Vaccination: A Robust Ecological Analysis Using Unsupervised Machine Learning. Sci. Rep. 2021, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.M.; Gutiérrez, A.H.; Boyle, C.M.; Terry, F.; McGonnigal, B.G.; Salazar, A.; Princiotta, M.F.; Martin, W.D.; De Groot, A.S.; Moise, L. Highly Conserved, Non-Human-like, and Cross-Reactive SARS-CoV-2 T Cell Epitopes for COVID-19 Vaccine Design and Validation. NPJ Vaccines 2021, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Alaran, A.J.; Adebisi, Y.A.; Badmos, A.; Khalid-Salako, F.; Gaya, S.K.; Ilesanmi, E.B.; Olaoye, D.Q.; Bamisaiye, A.; Lucero-Prisno, D.E., III. Uneven Power Dynamics Must Be Levelled in COVID-19 Vaccines Access and Distribution. Public Health Pract. 2021, 2, 100096. [Google Scholar] [CrossRef]
- Ahmed Saeed AL-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef]
- Victora, C.G.; Castro, M.C.; Gurzenda, S.; Barros, A.J.D. Estimating the Early Impact of Immunization against COVID-19 on Deaths among Elderly People in Brazil: Analyses of Secondary Data on Vaccine Coverage and Mortality. medRxiv 2021. [Google Scholar] [CrossRef]
- Al-Metwali, B.Z.; Al-Jumaili, A.A.; Al-Alag, Z.A.; Sorofman, B. Exploring the Acceptance of COVID-19 Vaccine among Healthcare Workers and General Population Using Health Belief Model. J. Eval. Clin. Pract. 2021, 27, 1112–1122. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Qi, F.; Lv, Q.; Song, Z.; Liu, J.; Gao, H.; Wei, Q.; Yu, P.; Xu, Y.; et al. Sequential Infection with H1N1 and SARS-CoV-2 Aggravated COVID-19 Pathogenesis in a Mammalian Model, and Co-Vaccination as an Effective Method of Prevention of COVID-19 and Influenza. Signal Transduct. Target. Ther. 2021, 6, 200. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.Z.; Sakamuru, S.; Zhao, J.; Ngan, D.K.; Simeonov, A.; Hall, M.D.; Xia, M.; Zheng, W.; Huang, R. Mining of High Throughput Screening Database Reveals AP-1 and Autophagy Pathways as Potential Targets for COVID-19 Therapeutics. Sci. Rep. 2021, 11, 6725. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mongia, A.; Saha, S.K.; Chouzenoux, E.; Majumdar, A. A Computational Approach to Aid Clinicians in Selecting Anti-Viral Drugs for COVID-19 Trials. Sci. Rep. 2021, 11, 9047. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Owusu, P.A. Impact of COVID-19 pandemic on waste management. Environ. Dev. Sustain. 2021, 23, 7951–7960. [Google Scholar] [CrossRef]
- Kumar, V.M.; Pandi-Perumal, S.R.; Trakht, I.; Thyagarajan, S.P. Strategy for COVID-19 Vaccination in India: The Country with the Second Highest Population and Number of Cases. NPJ Vaccines 2021, 6, 60. [Google Scholar] [CrossRef]
- Kashte, S.; Gulbake, A.; El-Amin, S.F., III; Gupta, A. COVID-19 Vaccines: Rapid Development, Implications, Challenges and Future Prospects. Hum. Cell 2021, 34, 711. [Google Scholar] [CrossRef]
- Yadav, P.R.; Han, T.; Olatunji, O.; Pattanayek, S.K. Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress. Pharmaceutics 2020, 12, 693. [Google Scholar] [CrossRef]
- Kuwentrai, C.; Yu, J.; Rong, L.; Zhang, B.; Hu, Y.; Gong, H.; Dou, Y.; Deng, J.; Huang, J.; Xu, C. Intradermal Delivery of Receptor-binding Domain of SARS-CoV-2 Spike Protein with Dissolvable Microneedles to Induce Humoral and Cellular Responses in Mice. Bioeng. Transl. Med. 2021, 6, e10202. [Google Scholar] [CrossRef]
- Kiem, C.T.; Massonnaud, C.; Levy-Bruhl, D.; Poletto, C.; Colizza, V.; Bosetti, P.; Fontanet, A.; Gabet, A.; Olie, V.; Zanetti, L. Short and Medium-Term Challenges for COVID-19 Vaccination: From Prioritisation to the Relaxation of Measures. eClinicalMedicine 2021, 38, 101001. [Google Scholar] [CrossRef] [PubMed]
- Campi, G.; Mazziotti, M.V.; Valletta, A.; Ravagnan, G.; Marcelli, A.; Perali, A.; Bianconi, A. Metastable States in Plateaus and Multi-Wave Epidemic Dynamics of COVID-19 Spreading in Italy. Sci. Rep. 2021, 11, 12412. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Brown, E.S.; Cheeseman, H.M.; Flight, K.E.; Higham, S.L.; Lemm, N.M.; Pierce, B.F.; Stirling, D.C.; Wang, Z.; Pollock, K.M. Vaccines for COVID-19. Clin. Exp. Immunol. 2020, 202, 162–192. [Google Scholar] [CrossRef] [PubMed]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.; Fernandes, A.P.S.; Frézard, F.; Moore, A.C. A TLR9-Adjuvanted Vaccine Formulated into Dissolvable Microneedle Patches or Cationic Liposomes Protects against Leishmaniasis after Skin or Subcutaneous Immunization. Int. J. Pharm. 2020, 586, 119390. [Google Scholar] [CrossRef]
- Antunez Muiños, P.J.; López Otero, D.; Amat-Santos, I.J.; López País, J.; Aparisi, A.; Cacho Antonio, C.E.; Catalá, P.; González Ferrero, T.; Cabezón, G.; Otero García, O.; et al. The COVID-19 Lab Score: An Accurate Dynamic Tool to Predict in-Hospital Outcomes in COVID-19 Patients. Sci. Rep. 2021, 11, 9361. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A Global Survey of Potential Acceptance of a COVID-19 Vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Castanon, A.; Rebolj, M.; Pesola, F.; Sasieni, P. Recovery Strategies Following COVID-19 Disruption to Cervical Cancer Screening and Their Impact on Excess Diagnoses. Br. J. Cancer 2021, 124, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, A.V.; Modi, N.; de Wildt, S.N.; Aurich, B.; Bakhtadze, S.; Sirvent, F.J.B.; Cabañas, F.; Campbell, L.; Casanova, M.; Charlton, P.; et al. Improving clinical paediatric research and learning from COVID-19: Recommendations by the Conect4Children expert advice group. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Razai, M.S.; Oakeshott, P.; Esmail, A.; Wiysonge, C.S.; Viswanath, K.; Mills, M.C. COVID-19 Vaccine Hesitancy: The Five Cs to Tackle Behavioural and Sociodemographic Factors. J. R. Soc. Med. 2021, 114, 295–298. [Google Scholar] [CrossRef]
- Longchamps, C.; Ducarroz, S.; Crouzet, L.; Vignier, N.; Pourtau, L.; Allaire, C.; Colleville, A.C.; El Aarbaoui, T.; Melchior, M. COVID-19 Vaccine Hesitancy among Persons Living in Homeless Shelters in France. Vaccine 2021, 39, 3315–3318. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in Ensuring Global Access to COVID-19 Vaccines: Production, Affordability, Allocation, and Deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Wibawa, T. COVID-19 Vaccine Research and Development: Ethical Issues. Trop. Med. Int. Health 2021, 26, 14–19. [Google Scholar] [CrossRef]
- Marian, A.J. Current state of vaccine development and targeted therapies for COVID-19: Impact of basic science discoveries. Cardiovasc. Pathol. 2021, 50, 107278. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of MRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 Vaccine: Where Are We Now and Where Should We Go? Expert Rev. Vaccines 2021, 20, 23–44. [Google Scholar] [CrossRef]
- Al-kassmy, J.; Pedersen, J.; Kobinger, G. Vaccine Candidates against Coronavirus Infections. Viruses 2020, 12, 861. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 Randomized Trial of a Plant-Derived Virus-like Particle Vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Santos-Beneit, F.; Raškevičius, V.; Skeberdis, V.A.; Bordel, S. A Metabolic Modeling Approach Reveals Promising Therapeutic Targets and Antiviral Drugs to Combat COVID-19. Sci. Rep. 2021, 11, 11982. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Seneviratne, C.J.; Fakhruddin, K.S. Coronavirus disease 2019 (COVID-19) vaccines: A concise review. Oral Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Yadav, T.; Srivastava, N.; Mishra, G.; Dhama, K.; Kumar, S.; Puri, B.; Saxena, S.K. Recombinant Vaccines for COVID-19. Hum. Vaccines Immunother. 2020, 16, 2905–2912. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; He, F.; Souho, T.; Lamboni, L.; Ullah, M.W.; Boni, B.O.; Ahmed, A.A.Q.; Mukole, B.M.; Yang, G. Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine. Biomed. Pharmacother. 2021, 133, 111008. [Google Scholar] [CrossRef]
- Bøhler, A.D.; Strøm, M.E.; Sandvig, K.U.; Moe, M.C.; Jørstad, Ø.K. Acute Macular Neuroretinopathy Following COVID-19 Vaccination. Eye 2021, 36, 644–645. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine Release Syndrome in a Patient with Colorectal Cancer after Vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Holzworth, A.; Couchot, P.; Cruz-Knight, W.; Brucculeri, M. Minimal Change Disease Following the Moderna MRNA-1273 SARS-CoV-2 Vaccine. Kidney Int. 2021, 100, 463. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Gethings, O.; Vihta, K.D.; Jones, J.; House, T.; VanSteenHouse, H.; Bell, I.; et al. Impact of Vaccination on New SARS-CoV-2 Infections in the United Kingdom. Nat. Med. 2021, 27, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.J.; Psevdos, G. Asymptomatic SARS-CoV-2 Infection Following First Dose MRNA-1273 COVID-19 Vaccine in a Veterans Affairs Long Term Care Facility. Am. J. Infect. Control 2021, 49, 1210–1211. [Google Scholar] [CrossRef]
- Adetifa, I.M.O.; Uyoga, S.; Gitonga, J.N.; Mugo, D.; Otiende, M.; Nyagwange, J.; Karanja, H.K.; Tuju, J.; Wanjiku, P.; Aman, R.; et al. Temporal Trends of SARS-CoV-2 Seroprevalence during the First Wave of the COVID-19 Epidemic in Kenya. Nat. Commun. 2021, 12, 3966. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 Variant of Concern 202012/01 (B.1.1.7): An Exploratory Analysis of a Randomised Controlled Trial. Lancet 2021, 397, 1351. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38. [Google Scholar] [CrossRef]
- Kukar, M.; Gunčar, G.; Vovko, T.; Podnar, S.; Černelč, P.; Brvar, M.; Zalaznik, M.; Notar, M.; Moškon, S.; Notar, M. COVID-19 Diagnosis by Routine Blood Tests Using Machine Learning. Sci. Rep. 2021, 11, 10738. [Google Scholar] [CrossRef]
- Ye, T.; Zhong, Z.; García-Sastre, A.; Schotsaert, M.; De Geest, B.G. Current Status of COVID-19 (Pre)Clinical Vaccine Development. Angew. Chem.-Int. Ed. 2020, 59, 18885–18897. [Google Scholar] [CrossRef]
- Bost, P.; De Sanctis, F.; Canè, S.; Ugel, S.; Donadello, K.; Castellucci, M.; Eyal, D.; Fiore, A.; Anselmi, C.; Barouni, R.M.; et al. Deciphering the State of Immune Silence in Fatal COVID-19 Patients. Nat. Commun. 2021, 12, 1428. [Google Scholar] [CrossRef]
- Otu, A.; Osifo-Dawodu, E.; Atuhebwe, P.; Agogo, E.; Ebenso, B. Beyond Vaccine Hesitancy: Time for Africa to Expand Vaccine Manufacturing Capacity amidst Growing COVID-19 Vaccine Nationalism. Lancet Microbe 2021, 2, e347–e348. [Google Scholar] [CrossRef]
- Huang, K.; Lin, S.W.; Sheng, W.H.; Wang, C.C. Influenza Vaccination and the Risk of COVID-19 Infection and Severe Illness in Older Adults in the United States. Sci. Rep. 2021, 11, 11025. [Google Scholar] [CrossRef]
- Galindez, G.; Matschinske, J.; Rose, T.D.; Sadegh, S.; Salgado-Albarrán, M.; Späth, J.; Baumbach, J.; Pauling, J.K. Lessons from the COVID-19 Pandemic for Advancing Computational Drug Repurposing Strategies. Nat. Comput. Sci. 2021, 1, 33–41. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Safe and Proper Sharps Disposal during the COVID-19 Mass Vaccination Campaign | CDC. Available online: https://www.cdc.gov/vaccines/covid-19/training-education/safe-proper-sharps-disposal.html (accessed on 12 April 2021).
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Chen, J.; Dai, L.; Barrett, L.; James, J.; Plaisance-Bonstaff, K.; Post, S.R.; Qin, Z. SARS-CoV-2 Proteins and Anti-COVID-19 Drugs Induce Lytic Reactivation of an Oncogenic Virus. Commun. Biol. 2021, 4, 2–7. [Google Scholar] [CrossRef]
- Kwon, S.; Joshi, A.D.; Lo, C.H.; Drew, D.A.; Nguyen, L.H.; Guo, C.G.; Ma, W.; Mehta, R.S.; Shebl, F.M.; Warner, E.T.; et al. Association of Social Distancing and Face Mask Use with Risk of COVID-19. Nat. Commun. 2021, 12, 3737. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Nemoto, K.; Matsumoto, S.; Nakamura, Y.; Kiyotani, K. SARS-CoV-2 Genomic Variations Associated with Mortality Rate of COVID-19. J. Hum. Genet. 2020, 65, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A Materials-Science Perspective on Tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Baker, M.G.; Blakely, T.; Eichner, M. Estimating the Impact of Control Measures to Prevent Outbreaks of COVID-19 Associated with Air Travel into a COVID-19-Free Country. Sci. Rep. 2021, 11, 10766. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S. Face Mask and Medical Waste Disposal during the Novel COVID-19 Pandemic in Asia. Case Stud. Chem. Environ. Eng. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition and Immunity: Lessons for COVID-19. Eur. J. Clin. Nutr. 2021, 75, 1309–1318. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Imami, A.; Eby, H.; Henkel, N.D.; Creeden, J.F.; Asah, S.; Zhang, X.; Wu, X.; Alnafisah, R.; Taylor, R.T.; et al. Identification of Candidate Repurposable Drugs to Combat COVID-19 Using a Signature-Based Approach. Sci. Rep. 2021, 11, 4495. [Google Scholar] [CrossRef]
- Ramakanth, D.; Singh, S.; Maji, P.K.; Lee, Y.S.; Gaikwad, K.K. Advanced Packaging for Distribution and Storage of COVID-19 Vaccines: A Review. Environ. Chem. Lett. 2021, 19, 3597–3608. [Google Scholar] [CrossRef]
- Wedlund, L.; Kvedar, J. New Machine Learning Model Predicts Who May Benefit Most from COVID-19 Vaccination. npj Digit. Med. 2021, 4, 2021. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological Considerations for COVID-19 Vaccine Strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Cannistraci, C.V.; Valsecchi, M.G.; Capua, I. Age-Sex Population Adjusted Analysis of Disease Severity in Epidemics as a Tool to Devise Public Health Policies for COVID-19. Sci. Rep. 2021, 11, 11787. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological Characteristics Associated with COVID-19 Vaccine Hesitancy and Resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef]
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O’Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory Analysis of Immunization Records Highlights Decreased SARS-CoV-2 Rates in Individuals with Recent Non-COVID-19 Vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef]
- Wu, J.; Liang, B.; Chen, C.; Wang, H.; Fang, Y.; Shen, S.; Yang, X.; Wang, B.; Chen, L.; Chen, Q.; et al. SARS-CoV-2 Infection Induces Sustained Humoral Immune Responses in Convalescent Patients Following Symptomatic COVID-19. Nat. Commun. 2021, 12, 1813. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.Y. A High-Throughput Neutralizing Antibody Assay for COVID-19 Diagnosis and Vaccine Evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H. Latest Updates on COVID-19 Vaccines. Biosci. Trends 2020, 14, 463–466. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a Prospective Cohort of Home-Isolated Patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Haynes, B.F.; Corey, L.; Fernandes, P.; Gilbert, P.B.; Hotez, P.J.; Rao, S.; Santos, M.R.; Schuitemaker, H.; Watson, M.; Arvin, A. Prospects for a Safe COVID-19 Vaccine. Sci. Transl. Med. 2020, 12, eabe0948. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar Sharma, S.; Shi, Y.; Bucci, E.; Carafoli, E.; Melino, G.; Bhattacherjee, A.; Das, G. BCG Vaccination Policy and Preventive Chloroquine Usage: Do They Have an Impact on COVID-19 Pandemic? Cell Death Dis. 2020, 11, 516. [Google Scholar] [CrossRef]
- Vanderbeke, L.; Van Mol, P.; Van Herck, Y.; De Smet, F.; Humblet-Baron, S.; Martinod, K.; Antoranz, A.; Arijs, I.; Boeckx, B.; Bosisio, F.M.; et al. Monocyte-Driven Atypical Cytokine Storm and Aberrant Neutrophil Activation as Key Mediators of COVID-19 Disease Severity. Nat. Commun. 2021, 12, 4117. [Google Scholar] [CrossRef]
- Silva, J.; Bratberg, J.; Lemay, V. COVID-19 and Influenza Vaccine Hesitancy among College Students. J. Am. Pharm. Assoc. 2021, 61, 709–714.e1. [Google Scholar] [CrossRef]
- Nisa, C.F.; Bélanger, J.J.; Faller, D.G.; Buttrick, N.R.; Mierau, J.O.; Austin, M.M.K.; Schumpe, B.M.; Sasin, E.M.; Agostini, M.; Gützkow, B.; et al. Lives versus Livelihoods? Perceived Economic Risk Has a Stronger Association with Support for COVID-19 Preventive Measures than Perceived Health Risk. Sci. Rep. 2021, 11, 9669. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021, 892, 173751. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.; Earnest-Silveira, L.; Grubor-Bauk, B.; Wijesundara, D.K.; Boo, I.; Ramsland, P.A.; Vincan, E.; Drummer, H.E.; Gowans, E.J.; Torresi, J. Pre-Clinical Evaluation of a Quadrivalent HCV VLP Vaccine in Pigs Following Microneedle Delivery. Sci. Rep. 2019, 9, 9251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulit-Penaloza, J.A.; Sapkota, B.; Stein Esser, E.; Compans, R.W.; Pollack, B.P.; Skountzou, I. Modulation of Influenza Vaccine Immune Responses Using an Epidermal Growth Factor Receptor Kinase Inhibitor. Sci. Rep. 2015, 5, 12321. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zhu, W.; Wang, B.Z. Influenza Vaccines toward Universality through Nanoplatforms and given by Microneedle Patches. Viruses 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.V.; Lau, W.M.; Moghimi, S.M.; Ng, K.W. The Diagnostic Potential of Microneedles in Infectious Diseases. Precis. Nanomed. 2020, 3, 629–640. [Google Scholar] [CrossRef]
- Plamadeala, C.; Gosain, S.R.; Hischen, F.; Buchroithner, B.; Puthukodan, S.; Jacak, J.; Bocchino, A.; Whelan, D.; O’Mahony, C.; Baumgartner, W.; et al. Bio-Inspired Microneedle Design for Efficient Drug/Vaccine Coating. Biomed. Microdevices 2020, 22, 8. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly Dissolving Polymeric Microneedles for Minimally Invasive Intraocular Drug Delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef] [Green Version]
- Staples, M.; Daniel, K.; Cima, M.J.; Langer, R. Application of Micro- and Nano-Electromechanical Devices to Drug Delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.M.; Kim, J.H.; Ryu, W. Impact Insertion of Transfer-Molded Microneedle for Localized and Minimally Invasive Ocular Drug Delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef]
- Onesto, V.; Di Natale, C.; Profeta, M.; Netti, P.A.; Vecchione, R. Engineered PLGA-PVP/VA Based Formulations to Produce Electro-Drawn Fast Biodegradable Microneedles for Labile Biomolecule Delivery. Prog. Biomater. 2020, 9, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent Advances of Microneedles for Biomedical Applications: Drug Delivery and Beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Chew, S.W.T.; Xu, C. Advances in the Formulations of Microneedles for Manifold Biomedical Applications. Adv. Mater. Technol. 2020, 5, 1900552. [Google Scholar] [CrossRef]
- Jung, J.H.; Jin, S.G. Microneedle for Transdermal Drug Delivery: Current Trends and Fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef]
- Bok, M.; Zhao, Z.J.; Jeon, S.; Jeong, J.H.; Lim, E. Ultrasonically and Iontophoretically Enhanced Drug-Delivery System Based on Dissolving Microneedle Patches. Sci. Rep. 2020, 10, 2027. [Google Scholar] [CrossRef]
- Niu, L.; Chu, L.Y.; Burton, S.A.; Hansen, K.J.; Panyam, J. Intradermal Delivery of Vaccine Nanoparticles Using Hollow Microneedle Array Generates Enhanced and Balanced Immune Response. J. Control. Release 2019, 294, 268–278. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
- Meng, F.; Hasan, A.; Mahdi Nejadi Babadaei, M.; Hashemi Kani, P.; Jouya Talaei, A.; Sharifi, M.; Cai, T.; Falahati, M.; Cai, Y. Polymeric-Based Microneedle Arrays as Potential Platforms in the Development of Drugs Delivery Systems. J. Adv. Res. 2020, 26, 137–147. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Tekko, I.A.; Jomaa, M.H.; Vora, L.; McAlister, E.; Volpe-Zanutto, F.; Nethery, M.; Baine, P.T.; Mitchell, N.; McNeill, D.W.; et al. Two-Photon Polymerisation 3D Printing of Microneedle Array Templates with Versatile Designs: Application in the Development of Polymeric Drug Delivery Systems. Pharm. Res. 2020, 37, 174. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Henricson, J.; Baker, S.B.; Togö, T.; Jayashi Flores, C.M.; Lemaire, P.A.; Forster, A.; Anderson, C.D. Innate Local Response and Tissue Recovery Following Application of High Density Microarray Patches to Human Skin. Sci. Rep. 2020, 10, 18468. [Google Scholar] [CrossRef]
- Xie, L.; Zeng, H.; Sun, J.; Qian, W. Engineering Microneedles for Therapy and Diagnosis: A Survey. Micromachines 2020, 11, 271. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Bonfante, G.; Sasaki, Y.; Takama, N.; Minami, T.; Kim, B. Porous Microneedles on a Paper for Screening Test of Prediabetes. Med. Devices Sens. 2020, 3, e10109. [Google Scholar] [CrossRef]
- Ullah, A.; Choi, H.J.; Jang, M.; An, S.; Kim, G.M. Smart Microneedles with Porous Polymer Layer for Glucose-Responsive Insulin Delivery. Pharmaceutics 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Makvandi, P.; Yiu, C.K.Y.; Agarwal, T.; Vecchione, R.; Sun, W.; Maiti, T.K.; Tay, F.R.; Netti, P.A. Engineered Microneedle Patches for Controlled Release of Active Compounds: Recent Advances in Release Profile Tuning. Adv. Ther. 2020, 3, 2000171. [Google Scholar] [CrossRef]
- Yeung, C.; Chen, S.; King, B.; Lin, H.; King, K.; Akhtar, F.; Diaz, G.; Wang, B.; Zhu, J.; Sun, W.; et al. A 3D-Printed Microfluidic-Enabled Hollow Microneedle Architecture for Transdermal Drug Delivery. Biomicrofluidics 2019, 13, 064125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle Arrays as Transdermal and Intradermal Drug Delivery Systems: Materials Science, Manufacture and Commercial Development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent Advances in Microneedle Composites for Biomedical Applications: Advanced Drug Delivery Technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Kalluri, H.; McAllister, D.; Taherbhai, M.T.; Esser, E.S.; Pewin, W.P.; Pulit-Penaloza, J.A.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Improved Immunogenicity of Individual Influenza Vaccine Components Delivered with a Novel Dissolving Microneedle Patch Stable at Room Temperature. Drug Deliv. Transl. Res. 2015, 5, 360–371. [Google Scholar] [CrossRef]
- Chang, L.; Wang, Y.C.; Ershad, F.; Yang, R.; Yu, C.; Fan, Y. Wearable Devices for Single-Cell Sensing and Transfection. Trends Biotechnol. 2019, 37, 1175–1188. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat Photopolymerization 3D Printing for Advanced Drug Delivery and Medical Device Applications. J. Control. Release 2020, 329, 743–757. [Google Scholar] [CrossRef]
- Lee, Y.; Dugansani, S.R.; Jeon, S.H.; Hwang, S.H.; Kim, J.H.; Park, S.H.; Jeong, J.H. Drug-Delivery System Based on Salmon DNA Nano- and Micro-Scale Structures. Sci. Rep. 2017, 7, 9724. [Google Scholar] [CrossRef] [Green Version]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhang, X.P.; Yu, H.L.; Liu, R.X.; Shen, C.B.; Zhang, W.F.; Cui, Y.; Guo, X.D. Kinetic Stability Studies of HBV Vaccine in a Microneedle Patch. Int. J. Pharm. 2019, 567, 118489. [Google Scholar] [CrossRef]
- Sarabi, M.R.; Ahmadpour, A.; Yetisen, A.K.; Tasoglu, S. Finger-Actuated Microneedle Array for Sampling Body Fluids. Appl. Sci. 2021, 11, 5329. [Google Scholar] [CrossRef]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.; Zare, E.; Makvandi, P.; Vecchione, R.; Netti, P. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Takahashi, T.; Aoyagi, S. Fabrication and Characterization of a Biodegradable Hollow Microneedle from Chitosan. J. Robot. Mechatron. 2020, 32, 401–407. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R. Novel Delivery Systems for Transdermal and Intradermal Drug Delivery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; ISBN 9781118734506. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, M.; Kharat, P.; Thakor, P.; Bhavana, V.; Singh, S.B.; Mehra, N.K. Panorama of Dissolving Microneedles for Transdermal Drug Delivery. Life Sci. 2021, 284, 119877. [Google Scholar] [CrossRef]
- Zhu, W.; Li, S.; Wang, C.; Yu, G.; Prausnitz, M.R.; Wang, B.-Z. Enhanced Immune Responses Conferring Cross-Protection by Skin Vaccination With a Tri-Component Influenza Vaccine Using a Microneedle Patch. Front. Immunol. 2018, 9, 1705. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The Safety, Immunogenicity, and Acceptability of Inactivated Influenza Vaccine Delivered by Microneedle Patch (TIV-MNP 2015): A Randomised, Partly Blinded, Placebo-Controlled, Phase 1 Trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Zaric, M.; Becker, P.D.; Hervouet, C.; Kalcheva, P.; Yus, B.I.; Cocita, C.; O’Neill, L.A.; Kwon, S.-Y.; Klavinskis, L.S. Long-Lived Tissue Resident HIV-1 Specific Memory CD8+ T Cells Are Generated by Skin Immunization with Live Virus Vectored Microneedle Arrays. J. Control. Release 2017, 268, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latanova, A.A.; Petkov, S.; Kilpelainen, A.; Jansons, J.; Latyshev, O.E.; Kuzmenko, Y.V.; Hinkula, J.; Abakumov, M.A.; Valuev-Elliston, V.T.; Gomelsky, M.; et al. Codon Optimization and Improved Delivery/Immunization Regimen Enhance the Immune Response against Wild-Type and Drug-Resistant HIV-1 Reverse Transcriptase, Preserving Its Th2-Polarity. Sci. Rep. 2018, 8, 8078. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, L.; Lin, F.; Gomaa, Y.; Flyer, D.; Carrion, R.; Patterson, J.L.; Prausnitz, M.R.; Smith, G.; Glenn, G.; et al. Intradermal Immunization by Ebola Virus GP Subunit Vaccines Using Microneedle Patches Protects Mice against Lethal EBOV Challenge. Sci. Rep. 2018, 8, 11193. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Ye, L.; Guo, X.D.; Yang, C.; Compans, R.W.; Prausnitz, M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/ΓPGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017, 6, 1600750. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef] [PubMed]
- Hensel, J.; McAndrews, K.M.; McGrail, D.J.; Dowlatshahi, D.P.; LeBleu, V.S.; Kalluri, R. Protection against SARS-CoV-2 by BCG Vaccination Is Not Supported by Epidemiological Analyses. Sci. Rep. 2020, 10, 18377. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An Emerging Transdermal Drug Delivery System. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and Biodegradable Microneedle Technologies for Transdermal Sustained Delivery of Drug and Vaccine. Drug Des. Devel. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Luo, G.; Xing, M. Biomedical Applications of Polymeric Microneedles for Transdermal Therapeutic Delivery and Diagnosis: Current Status and Future Perspectives. Adv. Ther. 2020, 3, 1900140. [Google Scholar] [CrossRef]
- Matsuo, K.; Hirobe, S.; Yokota, Y.; Ayabe, Y.; Seto, M.; Quan, Y.-S.; Kamiyama, F.; Tougan, T.; Horii, T.; Mukai, Y.; et al. Transcutaneous Immunization Using a Dissolving Microneedle Array Protects against Tetanus, Diphtheria, Malaria, and Influenza. J. Control. Release 2012, 160, 495–501. [Google Scholar] [CrossRef]
- Chen, F.; Yan, Q.; Yu, Y.; Wu, M.X. BCG Vaccine Powder-Laden and Dissolvable Microneedle Arrays for Lesion-Free Vaccination. J. Control. Release 2017, 255, 36–44. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, L.; Zhang, S.; Xu, B.; Gao, Y.; Hu, Y.; Hou, J.; Bai, B.; Shen, H.; Mao, P. DNA-Based Vaccination against Hepatitis B Virus Using Dissolving Microneedle Arrays Adjuvanted by Cationic Liposomes and CpG ODN. Drug Deliv. 2016, 23, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B Surface Antigen Incorporated in Dissolvable Microneedle Array Patch Is Antigenic and Thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef]
- Cuevas, M.B.P.; Kodani, M.; Choi, Y.; Joyce, J.; O’Connor, S.M.; Kamili, S.; Prausnitz, M.R. Hepatitis B Vaccination Using a Dissolvable Microneedle Patch Is Immunogenic in Mice and Rhesus Macaques. Bioeng. Transl. Med. 2018, 3, 186–196. [Google Scholar] [CrossRef]
- Edens, C.; Dybdahl-Sissoko, N.C.; Weldon, W.C.; Oberste, M.S.; Prausnitz, M.R. Inactivated Polio Vaccination Using a Microneedle Patch Is Immunogenic in the Rhesus Macaque. Vaccine 2015, 33, 4683–4690. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.A.; Fernando, G.J.P.; Owens, N.S.; Agyei-Yeboah, C.; Wei, J.C.J.; Depelsenaire, A.C.I.; Forster, A.; Fahey, P.; Weldon, W.C.; Oberste, M.S.; et al. High-Density Microprojection Array Delivery to Rat Skin of Low Doses of Trivalent Inactivated Poliovirus Vaccine Elicits Potent Neutralising Antibody Responses. Sci. Rep. 2017, 7, 12644. [Google Scholar] [CrossRef]
- Esser, E.S.; Romanyuk, A.; Vassilieva, E.V.; Jacob, J.; Prausnitz, M.; Compans, R.W.; Skountzou, I. Tetanus Vaccination with a Dissolving Microneedle Patch Confers Protective Immune Responses in Pregnancy. J. Control. Release 2016, 236, 47–56. [Google Scholar] [CrossRef]
- De Swart, R.L.; De Vries, R.D.; Rennick, L.J.; Van Amerongen, G.; McQuaid, S.; Verburgh, R.J.; Yüksel, S.; De Jong, A.; Lemon, K.; Nguyen, D.T.; et al. Needle-Free Delivery of Measles Virus Vaccine to the Lower Respiratory Tract of Non-Human Primates Elicits Optimal Immunity and Protection. NPJ Vaccines 2017, 2, 22. [Google Scholar] [CrossRef]
- Edens, C.; Collins, M.L.; Ayers, J.; Rota, P.A.; Prausnitz, M.R. Measles Vaccination Using a Microneedle Patch. Vaccine 2013, 31, 3403–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the Clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Wang, K.; Ross Ethier, C.; Prausnitz, M.R. Clearance Kinetics and Clearance Routes of Molecules from the Suprachoroidal Space after Microneedle Injection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Lillehoj, P.B. Microneedle-Based Skin Patch for Blood-Free Rapid Diagnostic Testing. Microsyst. Nanoeng. 2020, 6, 96. [Google Scholar] [CrossRef]
- Rad, Z.F.; Nordon, R.E.; Anthony, C.J.; Bilston, L.; Prewett, P.D.; Arns, J.Y.; Arns, C.H.; Zhang, L.; Davies, G.J. High-Fidelity Replication of Thermoplastic Microneedles with Open Microfluidic Channels. Microsyst. Nanoeng. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, J.; Lee, C. A Flexible Three-Dimensional Electrode Mesh: An Enabling Technology for Wireless Brain–Computer Interface Prostheses. Microsyst. Nanoeng. 2016, 2, 16012. [Google Scholar] [CrossRef]
- Pireddu, R.; Schlich, M.; Marceddu, S.; Valenti, D.; Pini, E.; Fadda, A.M.; Lai, F.; Sinico, C. Nanosuspensions and Microneedles Roller as a Combined Approach to Enhance Diclofenac Topical Bioavailability. Pharmaceutics 2020, 12, 1140. [Google Scholar] [CrossRef]
- Lutton, R.E.M.; Moore, J.; Larrañeta, E.; Ligett, S.; Woolfson, A.D.; Donnelly, R.F. Microneedle Characterisation: The Need for Universal Acceptance Criteria and GMP Specifications When Moving towards Commercialisation. Drug Deliv. Transl. Res. 2015, 5, 313–331. [Google Scholar] [CrossRef] [Green Version]
- Rezaei Nejad, H.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-Cost and Cleanroom-Free Fabrication of Microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Lahiji, S.F.; Dangol, M.; Jung, H. A Patchless Dissolving Microneedle Delivery System Enabling Rapid and Efficient Transdermal Drug Delivery. Sci. Rep. 2015, 5, 7914. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Balmert, S.C.; Carey, C.D.; Falo, G.D.; Sethi, S.K.; Erdos, G.; Korkmaz, E.; Falo, L.D. Dissolving Undercut Microneedle Arrays for Multicomponent Cutaneous Vaccination. J. Control. Release 2020, 317, 336–346. [Google Scholar] [CrossRef]
- Leone, M.; Mönkäre, J.; Bouwstra, J.A.; Kersten, G. Dissolving Microneedle Patches for Dermal Vaccination. Pharm. Res. 2017, 34, 2223–2240. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of Flexible Microneedle Array Electrodes for Wearable Bio-Signal Recording. Sensors 2018, 18, 1191. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, U.; Ning, S.; Wang, Y.; Kong, Y.L. Addressing Unmet Clinical Needs with 3D Printing Technologies. Adv. Healthc. Mater. 2018, 7, e1800417. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.Y.; Lee, S.G.; Park, S.H.; Yang, D.S.; Lee, J.J.; Khademhosseini, A.; Kim, J.S.; Ryu, W.H. Microneedle Drug Eluting Balloon for Enhanced Drug Delivery to Vascular Tissue. J. Control. Release 2020, 321, 174–183. [Google Scholar] [CrossRef]
- Satti, A.T.; Park, J.; Park, J.; Kim, H.; Cho, S. Fabrication of Parylene-Coated Microneedle Array Electrode for Wearable ECG Device. Sensors 2020, 20, 5183. [Google Scholar] [CrossRef]
- Tekko, I.A.; Raj Singh, T.R. Microneedles for Ocular Drug Delivery and Targeting: Challenges and Opportunities. In Microneedles for Drug and Vaccine Delivery and Patient Monitoring; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2018; pp. 283–306. [Google Scholar] [CrossRef]
- Maqsood, M.; Maqsood, H.; Kousar, R.; Jabeen, C.; Waqas, A.; Gillani, S. Effects of Hospital Service Quality on Patients Satisfaction and Behavioural Intention of Doctors and Nurses. Saudi J. Med. Pharm. Sci 2017, 3, 772–776. [Google Scholar] [CrossRef]
- Kusama, S.; Sato, K.; Matsui, Y.; Kimura, N.; Abe, H.; Yoshida, S.; Nishizawa, M. Transdermal Electroosmotic Flow Generated by a Porous Microneedle Array Patch. Nat. Commun. 2021, 12, 658. [Google Scholar] [CrossRef]
- Lee, K.J.; Goudie, M.J.; Tebon, P.; Sun, W.; Luo, Z.; Lee, J.; Zhang, S.; Fetah, K.; Kim, H.J.; Xue, Y.; et al. Non-Transdermal Microneedles for Advanced Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 41–59. [Google Scholar] [CrossRef]
- Jung, C.R.; Lahiji, S.F.; Jung, H.; Kim, Y.; Kim, H. Rapidly Separable Micropillar Integrated Dissolving Microneedles. Pharmaceutics 2020, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, S.N.; Federspiel, W.J.; Little, S.R. A simple model framework for the prediction of controlled release from bulk eroding polymer matrices. J. Mater. Chem. 2008, 16, 1873–1880. [Google Scholar] [CrossRef]
- Langer, R.S.; Peppas, N.A. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 Vaccine Development and a Potential Nanomaterial Path Forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Galigama, R.D.; Thorat, V.S.; Garg, P.; Venuganti, V.V.K. Microneedle Ocular Patch: Fabrication, Characterization, and Ex-Vivo Evaluation Using Pilocarpine as Model Drug. Drug Dev. Ind. Pharm. 2020, 46, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Yang, J.; Li, Y.; Zheng, Y.; Yang, J.; Li, Y.; Liu, B.; Jiang, L. Fabrication of Tip-Hollow and Tip-Dissolvable Microneedle Arrays for Transdermal Drug Delivery. ACS Biomater. Sci. Eng. 2020, 6, 2487–2494. [Google Scholar] [CrossRef]
- Yuzhakov, V.V. Microneedle Array, Patch, and Applicator for Transdermal Drug Delivery. U.S. Patent 7658728 B2, 9 February 2010. [Google Scholar]
- Dardano, P.; De Martino, S.; Battisti, M.; Miranda, B.; Rea, I.; De Stefano, L. One-Shot Fabrication of Polymeric Hollow Microneedles by Standard Photolithography. Polymers 2021, 13, 520. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Ye, R.; Zheng, Y.; Li, X.; Chen, Y.; Xie, X.; Jiang, L. Smartphone-Powered Iontophoresis-Microneedle Array Patch for Controlled Transdermal Delivery. Microsyst. Nanoeng. 2020, 6, 112. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Cho, S. Porous Platinum Black-Coated Minimally Invasive Microneedles for Non-Enzymatic Continuous Glucose Monitoring in Interstitial Fluid. Nanomaterials 2021, 11, 37. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with Controlled Bubble Sizes and Drug Distributions for Efficient Transdermal Drug Delivery. Sci. Rep. 2016, 6, 38755. [Google Scholar] [CrossRef] [Green Version]
- Wen, F.; He, T.; Liu, H.; Chen, H.Y.; Zhang, T.; Lee, C. Advances in Chemical Sensing Technology for Enabling the Next-Generation Self-Sustainable Integrated Wearable System in the IoT Era. Nano Energy 2020, 78, 105155. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D Printed Microneedle Patches Using Stereolithography (SLA)for Intradermal Insulin Delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; Fallows, S.J.; McMillan, H.L.; Donnelly, R.F.; Jones, D.S. Microneedle-Mediated Intrascleral Delivery of in Situ Forming Thermoresponsive Implants for Sustained Ocular Drug Delivery. J. Pharm. Pharmacol. 2014, 66, 584–595. [Google Scholar] [CrossRef]
- Lutton, R.E.M.; Larrañeta, E.; Kearney, M.C.; Boyd, P.; Woolfson, A.D.; Donnelly, R.F. A Novel Scalable Manufacturing Process for the Production of Hydrogel-Forming Microneedle Arrays. Int. J. Pharm. 2015, 494, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Eum, J.; Kim, Y.; Um, D.J.; Shin, J.; Yang, H.; Jung, H. Solvent-Free Polycaprolactone Dissolving Microneedles Generated via the Thermal Melting Method for the Sustained Release of Capsaicin. Micromachines 2021, 12, 167. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Leone, M.; Romeijn, S.; Du, G.; Le Dévédec, S.; Vrieling, H.; O’Mahony, C.; Bouwstra, J.; Kersten, G. Diphtheria Toxoid Dissolving Microneedle Vaccination: Adjuvant Screening and Effect of Repeated-Fractional Dose Administration. Int. J. Pharm. 2020, 580, 119182. [Google Scholar] [CrossRef]
- Hirobe, S.; Azukizawa, H.; Matsuo, K.; Zhai, Y.; Quan, Y.-S.; Kamiyama, F.; Suzuki, H.; Katayama, I.; Okada, N.; Nakagawa, S. Development and Clinical Study of a Self-Dissolving Microneedle Patch for Transcutaneous Immunization Device. Pharm. Res. 2013, 30, 2664–2674. [Google Scholar] [CrossRef]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.J.; McCrory, J.; Evans, S.L.; Allender, C.J. Structural characteristics and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef]
- Ronnander, P.; Simon, L.; Koch, A. Experimental and mathematical study of the transdermal delivery of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur. J. Pharm. Biopharm. 2020, 146, 32–40. [Google Scholar] [CrossRef]
- Chavoshi, S.; Rabiee, M.; Rafizadeh, M.; Rabiee, N.; Shamsabadi, A.S.; Bagherzadeh, M.; Salarian, R.; Tahriri, M.; Tayebi, L. Mathematical modeling of drug release from biodegradable polymeric microneedles. Bio-Des. Manuf. 2019, 2, 96–107. [Google Scholar] [CrossRef]
- Ronnander, P.; Simon, L.; Spilgies, H.; Koch, A. Modelling the in-vitro dissolution and release of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur. J. Pharm. Sci. 2018, 125, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ita, K.; Simon, L. Modelling of dissolving microneedles for transdermal drug delivery: Theoretical and experimental aspects. Eur. J. Pharm. Sci. 2015, 68, 137–143. [Google Scholar] [CrossRef]
- Zoudani, E.L.; Soltani, M. A New Computational Method of Modeling and Evaluation of Dissolving Microneedle for Drug Delivery Applications: Extension to Theoretical Modeling of a Novel Design of Microneedle (Array in Array) for Efficient Drug Delivery. Eur. J. Pharm. Sci. 2020, 150, 105339. [Google Scholar] [CrossRef]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A Microneedle Patch Containing Measles Vaccine Is Immunogenic in Non-Human Primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.H.; Liu, J.L.; Zhu, D.D.; Hao, Y.Y.; Guo, X.D. Multiscale simulations of drug distributions in polymer dissolvable microneedles. Colloids Surf. B Biointerfaces 2020, 189, 110844. [Google Scholar] [CrossRef] [PubMed]
- Suriyaamporn, P.; Opanasopit, P.; Ngawhirunpat, T.; Rangsimawong, W. Computer-aided rational design for optimally Gantrez® S-97 and hyaluronic acid-based dissolving microneedles as a potential ocular delivery system. J. Drug Deliv. Sci. Technol. 2021, 61, 102319. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Moore, L.E.; Vucen, S.; Moore, A.C. Trends in Drug- and Vaccine-Based Dissolvable Microneedle Materials and Methods of Fabrication. Eur. J. Pharm. Biopharm. 2022, 173, 54–72. [Google Scholar] [CrossRef]
- Koyani, R.D. Synthetic Polymers for Microneedle Synthesis: From Then to Now. J. Drug Deliv. Sci. Technol. 2020, 60, 102071. [Google Scholar] [CrossRef]
- Amodwala, S.; Kumar, P.; Thakkar, H.P. Statistically Optimized Fast Dissolving Microneedle Transdermal Patch of Meloxicam: A Patient Friendly Approach to Manage Arthritis. Eur. J. Pharm. Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, D.; Liu, Y.; Huang, Y.; Xu, H.; Gao, W.; Zhang, J.; Sun, J.; Zhuang, J. Optimal scaling analysis of polymeric microneedle length and its effect on transdermal insulin delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101547. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, Y.A.; Morrow, D.I.J.; Garland, M.J.; Donnelly, R.F.; El-Khordagui, L.K.; Meidan, V.M. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: Assessments by transepidermal water loss. Toxicol. In Vitro 2010, 24, 1971–1978. [Google Scholar] [CrossRef]
- Wang, C.; Han, B.; Zhao, T.; Liu, H.; Liu, B.; Chen, L.; Xie, M.; Liu, J.; Zheng, H.; Zhang, S.; et al. Vaccination Willingness, Vaccine Hesitancy, and Estimated Coverage at the First Round of COVID-19 Vaccination in China: A National Cross-Sectional Study. Vaccine 2021, 39, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-Based Vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [CrossRef] [Green Version]
- Prausnitz, M.R.; Goodson, J.L.; Rota, P.A.; Orenstein, W.A. A Microneedle Patch for Measles and Rubella Vaccination: A Game Changer for Achieving Elimination. Curr. Opin. Virol. 2020, 41, 68–76. [Google Scholar] [CrossRef]
- Ellison, T.J.; Talbott, G.C.; Henderson, D.R. VaxiPatchTM, a Novel Vaccination System Comprised of Subunit Antigens, Adjuvants and Microneedle Skin Delivery: An Application to Influenza B/Colorado/06/2017. Vaccine 2020, 38, 6839–6848. [Google Scholar] [CrossRef]
- Van Der Maaden, K.; Varypataki, E.M.; Yu, H.; Romeijn, S.; Jiskoot, W.; Bouwstra, J. Parameter Optimization toward Optimal Microneedle-Based Dermal Vaccination. Eur. J. Pharm. Sci. 2014, 64, 18–25. [Google Scholar] [CrossRef]
- Arya, J.; Prausnitz, M.R. Microneedle Patches for Vaccination in Developing Countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef] [Green Version]
- O’Shea, J.; Prausnitz, M.R.; Rouphael, N. Dissolvable Microneedle Patches to Enable Increased Access to Vaccines against SARS-CoV-2 and Future Pandemic Outbreaks. Vaccines 2021, 9, 320. [Google Scholar] [CrossRef]
- Arya, J.M.; Dewitt, K.; Scott-Garrard, M.; Chiang, Y.-W.; Prausnitz, M.R. Rabies Vaccination in Dogs Using a Dissolving Microneedle Patch. J. Control. Release 2016, 239, 19–26. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, H.; Cheng, Z.; Xue, Y.; Cheng, Z.; Dai, X.; Shan, W.; Chen, F. Immunotherapeutic Effect of BCG-Polysaccharide Nucleic Acid Powder on Mycobacterium Tuberculosis-Infected Mice Using Microneedle Patches. Drug Deliv. 2017, 24, 1648–1653. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.-F.; Lee, J.-C.; Lin, C.-K.; Hung-Wei, Y.; Chen, P.-Y.; Yang, H.-W. Self-Assembly DNA Polyplex Vaccine inside Dissolving Microneedles for High-Potency Intradermal Vaccination. Theranostics 2017, 7, 2593–2605. [Google Scholar] [CrossRef]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.; Falo, L.D., Jr.; Gambotto, A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine 2016, 13, 315. [Google Scholar] [CrossRef] [Green Version]
- Becker, P.D.; Hervouet, C.; Mason, G.M.; Kwon, S.-Y.; Klavinskis, L.S. Skin Vaccination with Live Virus Vectored Microneedle Arrays Induce Long Lived CD8(+) T Cell Memory. Vaccine 2015, 33, 4691–4698. [Google Scholar] [CrossRef]
- Bachy, V.; Hervouet, C.; Becker, P.D.; Chorro, L.; Carlin, L.M.; Herath, S.; Papagatsias, T.; Barbaroux, J.-B.; Oh, S.-J.; Benlahrech, A.; et al. Langerin Negative Dendritic Cells Promote Potent CD8+ T-Cell Priming by Skin Delivery of Live Adenovirus Vaccine Microneedle Arrays. Proc. Natl. Acad. Sci. USA 2013, 110, 3041–3046. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle Array Delivered Recombinant Coronavirus Vaccines: Immunogenicity and Rapid Translational Development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef]
- Pattani, A.; McKay, P.; Garland, M.J.; Curran, R.M.; Migalska, K.; Cassidy, C.M.; Malcolm, K.; Shattock, R.J.; McCarthy, H.; Donnelly, R.F. Microneedle Mediated Intradermal Delivery of Adjuvanted Recombinant HIV-1 CN54gp140 Effectively Primes Mucosal Boost Inoculations. J. Control. Release 2012, 162, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Pewin, W.; Wang, C.; Luo, Y.; Gonzalez, G.X.; Mohan, T.; Prausnitz, M.; Wang, B.-Z. A Boosting Skin Vaccination with Dissolving Microneedle Patch Encapsulating M2e Vaccine Broadens the Protective Efficacy of Conventional Influenza Vaccines. J. Control. Release 2017, 261, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chang, T.Z.; Wang, Y.; Li, S.; Wang, S.; Matsuyama, S.; Yu, G.; Compans, R.W.; Li, J.-D.; Prausnitz, M.R.; et al. Heterosubtypic Influenza Protection Elicited by Double-Layered Polypeptide Nanoparticles in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, E7758–E7767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, E.A.; O’Mahony, C.; Cronin, M.; O’Mahony, T.; Moore, A.C.; Crean, A.M. Dissolvable Microneedle Fabrication Using Piezoelectric Dispensing Technology. Int. J. Pharm. 2016, 500, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Oh, S.; Kwon, S.-Y.; Singh, M.; O’Hagan, D.T. Dissolvable Microneedle Patches for the Delivery of Cell-Culture-Derived Influenza Vaccine Antigens. J. Pharm. Sci. 2012, 101, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Mistilis, M.J.; Bommarius, A.S.; Prausnitz, M.R. Development of a Thermostable Microneedle Patch for Influenza Vaccination. J. Pharm. Sci. 2015, 104, 740–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, B.; Wu, M.X. Effective and Lesion-Free Cutaneous Influenza Vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 5005–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirobe, S.; Azukizawa, H.; Hanafusa, T.; Matsuo, K.; Quan, Y.-S.; Kamiyama, F.; Katayama, I.; Okada, N.; Nakagawa, S. Clinical Study and Stability Assessment of a Novel Transcutaneous Influenza Vaccination Using a Dissolving Microneedle Patch. Biomaterials 2015, 57, 50–58. [Google Scholar] [CrossRef]
- Long-Term Stability of Influenza Vaccine in a Dissolving Microneedle Patch. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5003766/ (accessed on 29 April 2021).
- Littauer, E.Q.; Mills, L.K.; Brock, N.; Esser, E.S.; Romanyuk, A.; Pulit-Penaloza, J.A.; Vassilieva, E.V.; Beaver, J.T.; Antao, O.; Krammer, F.; et al. Stable Incorporation of GM-CSF into Dissolvable Microneedle Patch Improves Skin Vaccination against Influenza. J. Control. Release 2018, 276, 1–16. [Google Scholar] [CrossRef]
- Esser, E.S.; Pulit-Penaloza, J.A.; Kalluri, H.; McAllister, D.; Vassilieva, E.V.; Littauer, E.; Lelutiu, N.; Prausnitz, M.; Compans, R.W.; Skountzou, I. Microneedle Patch Delivery of Influenza Vaccine during Pregnancy Enhances Maternal Immune Responses Promoting Survival and Long-Lasting Passive Immunity to Offspring. Sci. Rep. 2017, 7, 5705. [Google Scholar] [CrossRef] [Green Version]
- Vassilieva, E.V.; Wang, S.; Li, S.; Prausnitz, M.R.; Compans, R.W. Skin Immunization by Microneedle Patch Overcomes Statin-Induced Suppression of Immune Responses to Influenza Vaccine. Sci. Rep. 2017, 7, 17855. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhen, Y.; Ma, X.; Wei, B.; Li, S.; Wang, N. Mannosylated and Lipid A-Incorporating Cationic Liposomes Constituting Microneedle Arrays as an Effective Oral Mucosal HBV Vaccine Applicable in the Controlled Temperature Chain. Colloids Surf. B. Biointerfaces 2015, 126, 520–530. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Nakagawa, T.; Quan, Y.-S.; Kamiyama, F.; Hirobe, S.; Okada, N.; Nakagawa, S. Performance and Characteristics Evaluation of a Sodium Hyaluronate-Based Microneedle Patch for a Transcutaneous Drug Delivery System. Int. J. Pharm. 2013, 441, 570–579. [Google Scholar] [CrossRef]
- Chu, L.Y.; Ye, L.; Dong, K.; Compans, R.W.; Yang, C.; Prausnitz, M.R. Enhanced Stability of Inactivated Influenza Vaccine Encapsulated in Dissolving Microneedle Patches. Pharm. Res. 2016, 33, 868–878. [Google Scholar] [CrossRef]
- Chu, L.Y.; Prausnitz, M.R. Separable Arrowhead Microneedles. J. Control. Release 2011, 149, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Raphael, A.P.; Prow, T.W.; Crichton, M.L.; Chen, X.; Fernando, G.J.P.; Kendall, M.A.F. Targeted, Needle-Free Vaccinations in Skin Using Multilayered, Densely-Packed Dissolving Microprojection Arrays. Small 2010, 6, 1785–1793. [Google Scholar] [CrossRef]
- Donadei, A.; Kraan, H.; Ophorst, O.; Flynn, O.; O’Mahony, C.; Soema, P.C.; Moore, A.C. Skin Delivery of Trivalent Sabin Inactivated Poliovirus Vaccine Using Dissolvable Microneedle Patches Induces Neutralizing Antibodies. J. Control. Release 2019, 311–312, 96–103. [Google Scholar] [CrossRef]
- Kolluru, C.; Gomaa, Y.; Prausnitz, M.R. Development of a Thermostable Microneedle Patch for Polio Vaccination. Drug Deliv. Transl. Res. 2019, 9, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Joyce, J.C.; Carroll, T.D.; Collins, M.L.; Chen, M.-H.; Fritts, L.; Dutra, J.C.; Rourke, T.L.; Goodson, J.L.; McChesney, M.B.; Prausnitz, M.R.; et al. A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. J. Infect. Dis. 2018, 218, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Erdos, G.; Balmert, S.C.; Carey, C.D.; Falo, G.D.; Patel, N.A.; Zhang, J.; Gambotto, A.; Korkmaz, E.; Falo, L.D. Improved Cutaneous Genetic Immunization by Microneedle Array Delivery of an Adjuvanted Adenovirus Vaccine. J. Invest. Dermatol. 2020, 140, 2528–2531.e2. [Google Scholar] [CrossRef]
- Hsueh, K.-J.; Chen, M.-C.; Cheng, L.-T.; Lee, J.-W.; Chung, W.-B.; Chu, C.-Y. Transcutaneous Immunization of Streptococcus Suis Bacterin Using Dissolving Microneedles. Comp. Immunol. Microbiol. Infect. Dis. 2017, 50, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Vos, P.J.; Saelens, X.; van der Maaden, K. Vaccination with Influenza Hemagglutinin-Loaded Ceramic Nanoporous Microneedle Arrays Induces Protective Immune Responses. Eur. J. Pharm. Biopharm. 2019, 136, 259–266. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D Printed Microneedles for Anticancer Therapy of Skin Tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Subjects. Clin. J. Pain 2008, 24, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet Printing of Transdermal Microneedles for the Delivery of Anticancer Agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Liu, S.; Wang, Y.; Xu, M.; Cai, W.; Liu, J.; Bai, W.; Ye, S.; Ma, Y.; Hu, H.; et al. Rapid Isolation and Immune Profiling of SARS-CoV-2 Specific Memory B Cell in Convalescent COVID-19 Patients via LIBRA-Seq. Signal Transduct. Target. Ther. 2021, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Billah, M.M.; Sarker, A. COVID-19 Pandemic and Healthcare Solid Waste Management Strategy—A Mini-Review. Sci. Total Environ. 2021, 778, 146220. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.S.; Jayashi, C.M.F.; Reynolds, S.; Wun, C.; Calcutt, A.; Baker, S.B.; Murugappan, S.; Depelsenaire, A.C.I.; Dooley, J.; Fahey, P.V.; et al. M-Protein Based Vaccine Induces Immunogenicity and Protection from Streptococcus Pyogenes When Delivered on a High-Density Microarray Patch (HD-MAP). NPJ Vaccines 2020, 5, 74. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.A.; Esser, E.S.; Vassilieva, E.V.; Lee, J.W.; Taherbhai, M.T.; Pollack, B.P.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. A Protective Role of Murine Langerin+ Cells in Immune Responses to Cutaneous Vaccination with Microneedle Patches. Sci. Rep. 2014, 4, 6094. [Google Scholar] [CrossRef]
- Jeong, H.R.; Bae, J.Y.; Park, J.H.; Baek, S.K.; Kim, G.; Park, M.S.; Park, J.H. Preclinical Study of Influenza Bivalent Vaccine Delivered with a Two Compartmental Microneedle Array. J. Control. Release 2020, 324, 280–288. [Google Scholar] [CrossRef]
- Wong, Y.-L.; Sampson, S.; Germishuizen, W.A.; Goonesekera, S.; Caponetti, G.; Sadoff, J.; Bloom, B.R.; Edwards, D. Drying a Tuberculosis Vaccine without Freezing. Proc. Natl. Acad. Sci. USA 2007, 104, 2591–2595. [Google Scholar] [CrossRef] [Green Version]
- Bardel, E.; Doucet-Ladeveze, R.; Mathieu, C.; Harandi, A.M.; Dubois, B.; Kaiserlian, D. Intradermal Immunisation Using the TLR3-Ligand Poly (I:C) as Adjuvant Induces Mucosal Antibody Responses and Protects against Genital HSV-2 Infection. npj Vaccines 2016, 1, 16010. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.A.; Pearson, F.E.; Fernando, G.J.P.; Agyei-Yeboah, C.; Owens, N.S.; Corrie, S.R.; Crichton, M.L.; Wei, J.C.J.; Weldon, W.C.; Oberste, M.S.; et al. Inactivated Poliovirus Type 2 Vaccine Delivered to Rat Skin via High Density Microprojection Array Elicits Potent Neutralising Antibody Responses. Sci. Rep. 2016, 6, 22094. [Google Scholar] [CrossRef]
- Frolov, V.G.; Seid, R.C.; Odutayo, O.; Al-Khalili, M.; Yu, J.; Frolova, O.Y.; Vu, H.; Butler, B.A.; Look, J.L.; Ellingsworth, L.R.; et al. Transcutaneous Delivery and Thermostability of a Dry Trivalent Inactivated Influenza Vaccine Patch. Influenza Other Respir. Viruses 2008, 2, 53–60. [Google Scholar] [CrossRef]
- Nakagami, H.; Hayashi, H.; Shimamura, M.; Rakugi, H.; Morishita, R. Therapeutic Vaccine for Chronic Diseases after the COVID-19 Era. Hypertens. Res. 2021, 44, 1047–1053. [Google Scholar] [CrossRef]
- Lofano, G.; Mallett, C.P.; Bertholet, S.; O’Hagan, D.T. Technological Approaches to Streamline Vaccination Schedules, Progressing towards Single-Dose Vaccines. NPJ Vaccines 2020, 5, 88. [Google Scholar] [CrossRef]
- Brüssow, H. Efforts towards a COVID-19 Vaccine. Environ. Microbiol. 2020, 22, 4071–4084. [Google Scholar] [CrossRef]
- Creighton, R.L.; Phan, J.; Woodrow, K.A. In Situs 3D-Patterning of Electrospun Fibers Using Two-Layer Composite Materials. Sci. Rep. 2020, 10, 7949. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Serr, I.; Fürst, R.W.; Achenbach, P.; Scherm, M.G.; Gökmen, F.; Haupt, F.; Sedlmeier, E.M.; Knopff, A.; Shultz, L.; Willis, R.A.; et al. Type 1 Diabetes Vaccine Candidates Promote Human Foxp3+ Treg Induction in Humanized Mice. Nat. Commun. 2016, 7, 10991. [Google Scholar] [CrossRef]
- Yazie, T.D.; Tebeje, M.G.; Chufa, K.A. Healthcare Waste Management Current Status and Potential Challenges in Ethiopia: A Systematic Review. BMC Res. Notes 2019, 12, 285. [Google Scholar] [CrossRef]
- MacDonald, N.E.; The SAGE Working Group on Vaccine Hesitancy. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Salmon, D.A.; Dudley, M.Z.; Glanz, J.M.; Omer, S.B. Vaccine Hesitancy: Causes, Consequences, and a Call to Action. Am. J. Prev. Med. 2015, 49, S391–S398. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, M.J.; Lobera, J.; Díaz-Catalán, C. Vaccine Hesitancy Is Strongly Associated with Distrust of Conventional Medicine, and Only Weakly Associated with Trust in Alternative Medicine. Soc. Sci. Med. 2020, 255, 113019. [Google Scholar] [CrossRef]
- Stoeckel, F.; Carter, C.; Lyons, B.A.; Reifler, J. Association of Vaccine Hesitancy and Immunization Coverage Rates in the European Union. Vaccine 2021, 39, 3935–3939. [Google Scholar] [CrossRef] [PubMed]
- Abu Hammour, K.; Abu Farha, R.; Manaseer, Q.; Al-Manaseer, B. Factors Affecting Public Knowledge about COVID-19 Vaccines and the Influence of Knowledge on Their Acceptance to Get Vaccinated. J. Am. Pharm. Assoc. 2021, 62, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Luk, T.T.; Zhao, S.; Wu, Y.; Wong, J.Y.H.; Wang, M.P.; Lam, T.H. Prevalence and Determinants of SARS-CoV-2 Vaccine Hesitancy in Hong Kong: A Population-Based Survey. Vaccine 2021, 39, 3602–3607. [Google Scholar] [CrossRef]
- Paris, C.; Bénézit, F.; Geslin, M.; Polard, E.; Baldeyrou, M.; Turmel, V.; Tadié, É.; Garlantezec, R.; Tattevin, P. COVID-19 Vaccine Hesitancy among Healthcare Workers. Infect. Dis. Now 2021, 51, 484–487. [Google Scholar] [CrossRef]
- Pullan, S.; Dey, M. Vaccine Hesitancy and Anti-Vaccination in the Time of COVID-19: A Google Trends Analysis. Vaccine 2021, 39, 1877–1881. [Google Scholar] [CrossRef]
- Verger, P.; Dualé, C.; Lenzi, N.; Scronias, D.; Pulcini, C.; Launay, O. Vaccine Hesitancy among Hospital Staff Physicians: A Cross-Sectional Survey in France in 2019. Vaccine 2021, 39, 4481–4488. [Google Scholar] [CrossRef]
- Andrews, G.J. ‘I Had to Go to the Hospital and It Was Freaking Me out’: Needle Phobic Encounter Space. Health Place 2011, 17, 875–884. [Google Scholar] [CrossRef]
- Jenkins, K., II. Needle Phobia: A Psychological Perspective. Br. J. Anaesth. 2014, 113, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.P. Rapid Desensitization for Needle Phobia. Psychosomatics 2003, 44, 253–254. [Google Scholar] [CrossRef]
- Win, N.N.; Kohase, H.; Miyamoto, T.; Umino, M. Decreased Bispectral Index as an Indicator of Syncope before Hypotension and Bradycardia in Two Patients with Needle Phobia. Br. J. Anaesth. 2003, 91, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Turnage, J.R.; Logan, D.L. Treatment of a Hypodermic Needle Phobia by in Vivo Systematic Desensitization. J. Behav. Ther. Exp. Psychiatry 1974, 5, 67–69. [Google Scholar] [CrossRef]
- Sokolowski, C.J.; Giovannitti, J.A.; Boynes, S.G. Needle Phobia: Etiology, Adverse Consequences, and Patient Management. Dent. Clin. N. Am. 2010, 54, 731–744. [Google Scholar] [CrossRef]
- Noh, I.; Lee, K.; Rhee, Y.S. Microneedle Systems for Delivering Nucleic Acid Drugs. J. Pharm. Investig. 2022, 1, 273–292. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A Review of Recent Advances in Microneedle Technology for Transdermal Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Jiang, P.; Klemeš, J.J.; Van Fan, Y.; Fu, X.; Tan, R.R.; You, S.; Foley, A.M. Energy, Environmental, Economic and Social Equity (4E) Pressures of COVID-19 Vaccination Mismanagement: A Global Perspective. Energy 2021, 235, 121315. [Google Scholar] [CrossRef]
- Mallapaty, S. China Is Vaccinating a Staggering 20 Million People a Day. Nature 2021. [Google Scholar] [CrossRef]
- Burki, T.K. Challenges in the Rollout of COVID-19 Vaccines Worldwide. Lancet. Respir. Med. 2021, 9, e42. [Google Scholar] [CrossRef]
- So, A.D.; Woo, J. Special Paper: Reserving Coronavirus Disease 2019 Vaccines for Global Access: Cross Sectional Analysis. BMJ 2020, 371, m4750. [Google Scholar] [CrossRef] [PubMed]
- Eccleston-Turner, M.; Upton, H. International Collaboration to Ensure Equitable Access to Vaccines for COVID-19: The ACT-Accelerator and the COVAX Facility. Milbank Q. 2021, 99, 426–449. [Google Scholar] [CrossRef]
- Zaqout, A.; Daghfal, J.; Alaqad, I.; Hussein, S.A.N.; Aldushain, A.; Almaslamani, M.A.; Abukhattab, M.; Omrani, A.S. The Initial Impact of a National BNT162b2 MRNA COVID-19 Vaccine Rollout. Int. J. Infect. Dis. 2021, 108, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 MRNA COVID-19 Vaccine in Patients with Chronic Lymphocytic Leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.; Nayrac, M.; Benlarbi, M.; Gong, S.Y.; Gasser, R.; Beaudoin-Bussières, G.; Brassard, N.; Laumaea, A.; Vézina, D.; Prévost, J.; et al. A Single Dose of the SARS-CoV-2 Vaccine BNT162b2 Elicits Fc-Mediated Antibody Effector Functions and T Cell Responses. Cell Host Microbe 2021, 29, 1137–1150.e6. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial Observations on Age, Gender, BMI and Hypertension in Antibody Responses to SARS-CoV-2 BNT162b2 Vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chandrashekar, A.; Zahn, R.; Wegmann, F.; Yu, J.; Mercado, N.B.; McMahan, K.; Martinot, A.J.; Piedra-Mora, C.; Beecy, S.; et al. Low-Dose Ad26.COV2.S Protection against SARS-CoV-2 Challenge in Rhesus Macaques. Cell 2021, 184, 3467–3473.e11. [Google Scholar] [CrossRef]
- Karim, S.S.A.; de Oliveira, T. New SARS-CoV-2 Variants—Clinical, Public Health, and Vaccine Implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, D.; Broseta, J.J.; Maduell, F.; Bedini, J.L.; Vera, M. Humoral Response of the MRNA-1273 SARS-CoV-2 Vaccine in Peritoneal Dialysis Patients. Kidney Int. 2021, 100, 476. [Google Scholar] [CrossRef]
- Wu, K.; Werner, A.P.; Koch, M.; Choi, A.; Narayanan, E.; Stewart-Jones, G.B.E.; Colpitts, T.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; et al. Serum Neutralizing Activity Elicited by MRNA-1273 Vaccine. N. Engl. J. Med. 2021, 384, 1468–1470. [Google Scholar] [CrossRef]
- Silva-Cayetano, A.; Foster, W.S.; Innocentin, S.; Belij-Rammerstorfer, S.; Spencer, A.J.; Burton, O.T.; Fra-Bidó, S.; Le Lee, J.; Thakur, N.; Conceicao, C.; et al. A Booster Dose Enhances Immunogenicity of the COVID-19 Vaccine Candidate ChAdOx1 NCoV-19 in Aged Mice. Med 2021, 2, 243–262.e8. [Google Scholar] [CrossRef]
- Becerra-Flores, M.; Cardozo, T. SARS-CoV-2 Viral Spike G614 Mutation Exhibits Higher Case Fatality Rate. Int. J. Clin. Pract. 2020, 74, e13525. [Google Scholar] [CrossRef]
- Zhang, L.; Jackson, C.B.; Mou, H.; Ojha, A.; Peng, H.; Quinlan, B.D.; Rangarajan, E.S.; Pan, A.; Vanderheiden, A.; Suthar, M.S.; et al. SARS-CoV-2 Spike-Protein D614G Mutation Increases Virion Spike Density and Infectivity. Nat. Commun. 2020, 11, 6013. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, Q. Perspectives on Therapeutic Neutralizing Antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020, 16, 1718–1723. [Google Scholar] [CrossRef]
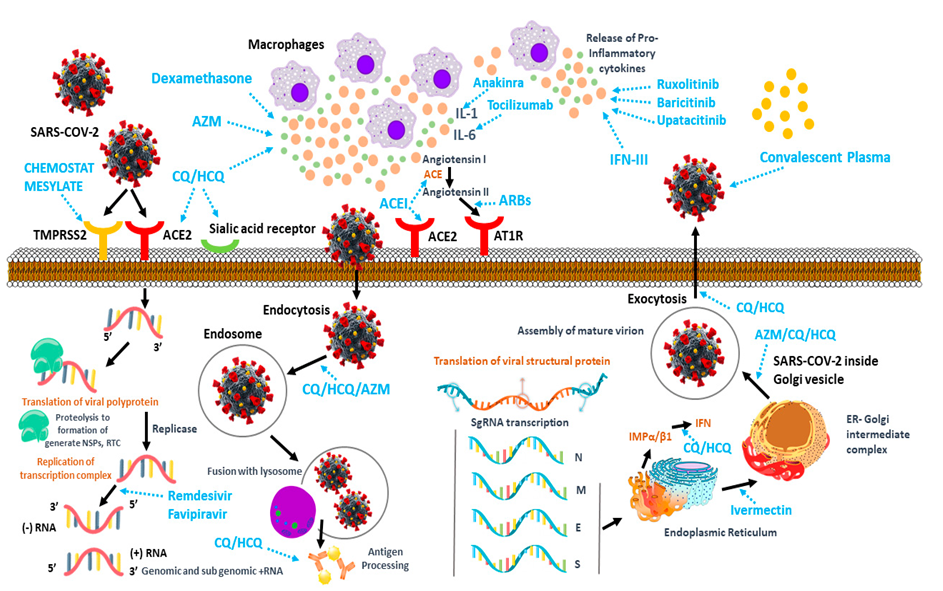











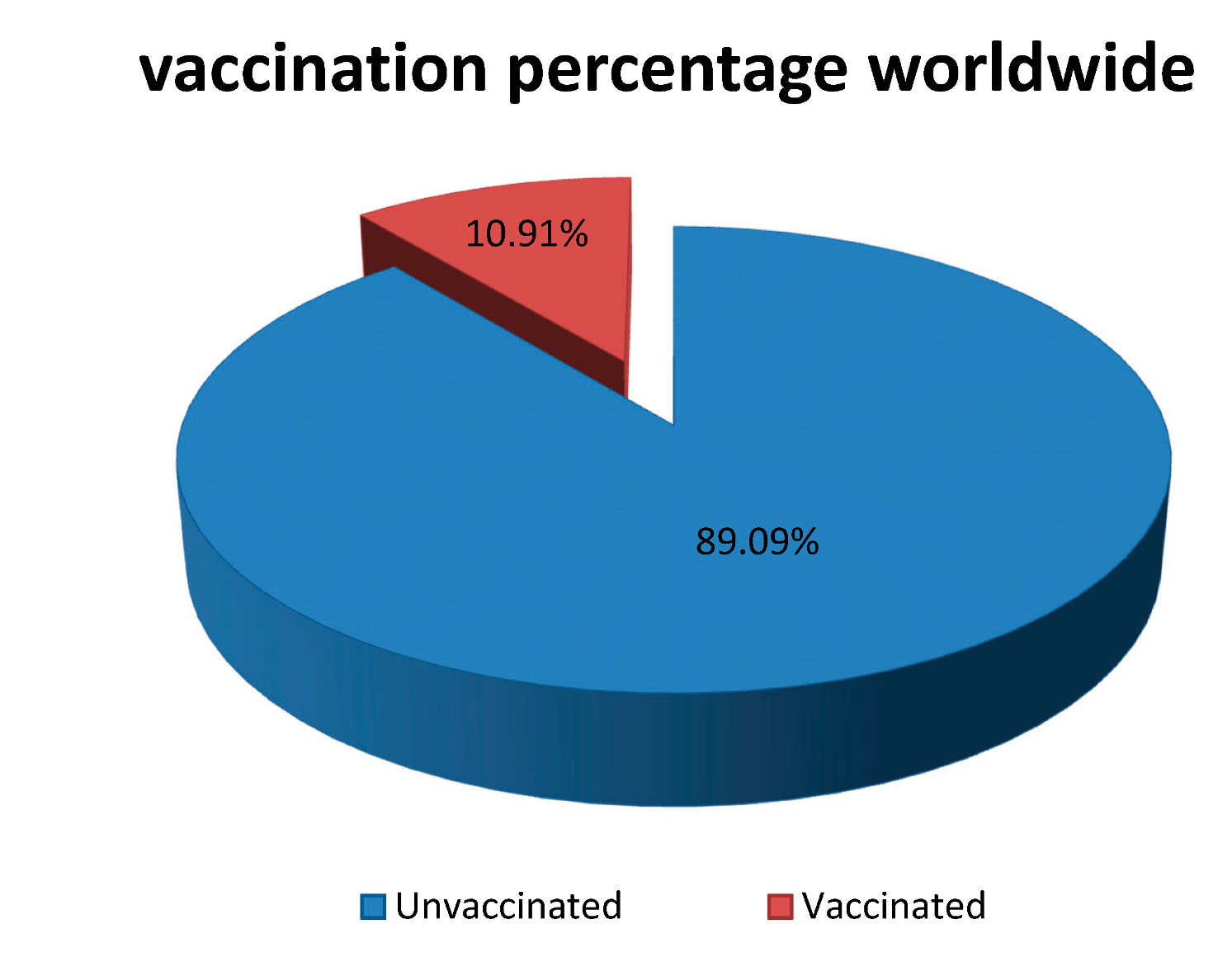


| Syringes and Needles (Limitations) | Microneedles in Vaccine Delivery | References | |
|---|---|---|---|
| Strengths | Weaknesses | ||
|
|
| [45,64] |
|
|
| [45,65] |
|
|
| [66,67] |
|
|
| [51,68,69] |
| |||
| SL No. | Organ System | Clinical Outcomes | Clinical Manifestations | References |
|---|---|---|---|---|
| 1. | Vascular system |
|
| [72,73] |
| 2. | Lungs |
|
| [69,74] |
| 3. | Kidneys |
|
| [75,76] |
| 4. | Liver |
|
| [77,78] |
| 5. | Heart |
|
| [73,79] |
| 6. | Intestine |
|
| [75,80] |
| 7. | Brain |
|
| [81,82] |
| SL No. | Vaccine Technology | Principle | Advantage | Disadvantage | References |
|---|---|---|---|---|---|
| 1. | mRNA-based | Delivery of modified mRNA |
|
| [27,77,156] |
| 2. | DNA-based | Vector-based delivery of a viral gene |
|
| [114,157,158] |
| 3. | Peptide-based | A fragment of whole-length viral peptide |
|
| [159,160] |
| 4. | Live attenuated virus | De-optimization of the genome (to reduce pathogenicity) |
|
| [157,161] |
| 5. | Inactivated virus | Chemically or UV-inactivated virus |
|
| [92,162,163] |
| Stability Profile | Manufacturer | References | |
|---|---|---|---|
| Moderna | Pfizer-BioNTech | ||
| Frozen State | −20 °C up to 6 months | −80 °C to −60 °C up to 6 months | [78,89,109,166,167,168] |
| 2–8 °C | 30 days | Up to 5 days | |
| Room Temperature | Up to 12 h. | Up to 2 h. (up to 6 h. after dilution) | |
| Dose | 100 µg (0.5 mL) | 30 µg (0.3 mL) | [109,159,169] |
| Dosing Schedule | Day 1, Day 29 | Day 1, Day 21 | |
| SL No. | Criteria | DMN Array Patch (Score) |
|---|---|---|
| 1. | Manufacturing cost | ** |
| 2. | Mass production | *** |
| 3. | Self-administration | ***** |
| 4. | Wear time | *** |
| 5. | Material biocompatibility | *** |
| 6. | Accurate dosage delivery | *** |
| 7. | Aseptic process | *** |
| 8. | Stability against humidity | ** |
| 9. | Waste generation | ***** |
| SL No. | Types of Vaccine | Animal Model | References |
|---|---|---|---|
| 1. | Influenza (inactivated) | Mouse | [245,246] |
| 2. | Hepatitis B (recombinant subunit) | Mouse | [78,159] |
| 3. | HIV (recombinant vector) | Mouse | [247,248] |
| 4. | Dengue virus (live attenuated) | Mouse | [63] |
| 5. | Ebola (DNA) | Mouse | [249,250] |
| 6. | Enterovirus (VLPs) | Mouse | [63] |
| 7. | Rotavirus (inactivated) | Mouse | [67] |
| 8. | Polio virus (inactivated) | Mouse | [128,251] |
| 9. | Streptococcus (inactivated) | Mouse | [63] |
| 10. | Staphylococcus (recombinant subunit) | Mouse | [63,166] |
| 11. | Shigella (BLP) | Mouse | [46,63] |
| 12. | Clostridium (toxoid) | Mouse | [46,63] |
| 13. | BCG (live attenuated) | Mouse | [107,252] |
| 14. | Neisseria gonorrhea (inactivated) | Mouse | [63,229,253,254] |
| 15. | Pseudomonas aeruginosa (inactivated) | Mouse | [64,253,254] |
| 16. | Orientia tsutsugamushi (recombinant subunit) | Mouse | [64,253,254] |
| 17. | Malaria (recombinant subunit) | Mouse | [63,253,255] |
| 18. | Influenza, DT, tetanus toxoid (inactivated) | Rat | [58,256] |
| 19. | BCG (live attenuated) | Mouse | [257] |
| 20. | Influenza (inactivated) | Guinea pig | [40,245] |
| 21. | Hepatitis B (recombinant subunit) | Pig | [258,259] |
| 22. | Hepatitis C (VLPs) | Mouse | [196,260] |
| 23. | Rabies (DNA) | Dog | [63,156] |
| 24. | IPV (inactivated) | Monkey | [261,262] |
| 25. | Measles (live attenuated) | Mouse | [63,128] |
| 26. | Hepatitis B (recombinant subunit) | Mouse | [258,259] |
| 27. | Tetanus toxoid (inactivated) | Pregnant mouse | [256,263] |
| 28. | Measles, rubella (live attenuated) | Infant monkey | [264,265] |
| Targeted Molecule | Disease Causing Microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Nucleic Acid | Rabies [330] | BCG [331] | Ebola [250] | Hepatitis B virus [258] | Porcine circovirus type 2 [332] | |||
| Viral Vector | SARS-CoV-2 [142] | Zika [333] | HIV [247,334,335] | |||||
| Protein-Based or VLP | SARS-CoV-2 [336] | HIV [337] | Influenza [33,233,245,338,339,340,341,342,343,344,345,346,347,348] | Hepatitis B [259,260,349] | Diphtheria [256,305,350] | HPV [65] | Tetanus [263,306,350] | Malaria [256] |
| Inactivated or Live Attenuated | Influenza [66,246,351,352,353,354] | Polio virus [261,355] | Rubella [325,356] | Adenovirus [33,357] | Streptococcus [358] | BCG [257] | Measles [325,356,359] | Rotavirus [67] |
| SL No. | Sections Where Huge Amounts of Costs Can Be Reduced | Dissolving MNs Patch | Conventional Syringes |
|---|---|---|---|
| 1. | Maintenance cost of cold chain system for storage after manufacturing vaccine | × | √ |
| 2. | Cold chain system cost during distribution of vaccine | × | √ |
| 3. | Syringes and vials’ manufacturing cost | × | √ |
| 4. | Sharp waste disposal procedure cost | × | √ |
| 5. | Plastic and glass waste management cost | × | √ |
| 6. | Training cost (training up the caregivers for syringe and vial handling, disposal, etc.) | × | √ |
| SL No. | Vaccine | Stabilizer | Temperature | Period | References |
|---|---|---|---|---|---|
| 1. | Influenza (inactivated) | Trehalose | 4 °C, 25 °C, 37 °C | 3 months | [40,69] |
| 2. | Influenza (inactivated) | Trehalose | 40 °C | 6 months | [40,69] |
| 3. | Influenza (inactivated) | Trehalose | 35 °C | 12 months | [40,69] |
| 4. | Rabies (DNA) | Sucrose | 4 °C | 3 weeks | [61,67,372] |
| 5. | Hepatitis B (recombinant subunit) | - | 4 °C | 3 months | [258,260] |
| 6. | Hepatitis B (recombinant subunit) | Sucrose | 45 °C | 6 months | [258,260] |
| 7. | Influenza (subunit) | Arginine + heptagluconate | 25 °C Freeze–thawing | 24 months5 cycles | [32,326] |
| 8. | BCG (live attenuated) | - | 25 °C | 2 months | [252] |
| 9. | Tetanus toxoid/Diphtheria toxoid (divalent subunit) | - | 4 °C | 24 weeks | [256,263] |
| 10. | Scrub typhus (recombinant subunit) | - | 25 °C | 4 weeks | [63] |
| SL No. | Assay | Purpose | References |
|---|---|---|---|
| 1. | Characterizing DNA templates and RNA transcripts | ||
| Identification of mRNA | [74,159] | |
| Quantification—purity dependent | [203] | |
| Quantification—purity assessment | [13] | |
| Molecular mass, RNA integrity, and quantification | [77] | |
| Identification and quantification of mRNA | [375] | |
| Quality assessment | [139] | |
| Quality assessment | [159] | |
| Quality assessment | [13] | |
| Quantity and quality assessment | [77] | |
| 2. | Characterizing mRNA-encoded translation products | ||
| Translation into target protein | [156] | |
| Translation product analysis and potential toxicity assay | [35,91] | |
| 3. | Characterizing mRNA-lipid/protein complexes | ||
| Particle size (distribution) | [72] | |
| Assessing bound/unbound mRNA and surface charge | [107] | |
| Zeta potential | [77] | |
| Quantification and integrity of carrier lipids/protein | [72] | |
| Encapsulation efficiency | [372] | |
| 4. | General pharmaceutical tests | Appearance, pH, osmolality, endotoxin concentration, and sterility | [107] |
| SL No. | Company | Type of Microneedle | Disease |
|---|---|---|---|
| 1. | Micron Biomedical | Dissolving microneedle | Inactivated rotavirus |
| 2. | 3M (Kindeva) | Hollow microneedle | Cancer vaccines |
| 3. | BD Technologies (BS Soluvia) | Stainless steel microneedle | Influenza |
| 4. | Flugen | Metal microneedles | Influenza |
| 5. | Debiotech | Hollow microneedles | COVID-19 |
| 6. | Vendari (Vaxipatch) | Stainless steel microneedle | Influenza and COVID-19 |
| 7. | Nanopass (MicrojetTM) | Silicon microneedles | Influenza, Polio, Varicella-Zoster, cancers, Hepatitis B, and COVID-19 |
| 8. | BioSeren Tach Inc. | Dissolving microneedles | Vaccine |
| 9. | Sorrento Therapeutics (Solusa) | Nanotopographical imprinted microneedles (coated) | Immuno-oncology |
| 10. | Vaxxas (NanopatchTM) | Coated microneedles array patch | Influenza, COVID-19 |
| 11. | Quadmedicine | Dissolving microneedles | Influenza, Canine Influenza |
| 12. | Vaxess | Dissolving microneedles | Influenza, COVID-19, and skin cancer |
| 13. | Raphas | Dissolving microneedles | HPV, Polio, T dap, HBV, IPV, and Hepatitis B |
| SL No. | Company | Current R&D Stage | Price Range per Course | Platform Technology |
|---|---|---|---|---|
| 1. | AstraZeneca/Oxford University | Phase II/III | USD 6-USD 8 | Non-replicating viral vector |
| 2. | Pfizer/BioNTech | USD 37-USD 39 | mRNA | |
| 3. | Johnson & Johnson/Janssen | Phase III | USD 20 | Non-replicating viral vector |
| 4. | Moderna | USD 30-USD 74 | mRNA | |
| 5. | Novavax | USD 6-USD 32 | Protein subunit | |
| 6. | SinoVac | Undisclosed | Inactivated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, J.; Haigh, C.; Ahmed, T.; Uddin, M.J.; Das, D.B. Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics 2022, 14, 1066. https://doi.org/10.3390/pharmaceutics14051066
Hassan J, Haigh C, Ahmed T, Uddin MJ, Das DB. Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics. 2022; 14(5):1066. https://doi.org/10.3390/pharmaceutics14051066
Chicago/Turabian StyleHassan, Jasmin, Charlotte Haigh, Tanvir Ahmed, Md Jasim Uddin, and Diganta B. Das. 2022. "Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges" Pharmaceutics 14, no. 5: 1066. https://doi.org/10.3390/pharmaceutics14051066
APA StyleHassan, J., Haigh, C., Ahmed, T., Uddin, M. J., & Das, D. B. (2022). Potential of Microneedle Systems for COVID-19 Vaccination: Current Trends and Challenges. Pharmaceutics, 14(5), 1066. https://doi.org/10.3390/pharmaceutics14051066










