Hydroxychloroquine Does Not Function as a Direct Zinc Ionophore
Abstract
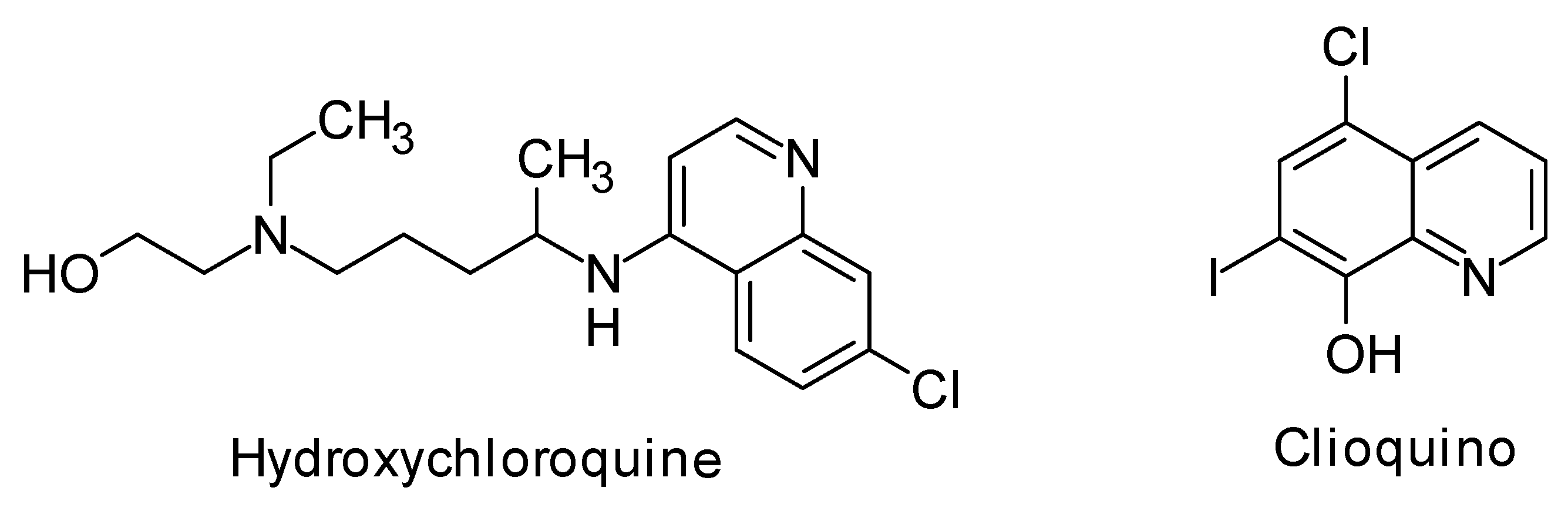
:1. Introduction
2. Results and Discussion
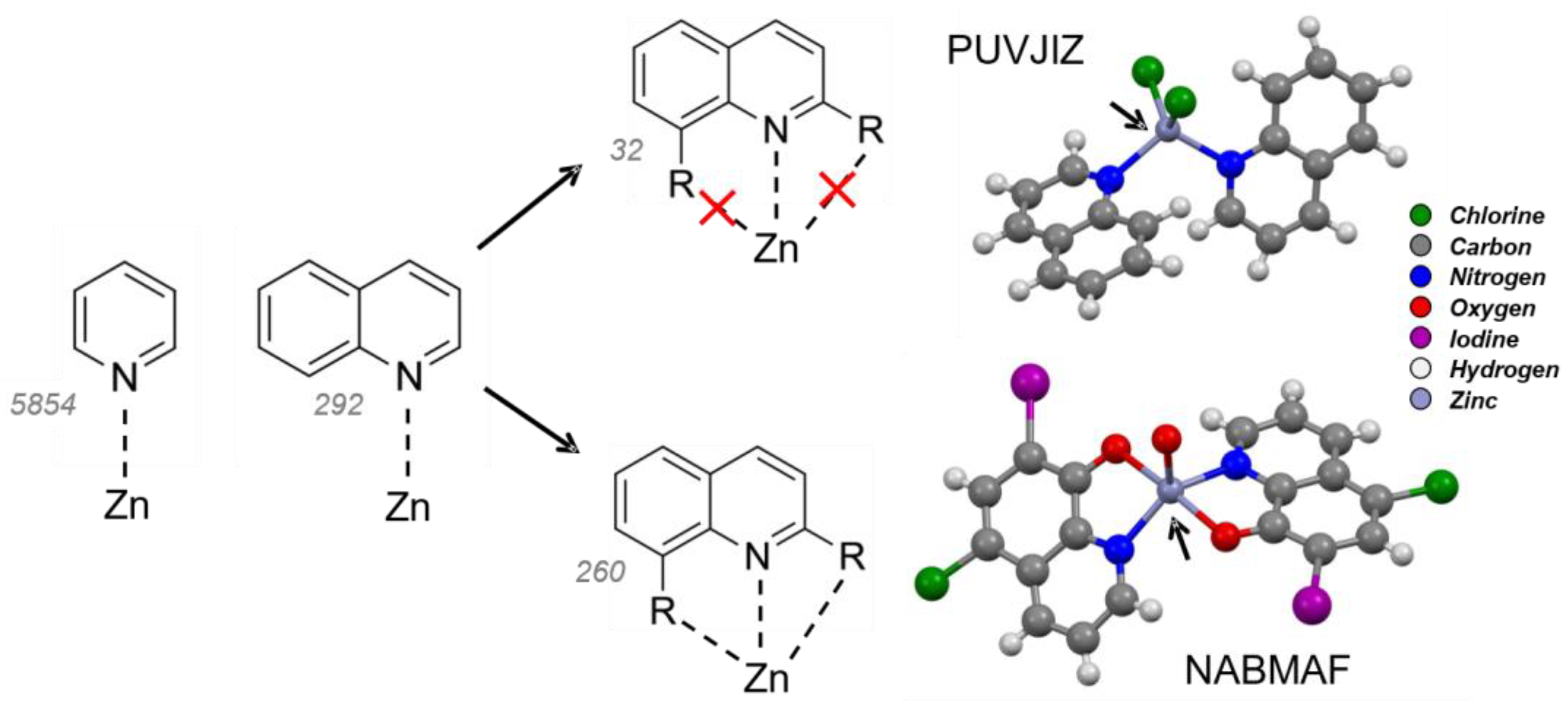
2.1. Quinoline–Zinc Structures in the Cambridge Structural Database
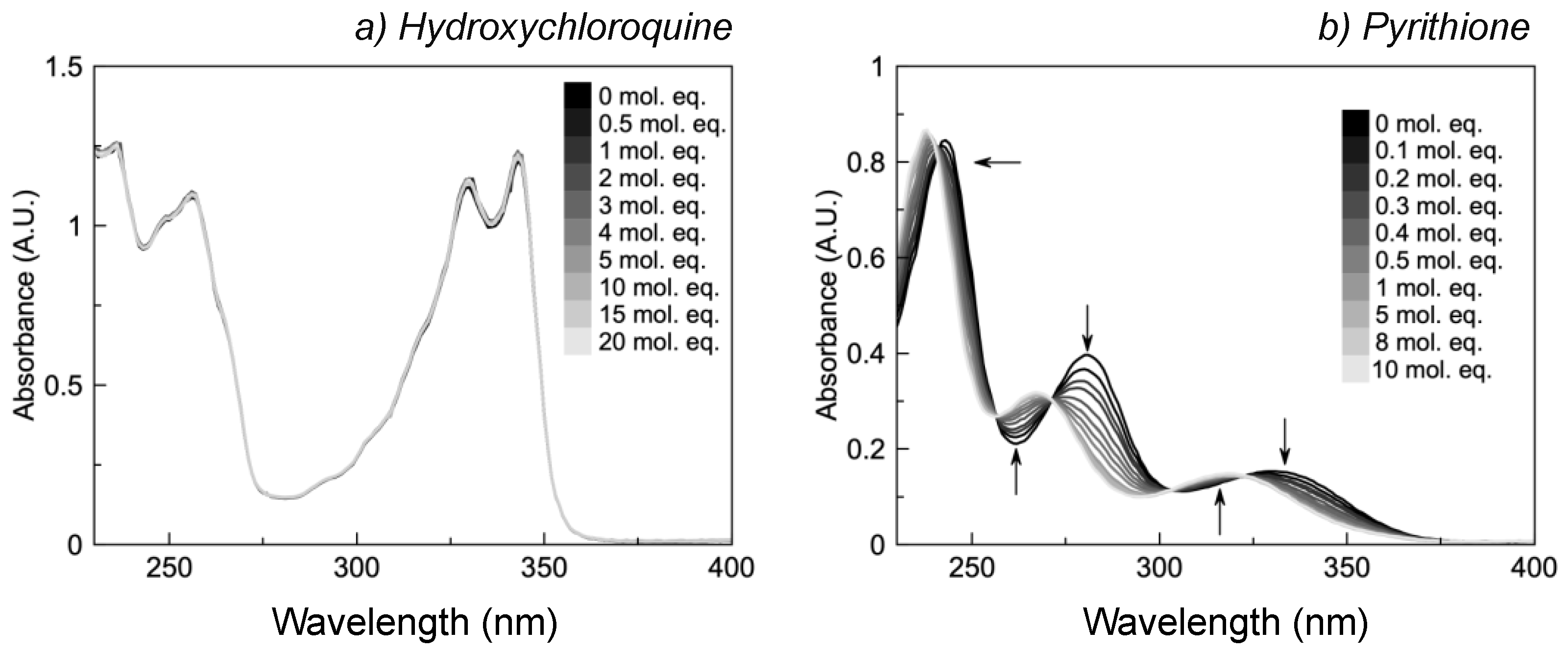
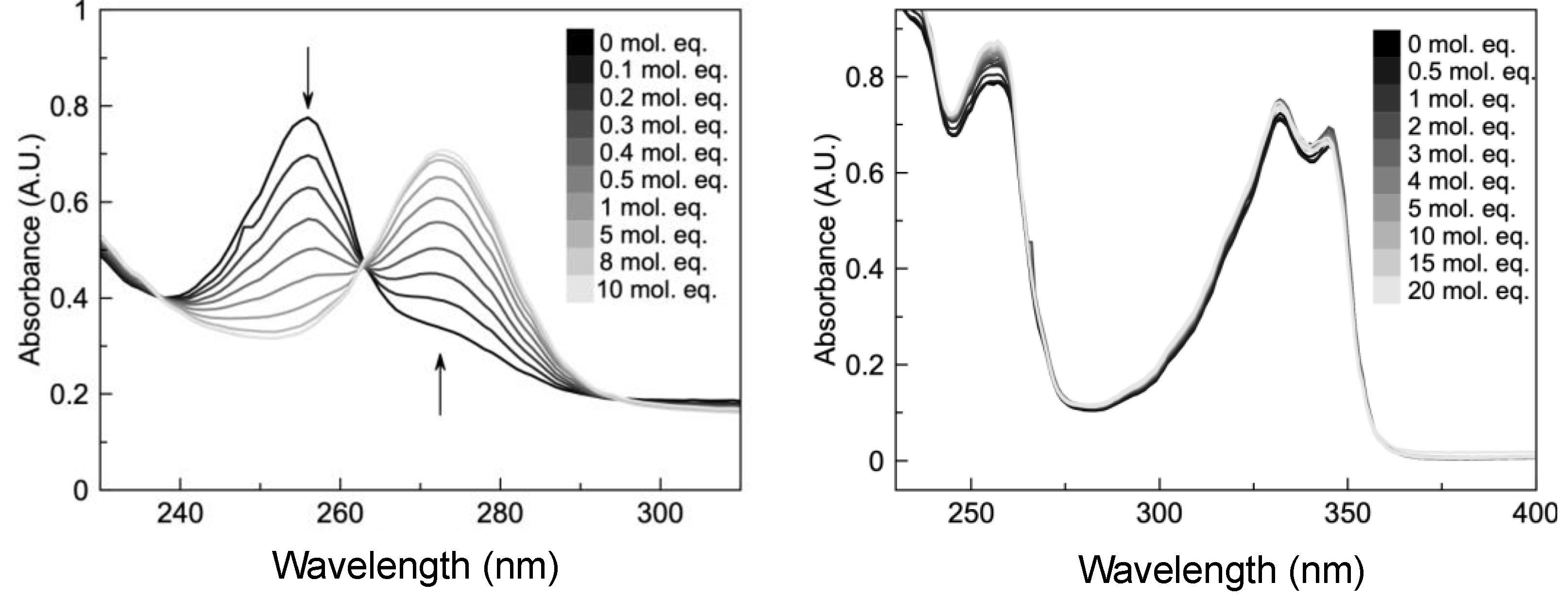
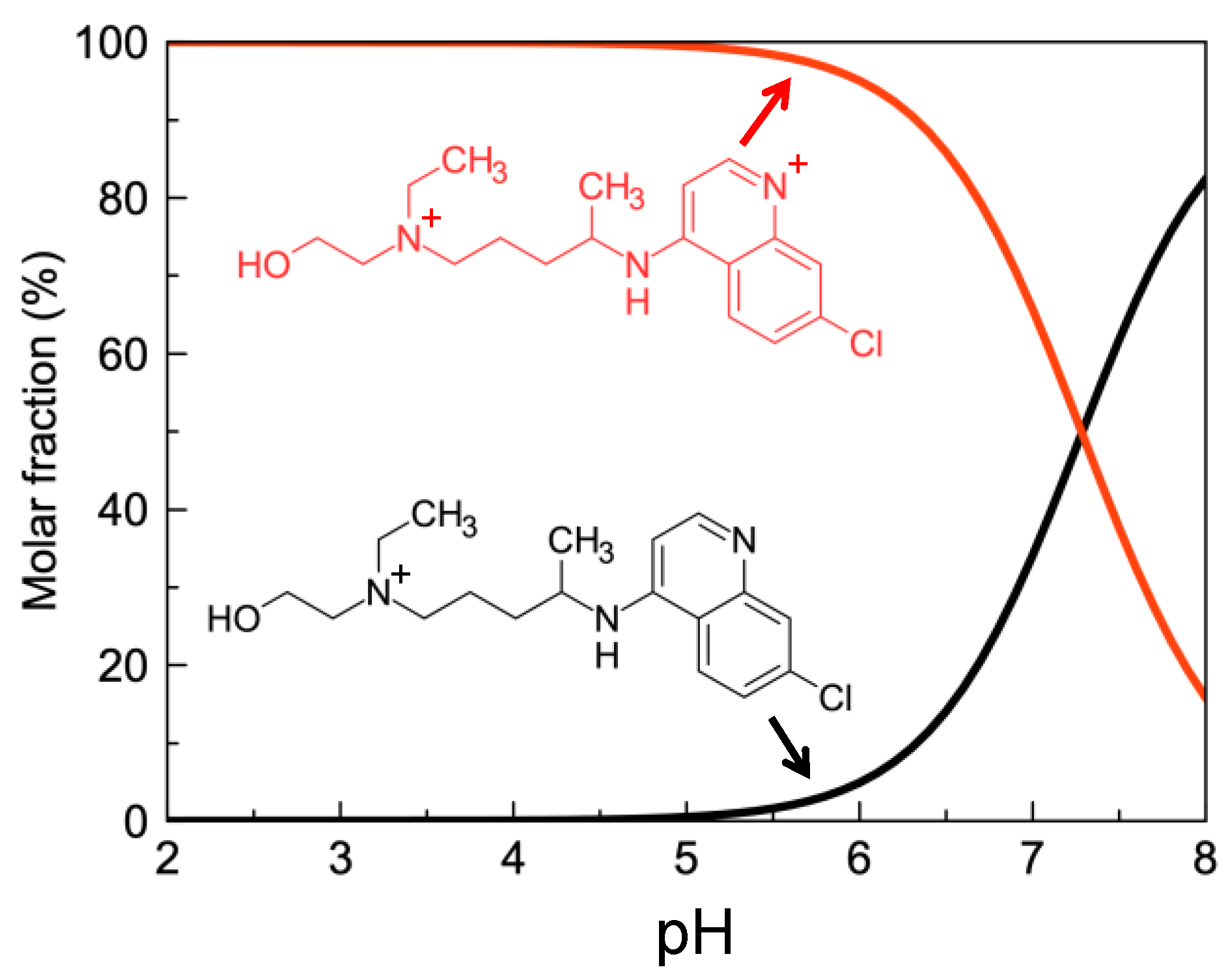
2.2. HCQ Does Not Exhibit Complexation Behavior with Zinc
2.3. Liposomal Assays Confirm Lack of Ionophoric Activity by HCQ
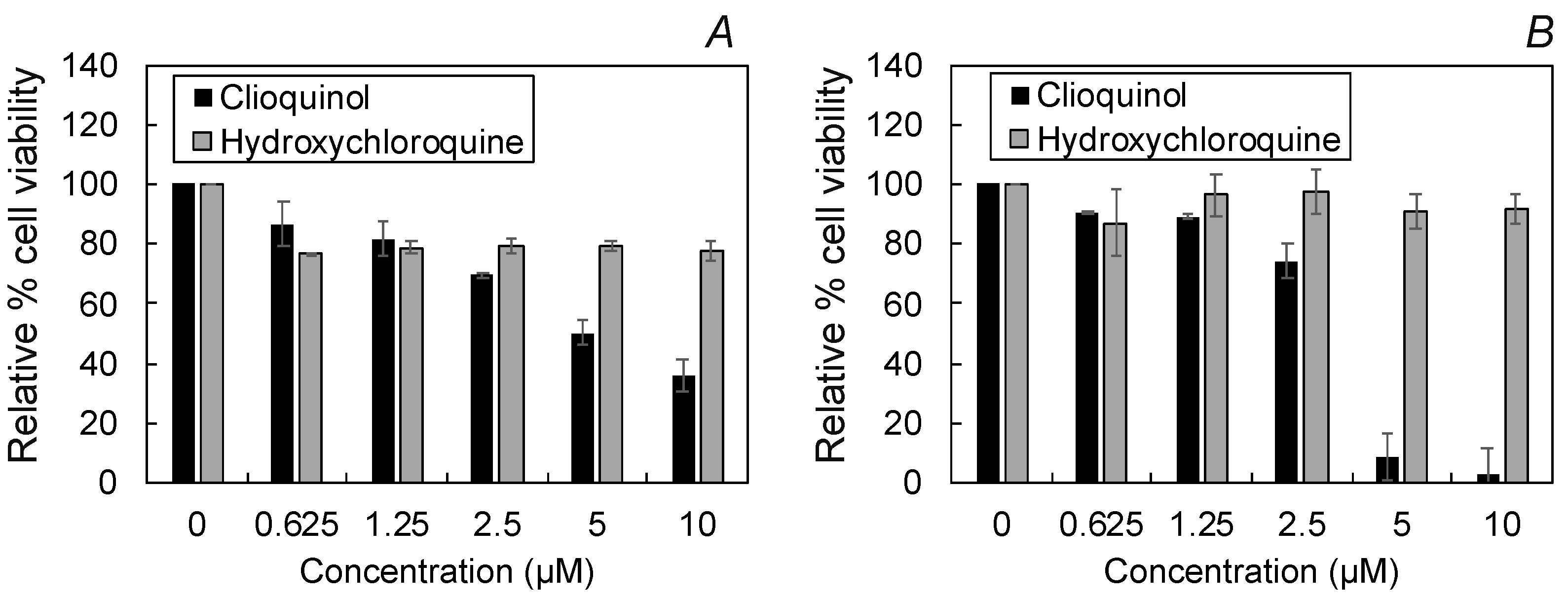
2.4. Cellular Toxicity and Fluorescence Imaging of HCQ Treated Cells
2.5. Molecular Models Predict Non-Ionophoric Mechanism for Future Study
3. Conclusions
4. Materials and Methods
4.1. Cambridge Structural Database
4.2. Complexation Studies
4.3. Synthesis of FluoZin-3 Loaded Liposomes
4.4. Liposomal Penetration Assay
4.5. Cell Culture Conditions
4.6. Cell Toxicity Assay
4.7. Fluoresczence Imaging
4.8. Molecular Dynamics Simulations and Supplementary Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohlmann, L.; de Oliveira, D.M.P.; El-Deeb, I.M.; Brazel, E.B.; Harbison-Price, N.; Ong, C.L.Y.; Rivera-Hernandez, T.; Ferguson, S.A.; Cork, A.J.; Phan, M.D.; et al. Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance. MBio 2018, 9, e02391-18. [Google Scholar] [CrossRef] [Green Version]
- Harbison-Price, N.; Ferguson, S.A.; Heikal, A.; Taiaroa, G.; Hards, K.; Nakatani, Y.; Rennison, D.; Brimble, M.A.; El-Deeb, I.M.; Bohlmann, L.; et al. Multiple Bactericidal Mechanisms of the Zinc Ionophore PBT2. mSphere 2020, 5, e00157-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, M.; Cossu, C.; Capurro, V.; Picco, C.; Ludovico, A.; Mielczarek, M.; Carreira-Barral, I.; Caci, E.; Baroni, D.; Quesada, R.; et al. Small molecule-facilitated anion transporters in cells for a novel therapeutic approach to cystic fibrosis. Pharmacology 2019, 176, 1764–1779. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Yoshizawa, H.; Yamawaki, K.; Yokoo, K.; Sato, J.; Hisakawa, S.; Hasegawa, Y.; Kusano, H.; Sano, M.; Sugimoto, H.; et al. Cefiderocol (S-649266), a new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur. J. Med. Chem. 2018, 155, 847–868. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Busschaert, N.; Wenzel, M.; Haynes, C.J.E.; Hiscock, J.R.; Kirby, I.L.; Karagiannidis, L.E.; Moore, S.J.; Wells, N.J.; Herniman, J.; et al. Towards predictable transmembrane transport: QSAR analysis of anion binding and transport. Chem. Sci. 2013, 4, 3036–3045. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.Q. Chloroquine Is a Zinc Ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, M.; Goitia, H.; Silva, P.; Velásquez, M.; Ojeda, L.E.; Fraile, G. Synthesis and characterization of new copper–and zinc–chloroquine complexes and their activities on respiratory burst of polymorphonuclear leukocytes. J. Inorg. Biochem. 2005, 99, 1630–1636. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency. Br. Med. J. 2003, 326, 409–410. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond iron: Non-classical biological functions of bacterial siderophores. Dalt. Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhou, Y.; Lind, S.E.; Ding, W.Q. Clioquinol targets zinc to lysosomes in human cancer cells. Biochem. J. 2009, 417, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, K.R.; Zhou, Z.L.; Ton-That, D.; Sensi, S.L.; Weiss, J.H. Measuring zinc in living cells.: A new generation of sensitive and selective fluorescent probes. Cell Calcium 2002, 31, 245–251. [Google Scholar] [CrossRef]
- Hecel, A.; Ostrowska, M.; Stokowa-Sołtys, K.; Wątły, J.; Dudek, D.; Miller, A.; Potocki, S.; Matera-Witkiewicz, A.; Dominguez-Martin, A.; Kozłowski, H. Zinc (II)—The overlooked éminence grise of chloroquine’s fight against COVID-19? Pharmaceuticals 2020, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, D.; Huang, M.; Huang, J.; Wang, D.; Liu, X.; Nguyen, M.; Vendier, L.; Mazères, S.; Robert, A.; et al. Preparation of Tetradentate Copper Chelators as Potential Anti-Alzheimer Agents. Chem. Med. Chem. 2018, 13, 684–704. [Google Scholar] [CrossRef]
- Navarro, M.; Hernández, C.; Vásquez, F.; Goitia, H.; Ojeda, L.E.; Velásquez, M.; Fraile, G. Syntheses, characterization, and biological evaluation of new zinc-and gold-chloroquine diphosphate complexes. Transit. Met. Chem. 2008, 33, 893–898. [Google Scholar] [CrossRef]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.K.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef]
- Sharma, P.; Reddy, P.K.; Kumar, B. Trace Element Zinc, a Nature’s Gift to Fight Unprecedented Global Pandemic COVID-19. Biol. Trace Elem. Res. 2021, 199, 3213–3221. [Google Scholar] [CrossRef]
- Ewing, D.F. The effect of solute concentration on the chemical shift of aromatic compounds. Some studies using 1-iodonaphthalene as a model compound. Org. Magn. Reson. 1973, 5, 321–325. [Google Scholar] [CrossRef]
- Levy, G.; Reuning, R.H. Effect of Complex Formation on Drug Absorption I: Complexes of Salicylic Acid with Absorbable and Nonabsorbable Compounds. J. Pharm. Sci. 1964, 53, 1471–1475. [Google Scholar] [CrossRef]
- Higuchi, T.; Gupta, M.; Busse, L.W. Influence of electrolytes, pH, and alcohol concentration on the solubilities of acidic drugs. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1953, 42, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. Optical Absorbance Spectroscopy. In Binding Constants: The Measurement of Molecular Complex Stability; Wiley: New York, NY, USA, 1987; pp. 141–145. [Google Scholar]
- Ovchinnikov, Y.A.; Ivanov, V.T. Specificity of Ionophore-Cation Interaction. In Specificity in Biological Interactions; Chagas, C., Pullman, B., Eds.; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Engl. 1972, 11, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, J.; Csizmadia, F. Abstracts of Papers of the American Chemical Society; ChemAxon: Budapest, Hungary, 2007; Volume 233. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Govorkova, E.A.; Murti, G.; Meignier, B.; de Taisne, C.; Webster, R.G. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J. Virol. 1996, 70, 5519–5524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MNicol, R.; Joshi, A.; Rizk, M.L.; Sabato, P.E.; Savic, R.M.; Wesche, D.; Zheng, J.H.; Cook, J. Pharmacokinetics and Pharmacological Properties of Chloroquine and Hydroxychloroquine in the Context of COVID-19 Infection. Clin. Pharmacol. Ther. 2020, 108, 1135–1149. [Google Scholar]
- Ramser, B.; Kokot, A.; Metze, D.; Weiß, N.; Luger, T.A.; Böhm, M. Hydroxychloroquine modulates metabolic activity and proliferation and induces autophagic cell death of human dermal fibroblasts. J. Investig. Dermatol. 2009, 129, 2419–2426. [Google Scholar] [CrossRef] [Green Version]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Wang, W.; Zhao, B.; Zhang, S.; Miao, J. Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorg. Med. Chem. 2006, 14, 3218–3222. [Google Scholar] [CrossRef]
- Macintrye, A.C.; Cutler, D.J. Role of lysosomes in hepatic accumulation of chloroquine. J. Pharm. Sci. 1988, 77, 196–199. [Google Scholar] [CrossRef]
- Sundelin, S.P.; Terman, A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. Apmis 2002, 110, 481–489. [Google Scholar] [CrossRef]
- Daniel, W.A.; Bickel, M.H.; Honegger, U.E. The contribution of lysosomal trapping in the uptake of desipramine and chloroquine by different tissues. Pharmacol. Toxicol. 1995, 77, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.; Horgan, K.; Clynes, M.; Sinkunaite, I.; Ward, P.; Murphy, R.; O’Sullivan, F. Unexpected fluctuations of trace element levels in cell culture medium in vitro: Caveat emptor. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.N.; Elmes, R.; O’Sullivan, F.; Farragher, J.; Robinson, S.; Walker, G. Investigating Structural Property Relationships to Enable Repurposing of Pharmaceuticals as Zinc Ionophores. Pharmaceutics 2021, 13, 2032. [Google Scholar] [CrossRef]
- Omichinski, J.G.; Clore, G.M.; Appella, E.; Sakaguchi, K.; Gronenborn, A.M. High-resolution three-dimensional structure of a single zinc finger from a human enhancer binding protein in solution. Biochemistry 1990, 29, 9324–9334. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmuller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond perception and atom typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A.J. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. Journal of molecular graphics and modelling. J. Mol. Graph Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Hockney, R.W. The potential calculation and some applications. Methods Comput. Phys. 1970, 9, 136. [Google Scholar]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.; Postma, J.V.; van Gunsteren, W.F.; DiNola, A.; Haak, J. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavanagh, O.N.; Bhattacharya, S.; Marchetti, L.; Elmes, R.; O’Sullivan, F.; Farragher, J.P.; Robinson, S.; Thompson, D.; Walker, G.M. Hydroxychloroquine Does Not Function as a Direct Zinc Ionophore. Pharmaceutics 2022, 14, 899. https://doi.org/10.3390/pharmaceutics14050899
Kavanagh ON, Bhattacharya S, Marchetti L, Elmes R, O’Sullivan F, Farragher JP, Robinson S, Thompson D, Walker GM. Hydroxychloroquine Does Not Function as a Direct Zinc Ionophore. Pharmaceutics. 2022; 14(5):899. https://doi.org/10.3390/pharmaceutics14050899
Chicago/Turabian StyleKavanagh, Oisín N., Shayon Bhattacharya, Luke Marchetti, Robert Elmes, Finbarr O’Sullivan, John P. Farragher, Shane Robinson, Damien Thompson, and Gavin M. Walker. 2022. "Hydroxychloroquine Does Not Function as a Direct Zinc Ionophore" Pharmaceutics 14, no. 5: 899. https://doi.org/10.3390/pharmaceutics14050899
APA StyleKavanagh, O. N., Bhattacharya, S., Marchetti, L., Elmes, R., O’Sullivan, F., Farragher, J. P., Robinson, S., Thompson, D., & Walker, G. M. (2022). Hydroxychloroquine Does Not Function as a Direct Zinc Ionophore. Pharmaceutics, 14(5), 899. https://doi.org/10.3390/pharmaceutics14050899









