Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent
Abstract
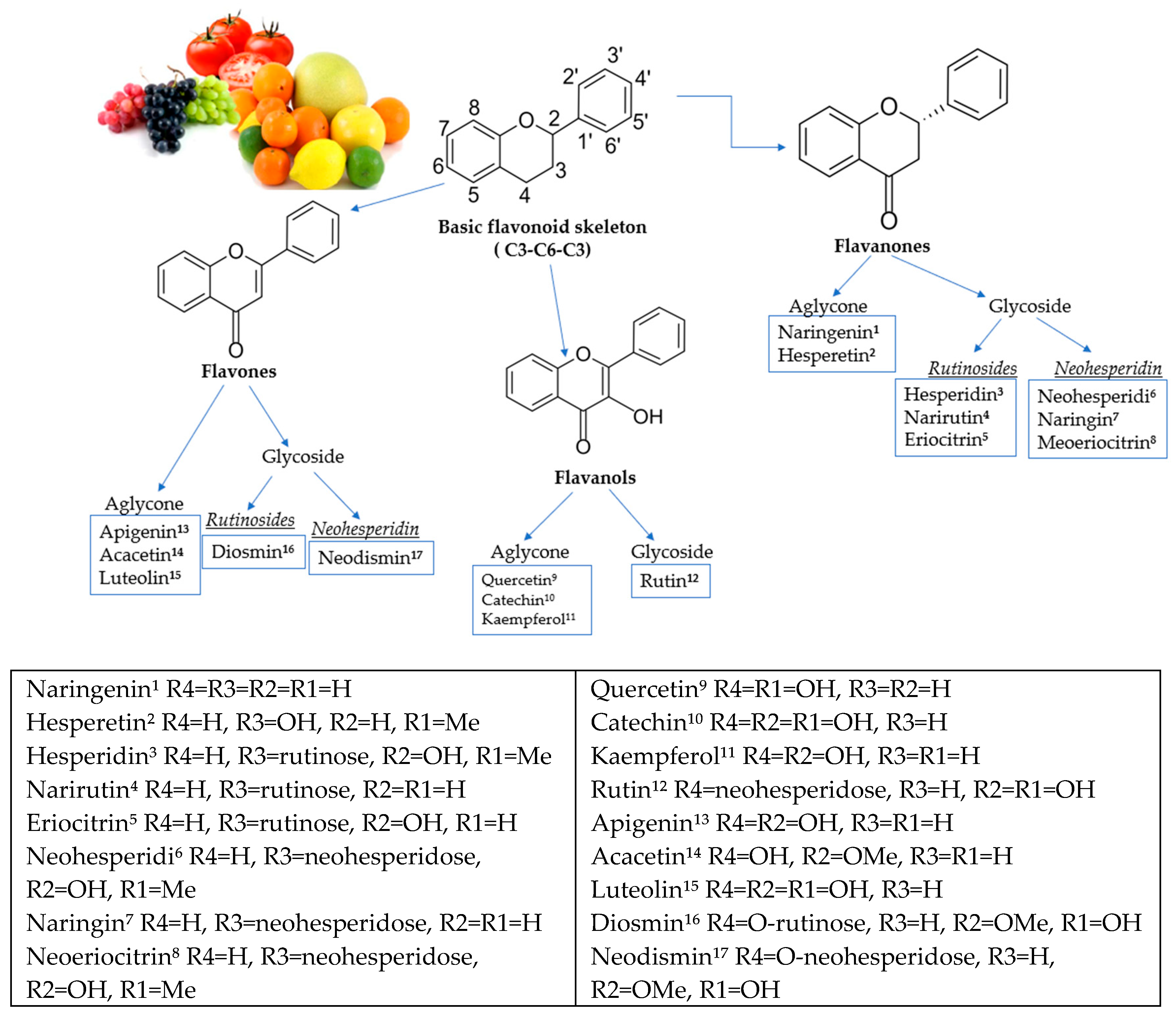
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Heat Reflux Extraction (HRE)
2.2.2. Ultrasound-Assisted Extraction Bath (UAE)
2.2.3. Ultrasound-Assisted Extraction Using an Ultrasonic Homogenizer (UAE*)
2.2.4. The Use of Magnesium Aluminometasilicate in the Preparation of Extracts
2.3. Hydrolysis and Neutralization
2.3.1. Acidic Hydrolysis and Neutralization Using Albedo, Flavedo, and Segmental Parts
2.3.2. Thermal Hydrolysis Using Albedo, Flavedo, and Segmental Parts
2.3.3. Alkaline Hydrolysis and Neutralization Using Albedo, Flavedo, and Segmental Parts
2.4. Hydro Distillation (HD)
2.5. HPLC–PDA Conditions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Flavanone Determination in Citrus × paradisi L. Extracts
3.1.1. Flavanones Extraction Using the UAE Method with Acidic, Alkaline, and Thermal Hydrolysis
3.1.2. Flavanone Extraction Using an Excipient as Adsorbent 1% Magnesium Aluminometasilicate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lü, Z.; Zhang, Z.; Wu, H.; Zhou, Z.; Yu, J. Phenolic Composition and Antioxidant Capacities of Chinese Local Pummelo Cultivars’ Peel. Hortic. Plant J. 2016, 2, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Durand-Hulak, M.; Dugrand, A.; Duval, T.; Bidel, L.P.R.; Jay-Allemand, C.; Froelicher, Y.; Bourgaud, F.; Fanciullino, A.-L. Mapping the genetic and tissular diversity of 64 phenolic compounds in Citrus species using a UPLC–MS approach. Ann. Bot. 2015, 115, 861–877. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Ribeiro, I.A.; Rocha, J.; Sepodes, B.; Mota-Filipe, H.; Ribeiro, M.H. Effect of naringin enzymatic hydrolysis towards naringenin on the anti-inflammatory activity of both compounds. J. Mol. Catal. B Enzym. 2008, 52–53, 13–18. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative Diseases: Might Citrus Flavonoids Play a Protective Role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [Green Version]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef]
- Di Majo, D.; Giammanco, M.; La Guardia, M.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure–antioxidant activity relationships. Food Res. Int. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Liver Physiol. 2000, 279, G1148–G1154. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Bronze, M.R.; Calado, A.R.T.; Ribeiro, M.H.L. Enzymatic Synthesis of the Flavone Glucosides, Prunin and Isoquercetin, and the Aglycones, Naringenin and Quercetin, with Selective α-L-Rhamnosidase and β-D-Glucosidase Activities of Naringinase. Enzym. Res. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 1–38. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Fernández Barbero, G. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Boggetti, H.J.; Nazareno, M.A.; Mishima, H.T. Supercritical fluid extraction of naringin from the peel of Citrus paradisi. Phytochem. Anal. 2003, 14, 221–223. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Paris, C.; Ghoul, M.; Mihoubi Boudhrioua, N. Ecophysiology and Agri Processes, Higher Institute of Biotechnology of Sidi Thabet, University of Manouba, BP-66, 2020 Ariana-Tunis, Tunisie, Boudhrioua N. Comparison of the Efficiency of Different Extraction Methods on Antioxidants of Maltease Orange Peel. IJFNS 2016, 3, 1–13. [Google Scholar]
- Rozzi, N.L.; Singh, R.K. Supercritical Fluids and the Food Industry. Compr. Rev. Food Sci. Food Saf. 2006, 1, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; M’Hiri, N.; Chaaban, H.; Boudhrioua, N.M.; Ghoul, M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. Int. J. Food Sci. Technol. 2018, 53, 2754–2760. [Google Scholar] [CrossRef]
- Nuutila, A.; Kammiovirta, K.; Oksman-Caldentey, K.-M. Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid Glucosides Are Hydrolyzed and Thus Activated in the Oral Cavity in Humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Kazlauskaite, J.; Ivanauskas, L.; Bernatoniene, J. Novel Extraction Method Using Excipients to Enhance Yield of Genistein and Daidzein in Trifolium pratensis L. Pharmaceutics 2021, 13, 777. [Google Scholar] [CrossRef]
- Matulyte, I.; Jekabsone, A.; Jankauskaite, L.; Zavistanaviciute, P.; Sakiene, V.; Bartkiene, E.; Ruzauskas, M.; Kopustinskiene, D.M.; Santini, A.; Bernatoniene, J. The Essential Oil and Hydrolats from Myristica fragrans Seeds with Magnesium Aluminometasilicate as Excipient: Antioxidant, Antibacterial, and Anti-inflammatory Activity. Foods 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Min, K.Y.; Kim, H.J.; Lee, K.A.; Kim, K.-T.; Paik, H.-D. Antimicrobial activity of acid-hydrolyzed Citrus unshiu peel extract in milk. J. Dairy Sci. 2014, 97, 1955–1960. [Google Scholar] [CrossRef] [Green Version]
- Wolfender, J.-L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Tu, X.; Ma, S.; Gao, Z.; Wang, J.; Huang, S.; Chen, W. One-Step Extraction and Hydrolysis of Flavonoid Glycosides in Rape Bee Pollen Based on Soxhlet-Assisted Matrix Solid Phase Dispersion: A Modified MSPD Method for the Determination of Flavonoid Aglycones. Phytochem. Anal. 2017, 28, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvėnienė, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Extract ID | Extraction Temp. °C | Extraction Time, min | Material:Solvent Ratio (g/mL) | Solvent | Excipient | Hydrolysis Methods |
|---|---|---|---|---|---|---|
| HRE—Heat-Reflux Extraction | ||||||
| 21-FHR | Ethanol 70% (v/v) | Magnesium aluminometasilicate | - | |||
| 22-AHR | 100 ± 2 | 60 | 1:10 | - | ||
| 23-SHR | - | |||||
| UAE—Ultrasound-Assisted Extraction Bath | ||||||
| 1-F | 50 ± 2 | 20 | 1:10 | Ethanol 50% (v/v) | Magnesium aluminometasilicate | - |
| 2-F | 50 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 3-F | 50 ± 2 | 20 | 1:10 | Ethanol 70% (v/v) | AC*/AL*/T* | |
| 4-F | 50 ± 2 | 30 | 1:10 | Ethanol 70% (v/v) | - | |
| 5-F | 70 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 6-F | 70 ± 2 | 30 | 1:10 | Ethanol 70% (v/v) | - | |
| 7-A | 50 ± 2 | 20 | 1:10 | Ethanol 50% (v/v) | - | |
| 8-A | 50 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 9-A | 50 ± 2 | 20 | 1:10 | Ethanol 70% (v/v) | AC*/AL*/T* | |
| 10-A | 50 ± 2 | 30 | 1:10 | Ethanol 70% (v/v) | - | |
| 11-A | 70 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 12-A | 70 ± 2 | 30 | 1:10 | Ethanol 70% (v/v) | - | |
| 13-S | 50 ± 2 | 20 | 1:10 | Ethanol 50% (v/v) | - | |
| 14-S | 50 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 15-S | 50 ± 2 | 20 | 1:10 | Ethanol 70% (v/v) | AC*/AL*/T* | |
| 16-S | 50 ± 2 | 30 | 1:10 | Ethanol 70% (v/v) | - | |
| 17-S | 70 ± 2 | 30 | 1:10 | Ethanol 50% (v/v) | - | |
| 18-S | 70 ± 2 | 20 | 1:10 | Ethanol 70% (v/v) | - | |
| UAE*—Ultrasound-Assisted Extraction Using an Ultrasonic Homogenizer | ||||||
| 27-SUX1 | from 33.2 to 40 ± 2 | 1 | 1:5 | Ethanol 70% (v/v) | - | - |
| 28-SUX2 | from 33.2 to 40 ± 2 | 3 | 1:5 | Ethanol 70% (v/v) | - | - |
| 29-SUX3 | from 33.2 to 40 ± 2 | 5 | 1:5 | Ethanol 70% (v/v) | - | - |
| 30-FUX1 | from 33.2 to 40 ± 2 | 1 | 1:5 | Ethanol 70% (v/v) | - | - |
| 31-FUX2 | from 33.2 to 40 ± 2 | 3 | 1:5 | Ethanol 70% (v/v) | - | - |
| 32-FUX3 | from 33.2 to 40 ± 2 | 5 | 1:5 | Ethanol 70% (v/v) | - | - |
| 33-AUX1 | from 33.2 to 40 ± 2 | 1 | 1:5 | Ethanol 70% (v/v) | - | - |
| 34-AUX2 | from 33.2 to 40 ± 2 | 3 | 1:5 | Ethanol 70% (v/v) | - | - |
| 35-AUX3 | from 33.2 to 40 ± 2 | 5 | 1:5 | Ethanol 70% (v/v) | - | - |
| Component | Calibration Equation | Coefficient of Determination R2 | Coefficient of Correlation R | LOD* µg/mL | LOQ** µg/mL |
|---|---|---|---|---|---|
| Naringin | Y = 25.500x + 6720 | 0.999923 | 0.99996 | 0.146 | 0.583 |
| Naringenin | ±Y = 33.300x + 3570 | 0.999924 | 0.99996 | 0.118 | 0.430 |
| Extraction Methods | Extract ID * | Naringin mg/g | Naringenin µg/g |
|---|---|---|---|
| Ultrasound-assisted extraction bath | 1-F | 5.41 ± 0.27 ᵈ | - |
| 2-F | 5.38 ± 0.267 | - | |
| 3-F | 5.59 ± 0.279 ᵈ | - | |
| 4-F | 6.08 ± 0.304 | - | |
| 5-F | 7.18 ± 0.359 | - | |
| 6-F | 4.82 ± 0.241 ᵇ | - | |
| 7-A | 14.79 ± 0.739 ᵈ | 3.36 ± 0.168 ᵈ,ᵇ | |
| 8-A | 17.45 ± 0.872 | 3.55 ± 0.1775 ᵇ | |
| 9-A | 17.39 ± 0.869 ᵈ | 4.57 ± 0.228 ᵈ,ᵇ | |
| 10-A | 16.46 ± 0.823 | 4.63 ± 0.231 ᵇ | |
| 11-A | 16.08 ± 0.820 | 3.53 ± 0.176 ᵇ | |
| 12-A | 15.86 ± 0.793 | 4.34 ± 0.207 ᵇ | |
| 13-S | 5.91 ± 0.295 ᵇ,ᵈ,ᵉ | - ᵇ,ᵉ | |
| 14-S | 5.06 ± 0.253 ᵇ,ᵉ | - ᵇ,ᵉ | |
| 15-S | 5.26 ± 0.263 ᵈ,ᵇ,ᵉ | - ᵇ,ᵉ | |
| 16-S | 5.40 ± 0.27 ᵇ,ᵉ | - ᵇ,ᵉ | |
| 17-S | 4.31 ± 0.215 ᵇ,ᵉ | - ᵇ,ᵉ | |
| 18-S | 5.65 ± 0.282 ᵇ,ᵉ | - ᵇ,ᵉ | |
| Heat reflux extraction | 21-FHR | 5.16 ± 0.258 ᵃ | - |
| 22-AHR | 14.17 ± 0.708 ᵃ | 12.60 ± 0.63 | |
| 23-SHR | 6.68 ± 0.334 | 35.80 ± 1.79 | |
| Ultrasound-assisted extraction using an ultrasonic homogenizer | 27-SUX1 | 5.15 ± 0.257 ᵃ,ᵇ | 4.39 ± 0.219 |
| 28-SUX2 | 6.38 ± 0.319 ᵇ | 7.40 ± 0.37 | |
| 29-SUX3 | 5.56 ± 0.279 ᵃ,ᵇ | 5.88 ± 0.294 | |
| 30-FUX1 | 0.96 ± 0.048 ᵃ,ᵇ | - | |
| 31-FUX2 | 1.05 ± 0.0525 ᵃ,ᵇ | - | |
| 32-FUX3 | 0.98 ± 0.049 ᵃ,ᵇ | - | |
| 33-AUX1 | 5.75 ± 0.287 ᵃ,ᵇ | - ᵃ,ᵇ | |
| 34-AUX2 | 6.67 ± 0.333 ᵃ,ᵇ | - ᵃ,ᵇ | |
| 35-AUX3 | 6.13 ± 0.306 ᵃ,ᵇ | - ᵃ,ᵇ |
| Naringin mg/g | Naringenin µg/g | |||||||
|---|---|---|---|---|---|---|---|---|
| Extract ID ** | No Hydrolysis | AC * | AK * | T * | No Hydrolysis | AC * | AK * | T * |
| 3-F | 5.59 ± 0.279 ᵃ | 2.14 ± 0.10 | 3.36 ± 0.168 | 6.25 ± 0.312 ᵃ | - | - | - | - |
| 9-A | 17.39 ± 0.869 ᵃ | 11.39 ± 0.56 | 12.59 ± 0.629 | 25.05 ± 1.25 ᵃ | 4.57 ± 0.249 | 1.78 ± 0.089 | - | 1.87 ± 0.09 |
| 15-S | 5.26 ± 0.263 ᵃ | 6.39 ± 0.319 | 5.13 ± 0.256 | 11.07 ± 0.55 ᵃ | 0 ᵃ | 1.12 ± 0.065 | - | 4.21 ± 0.21 ᵃ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. https://doi.org/10.3390/pharmaceutics14050890
Stabrauskiene J, Marksa M, Ivanauskas L, Bernatoniene J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics. 2022; 14(5):890. https://doi.org/10.3390/pharmaceutics14050890
Chicago/Turabian StyleStabrauskiene, Jolita, Mindaugas Marksa, Liudas Ivanauskas, and Jurga Bernatoniene. 2022. "Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent" Pharmaceutics 14, no. 5: 890. https://doi.org/10.3390/pharmaceutics14050890
APA StyleStabrauskiene, J., Marksa, M., Ivanauskas, L., & Bernatoniene, J. (2022). Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics, 14(5), 890. https://doi.org/10.3390/pharmaceutics14050890








